Abstract
Although many components of the cell division machinery in bacteria have been identified1,2, the mechanisms by which they work together to divide the cell remain poorly understood. Key among these components is the tubulin FtsZ, which forms a Z ring at the midcell. FtsZ recruits the other cell division proteins, collectively called the divisome, and the Z ring constricts as the cell divides. We applied live-cell single-molecule imaging to describe the dynamics of the divisome in detail, and to evaluate the individual roles of FtsZ-binding proteins (ZBPs), specifically FtsA and the ZBPs EzrA, SepF and ZapA, in cytokinesis. We show that the divisome comprises two subcomplexes that move differently: stationary ZBPs that transiently bind to treadmilling FtsZ filaments, and a moving complex that includes cell wall synthases. Our imaging analyses reveal that ZBPs bundle FtsZ filaments together and condense them into Z rings, and that this condensation is necessary for cytokinesis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request. Source data are provided with this paper.
Code availability
Custom MATLAB code is available at https://bitbucket.org/garnerlab/squyres-2020.
References
Erickson, H. P., Anderson, D. E. & Osawa, M. FtsZ in bacterial cytokinesis: cytoskeleton and force generator all in one. Microbiol. Mol. Biol. Rev. 74, 504–528 (2010).
Adams, D. W. & Errington, J. Bacterial cell division: assembly, maintenance and disassembly of the Z ring. Nat. Rev. Microbiol. 7, 642–653 (2009).
Gamba, P., Veening, J.-W., Saunders, N. J., Hamoen, L. W. & Daniel, R. A. Two-step assembly dynamics of the Bacillus subtilis divisome. J. Bacteriol. 191, 4186–4194 (2009).
Meier, E. L. & Goley, E. D. Form and function of the bacterial cytokinetic ring. Curr. Opin. Cell Biol. 26, 19–27 (2014).
Bisson-Filho, A. W. et al. Treadmilling by FtsZ filaments drives peptidoglycan synthesis and bacterial cell division. Science 355, 739–743 (2017).
Yang, X. et al. GTPase activity-coupled treadmilling of the bacterial tubulin FtsZ organizes septal cell wall synthesis. Science 355, 744–747 (2017).
Grimm, J. B. et al. A general method to improve fluorophores for live-cell and single-molecule microscopy. Nat. Methods 12, 244–250 (2015).
Perez, A. J. et al. Movement dynamics of divisome proteins and PBP2x:FtsW in cells of Streptococcus pneumoniae. Proc. Natl Acad. Sci. 116, 3211–3220 (2019).
Taguchi, A. et al. FtsW is a peptidoglycan polymerase that is functional only in complex with its cognate penicillin-binding protein. Nat. Microbiol 4, 587–594 (2019).
Noirclerc-Savoye, M. et al. In vitro reconstitution of a trimeric complex of DivIB, DivIC and FtsL, and their transient co-localization at the division site in Streptococcus pneumoniae. Mol. Microbiol. 55, 413–424 (2005).
Loose, M. & Mitchison, T. J. The bacterial cell division proteins FtsA and FtsZ self-organize into dynamic cytoskeletal patterns. Nat. Cell Biol. 16, 38–46 (2014).
Ramirez-Diaz, D. A. et al. Treadmilling analysis reveals new insights into dynamic FtsZ ring architecture. PLoS Biol. 16, e2004845 (2018).
Anderson, D. E., Gueiros-Filho, F. J. & Erickson, H. P. Assembly dynamics of FtsZ rings in Bacillus subtilis and Escherichia coli and effects of FtsZ-regulating proteins. J. Bacteriol. 186, 5775–5781 (2004).
Rodrigues, C. D. A. & Harry, E. J. The Min system and nucleoid occlusion are not required for identifying the division site in Bacillus subtilis but ensure its efficient utilization. PLoS Genet. 8, e1002561 (2012).
Bisson-Filho, A. W. et al. FtsZ filament capping by MciZ, a developmental regulator of bacterial division. Proc. Natl Acad. Sci. USA 112, E2130–E2138 (2015).
Northrup, S. H. & Erickson, H. P. Kinetics of protein–protein association explained by Brownian dynamics computer simulation. Proc. Natl Acad. Sci. USA 89, 3338–3342 (1992).
Romberg, L., Simon, M. & Erickson, H. P. Polymerization of Ftsz, a bacterial homolog of tubulin. Is assembly cooperative? J. Biol. Chem. 276, 11743–11753 (2001).
Gueiros-Filho, F. J. & Losick, R. A widely conserved bacterial cell division protein that promotes assembly of the tubulin-like protein FtsZ. Genes Dev. 16, 2544–2556 (2002).
Low, H. H., Moncrieffe, M. C. & Löwe, J. The crystal structure of ZapA and its modulation of FtsZ polymerisation. J. Mol. Biol. 341, 839–852 (2004).
Small, E. et al. FtsZ polymer-bundling by the Escherichia coli ZapA orthologue, YgfE, involves a conformational change in bound GTP. J. Mol. Biol. 369, 210–221 (2007).
Singh, J. K., Makde, R. D., Kumar, V. & Panda, D. SepF increases the assembly and bundling of FtsZ polymers and stabilizes FtsZ protofilaments by binding along its length. J. Biol. Chem. 283, 31116–31124 (2008).
Dajkovic, A., Pichoff, S., Lutkenhaus, J. & Wirtz, D. Cross-linking FtsZ polymers into coherent Z rings. Mol. Microbiol. 78, 651–668 (2010).
Gündoğdu, M. E. et al. Large ring polymers align FtsZ polymers for normal septum formation. EMBO J. 30, 617–626 (2011).
Pacheco-Gómez, R. et al. Tetramerization of ZapA is required for FtsZ bundling. Biochem. J. 449, 795–802 (2013).
Caldas, P. et al. Cooperative ordering of treadmilling filaments in cytoskeletal networks of FtsZ and its crosslinker ZapA. Nat. Commun. 10, 5744 (2019).
Monahan, L. G., Robinson, A. & Harry, E. J. Lateral FtsZ association and the assembly of the cytokinetic Z ring in bacteria. Mol. Microbiol. 74, 1004–1017 (2009).
Buss, J. et al. In vivo organization of the FtsZ-ring by ZapA and ZapB revealed by quantitative super-resolution microscopy. Mol. Microbiol. 89, 1099–1120 (2013).
Duman, R. et al. Structural and genetic analyses reveal the protein SepF as a new membrane anchor for the Z ring. Proc. Natl Acad. Sci. USA 110, E4601–E4610 (2013).
Hamoen, L. W., Meile, J.-C., de Jong, W., Noirot, P. & Errington, J. SepF, a novel FtsZ-interacting protein required for a late step in cell division. Mol. Microbiol. 59, 989–999 (2006).
Haeusser, D. P., Schwartz, R. L., Smith, A. M., Oates, M. E. & Levin, P. A. EzrA prevents aberrant cell division by modulating assembly of the cytoskeletal protein FtsZ. Mol. Microbiol. 52, 801–814 (2004).
Singh, J. K., Makde, R. D., Kumar, V. & Panda, D. A membrane protein, EzrA, regulates assembly dynamics of FtsZ by interacting with the C-terminal tail of FtsZ. Biochemistry 46, 11013–11022 (2007).
Cleverley, R. M. et al. Structure and function of a spectrin-like regulator of bacterial cytokinesis. Nat. Commun. 5, 5421 (2014).
Levin, P. A., Kurtser, I. G. & Grossman, A. D. Identification and characterization of a negative regulator of FtsZ ring formation in Bacillus subtilis. Proc. Natl Acad. Sci. USA 96, 9642–9647 (1999).
Haeusser, D. P., Garza, A. C., Buscher, A. Z. & Levin, P. A. The division inhibitor EzrA contains a seven-residue patch required for maintaining the dynamic nature of the medial FtsZ Ring. J. Bacteriol. 189, 9001–9010 (2007).
Guan, F. et al. Lateral interactions between protofilaments of the bacterial tubulin homolog FtsZ are essential for cell division. eLife 7, 30 (2018).
Shin, J. Y., Vollmer, W., Lagos, R. & Monasterio, O. Glutamate 83 and arginine 85 of helix H3 bend are key residues for FtsZ polymerization, GTPase activity and cellular viability of Escherichia coli: lateral mutations affect FtsZ polymerization and E. coli viability. BMC Microbiol. 13, 26 (2013).
Pichoff, S. & Lutkenhaus, J. Tethering the Z ring to the membrane through a conserved membrane targeting sequence in FtsA. Mol. Microbiol. 55, 1722–1734 (2005).
Beall, B. & Lutkenhaus, J. Impaired cell division and sporulation of a Bacillus subtilis strain with the ftsA gene deleted. J. Bacteriol. 174, 2398–2403 (1992).
Jensen, S. O., Thompson, L. S. & Harry, E. J. Cell division in Bacillus subtilis: FtsZ and FtsA association is Z-ring independent, and FtsA is required for efficient midcell Z-Ring assembly. J. Bacteriol. 187, 6536–6544 (2005).
Krupka, M. & Margolin, W. Unite to divide: oligomerization of tubulin and actin homologs regulates initiation of bacterial cell division. F1000Res 7, 235 (2018).
Huang, K.-H., Durand-Heredia, J. & Janakiraman, A. FtsZ ring stability: of bundles, tubules, crosslinks, and curves. J. Bacteriol. 195, 1859–1868 (2013).
Bhattacharya, D., Kumar, A. & Panda, D. WhmD promotes the assembly of Mycobacterium smegmatis FtsZ: a possible role of WhmD in bacterial cell division. Int. J. Biol. Macromol. 95, 582–591 (2017).
Eswara, P. J. et al. An essential Staphylococcus aureus cell division protein directly regulates FtsZ dynamics. eLife 7, e38856 (2018).
Sogues, A. et al. Essential dynamic interdependence of FtsZ and SepF for Z-ring and septum formation in Corynebacterium glutamicum. Nat. Commun. 11, 1641 (2020).
Rahman, M. U. et al. Assembly properties of bacterial tubulin homolog FtsZ regulated by the positive regulator protein ZipA and ZapA from Pseudomonas aeruginosa. Sci. Rep. 10, 21369 (2020).
Pende, N. et al. SepF is the FtsZ-anchor in Archaea: implications for cell division in the Last Universal Common Ancestor. Preprint at bioRxiv https://doi.org/10.1101/2020.10.06.328377 (2020).
Mateos-Gil, P., Tarazona, P. & Vélez, M. Bacterial cell division: modeling FtsZ assembly and force generation from single filament experimental data. FEMS Microbiol. Rev. 43, 73–87 (2019).
Osawa, M., Anderson, D. E. & Erickson, H. P. Reconstitution of contractile FtsZ rings in liposomes. Science 320, 792–794 (2008).
Szwedziak, P., Wang, Q., Bharat, T. A. M., Tsim, M. & Löwe, J. Architecture of the ring formed by the tubulin homologue FtsZ in bacterial cell division. eLife 3, e04601 (2014).
Guigas, G. & Weiss, M. Effects of protein crowding on membrane systems. Biochim. Biophys. Acta 1858, 2441–2450 (2016).
Cayley, D. S., Guttman, H. J. & Record, M. T. Biophysical characterization of changes in amounts and activity of Escherichia coli cell and compartment water and turgor pressure in response to osmotic stress. Biophys J. 78, 1748–1764 (2000).
Erickson, H. P. How bacterial cell division might cheat turgor pressure – a unified mechanism of septal division in Gram-positive and Gram-negative bacteria. Bioessays 39, 1700045 (2017).
Nguyen, L. T. et al. Simulations suggest a constrictive force is required for Gram-negative bacterial cell division. Nat. Commun. 10, 1259 (2019).
Kner, P., Chhun, B. B., Griffis, E. R., Winoto, L. & Gustafsson, M. G. L. Super-resolution video microscopy of live cells by structured illumination. Nat. Methods 6, 339–342 (2009).
Hoffmann, H. Violin plot. MathWorks https://www.mathworks.com/matlabcentral/fileexchange/45134-violin-plot (2015).
Van Valen, D. A. et al. Deep learning automates the quantitative analysis of individual cells in live-cell imaging experiments. PLoS Comput. Biol. 12, e1005177 (2016).
Tinevez, J.-Y. et al. TrackMate: an open and extensible platform for single-particle tracking. Methods https://doi.org/10.1016/j.ymeth.2016.09.016 (2016).
Bronson, J. E., Fei, J., Hofman, J. M., Gonzalez, R. L. Jr & Wiggins, C. H. Learning rates and states from biophysical time series: a Bayesian approach to model selection and Single-Molecule FRET data. Biophysj 97, 3196–3205 (2009).
Levin, P. A. & Losick, R. Characterization of a cell division gene from Bacillus subtilis that is required for vegetative and sporulation septum formation. J. Bacteriol. 176, 1451–1459 (1994).
Gamba, P., Hamoen, L. W. & Daniel, R. A. Cooperative recruitment of FtsW to the division site of Bacillus subtilis. Front. Microbiol. 7, 1808 (2016).
Daniel, R. A., Harry, E. J., Katis, V. L., Wake, R. G. & Errington, J. Characterization of the essential cell division gene ftsL (yIID) of Bacillus subtilis and its role in the assembly of the division apparatus. Mol. Microbiol. 29, 593–604 (1998).
Daniel, R. A., Harry, E. J. & Errington, J. Role of penicillin-binding protein PBP 2B in assembly and functioning of the division machinery of Bacillus subtilis. Mol. Microbiol. 35, 299–311 (2000).
Acknowledgements
We thank the Garner laboratory, especially A. Bisson-Filho, Y. Sun and S. Wilson, for discussions and strains, and L. Lavis for JF dyes. SIM–TIRF was performed at the Advanced Imaging Center at the Janelia Research Campus, a facility jointly supported by the Gordon and Betty Moore Foundation and Howard Hughes Medical Institute. This work was funded by the National Institutes of Health grant DP2AI117923-01 to E.C.G., as well as support from the Volkswagen Foundation, NSF GRFP (DGE1144152) to G.R.S., and the physiology course at the Marine Biological Laboratory at Woods Hole. This work was supported by the NSF-Simons Center for Mathematical and Statistical Analysis of Biology at Harvard (1764269) and the Harvard Quantitative Biology Initiative.
Author information
Authors and Affiliations
Contributions
G.R.S., M.J.H. and E.C.G. designed the experiments. G.R.S. conducted and analysed FtsZ lifetime experiments. M.J.H. conducted and analysed divisome dynamics experiments. G.R.S. and M.J.H. conducted and analysed ZBP perturbation experiments. S.R.B., B.R.P. and J.R. helped optimize the FtsZ lifetime assay. V.T.Y. helped initiate the ZBP perturbation experiments. G.R.S., M.J.H. and E.C.G. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Microbiology thanks Tanneke den Blaauwen, Liz Harry, Suliana Manley and the other, anonymous, reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
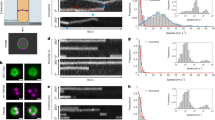
Extended Data Fig. 1 Cell lengths with fusions for fluorescence microscopy.
a Cell lengths in strains expressing HaloTag (HT) fusions used in this study. (sw) indicates a sandwich fusion. Cell lengths were measured from confocal microscopy of FM5-95 membrane stained cells. When cell division is inhibited, cell length increases; that cell lengths in each strain are equal to or less than that of wild type (WT) cells indicates that these fluorescent fusions do not strongly inhibit cell division. In some cases where the fluorescent fusion is merodiploid or expressed under inducible control, cells are shorter than WT, as might be expected when components of the cell division machinery are overexpressed. Blue: fusions to early-arriving division proteins, red: fusions to late-arriving division proteins. Gray lines: mean (solid line) ± standard deviation (dashed lines) for WT cell lengths. N>400 cells for each sample. b Lengths of cells with various division proteins knocked out, for comparison with a. We include all knockouts whose lengths can be measured in a straightforward way: ∆ftsA cells have severe division defects and are highly elongated (see Extended Data Fig. 10), and the remaining division proteins are essential to avoid lethal filamentation59,60,61,62. N>140 cells for each sample. c EzrA and ZapA HT fusions are functional and SepF HT fusion expressed at an ectopic site does not disrupt SepF function. EzrA is synthetically lethal with SepF and ZapA18,29. We therefore knocked out one of these proteins and then expressed our HT fusion to the other protein; if HT fusion induced a critical defect in protein function, this combination will be lethal. Instead, in each case cells remained viable, with comparable lengths to the knockout alone. N>200 cells for each sample.
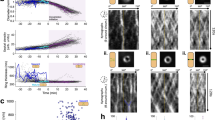
Extended Data Fig. 2 Controls for FtsZ lifetime measurements.
a FtsZ subunit lifetime is consistent across experimental replicates. To measure lifetimes, cells expressing FtsZ-HaloTag were induced with 20 µM IPTG for 2 hours, labelled with 40 pM JF549-HTL, and then imaged by TIRFM. Light curves: 4 experimental replicates for which N>200. Points represent combined data from all experimental replicates (17 replicates). Error bars: weighted standard deviation of distributions for all replicates. b FtsZ subunit lifetime is consistent across measurement techniques. Lifetime distributions were measured using an automated hidden Markov model (HMM) based analysis pipeline and manually for N = 265 particles (dashed line). c FtsZ subunit lifetime is not affected by Pbp2B tagging. The 1-colour strain (bAB309) contains labelled FtsZ-HaloTag, induced as a second copy with 20 µM IPTG for 2 hours; the 2-colour strain (bGS104) contain both this FtsZ-HaloTag construct and a native mNeonGreen-Pbp2B fusion, which was used to localize the division site. d FtsZ subunit lifetime is not affected by photobleaching. If the measurements were affected by photobleaching, the measured lifetimes would increase when we decrease the imaging interval; however, we see that the lifetime distributions are consistent for images taken at 0.5-second intervals and 1-second intervals. For images at 0.5-second intervals, images were acquired continuously with 0.5-second exposures. For images at 1-second intervals, images were acquired with the same settings, with 0.5 seconds of exposure and 0.5-second intervals without illumination. e Co-overexpression of FtsA and FtsZ increases the number of FtsZ filaments in the cell (left) but does not change FtsZ subunit lifetime (right). The increased number of filaments that form upon FtsAZ overexpression is consistent with steady-state treadmilling of FtsZ. The fact that the subunit lifetime does not change when FtsAZ is overexpressed further indicates that the additional FtsZ forms new filaments rather than elongating existing filaments. A second copy of ftsAZ is expressed from an IPTG-inducible promoter with 100 µM IPTG for 2 hours. Filament density is visualized by TIRFM for at least two replicates of each condition. Scale bar: 2 µm.
Extended Data Fig. 3 Effects of individual ZBP knockouts on cell and Z ring morphology.
Each pair of images shows cell morphology (phase-contrast imaging, left), and Z ring morphology (epifluorescence images of cells expressing FtsZ-mNeonGreen induced with 20 µM IPTG for 2 hours, right) in control cells, compared to cells with individual ZBPs deletions. ∆ezrA cells less condensed Z rings, along with the expected Z rings near their poles33; ∆sepF and ∆zapA cells have normal Z rings. FtsZ(T111A) mutant cells have excess Z rings and form mini-cells. The distribution of Z ring widths in each strain is plotted at bottom left. Representative images from at least two replicates of each condition. Scale bars: 2 µm.
Extended Data Fig. 4 Effects of ZBP overexpression on FtsZ.
a Each pair of images shows cell morphology (phase-contrast imaging, left), and Z ring morphology (epifluorescence images of cells expressing FtsZ-mNeonGreen induced with 20 µM IPTG for 2 hours, right), in cells overexpressing SepF and ZapA. These cells have normal Z ring morphology except for some polar Z rings in SepF-overexpressing cells. Second copies of sepF and zapA were expressed from a xylose-inducible promoter with 30 mM xylose for 2 hours. Representative images from at least two replicates of each condition. Scale bars: 2 µm. b sepF- and zapA-overexpressing cells have similar FtsZ treadmilling velocities (left) and subunit lifetimes (right) to control cells. For velocity measurements, FtsZ-mNeonGreen was induced with 20 µM IPTG for 2 hours, imaged by TIRFM, and analysed from kymographs. For lifetime measurements, FtsZ-HaloTag was induced with 20 µM IPTG for 2 hours and labelled with 40 pM JF549-HTL. c Each pair of images shows cell morphology (phase-contrast imaging, left), and Z ring morphology (epifluorescence images of cells expressing FtsZ-mNeonGreen induced with 20 µM IPTG for 2 hours, right), in control cells and cells with EzrA overexpressed. EzrA-overexpressing cells have perturbed Z ring morphology, as expected30, a phenotype exacerbated with increasing induction. A second copy of ezrA was expressed from a xylose-inducible promoter by adding xylose at the indicated mM concentration. The 0.1, 0.5, and 5 mM concentrations were selected for quantitative analysis as the 10 and 20 mM xylose overexpression yielded unstable FtsZ filaments whose lifetimes were too short to be measured accurately. Representative images from at least two replicates of each condition. Scale bars: 2 µm.
Extended Data Fig. 5 Effects of removing synthetically lethal combinations of ZBPs on cell and Z ring morphology.
Each pair of images shows cell morphology (phase-contrast imaging, left), and Z ring morphology (epifluorescence images of cells expressing FtsZ-mNeonGreen induced with 20 µM IPTG for 2 hours, right), in control cells and cells lacking synthetically lethal combinations of ZBPs. To achieve this, a combination of knockouts (indicated by ∆) and depletions (indicated by ↓) were used; depletions were performed by expressing each gene under an inducible promoter until the start of the experiment, then withdrawing the inducer for 7 hours. This was repeated for all permutations of synthetically lethal combinations of ZBPs; all of these combinations result in elongated cells and disrupted Z ring architecture. Representative images from at least two replicates of each condition. Scale bars: 2 µm.
Extended Data Fig. 6 Effects of removing synthetically lethal combinations of ZBPs on FtsZ.
Velocity, lifetime, and Z ring morphology measurements for cells missing each synthetic lethal combination of ZBPs. All synthetic lethal combinations were investigated by a combination of knockouts (indicated by ∆) and depletions (indicated by ↓); depletions were performed by expressing the gene under an inducible promoter until the start of the experiment, then withdrawing the inducer for 7 hours. a Velocity (left) and lifetime (right) of cells missing synthetically lethal combinations of ZBPs are unchanged from control. For velocity measurements, FtsZ-mNeonGreen was induced with 20 µM IPTG for 2 hours, imaged by TIRFM, and then analysed from kymographs. For lifetime measurements, FtsZ-HaloTag was induced with 20 µM IPTG for 2 hours and labelled with 40 pM JF549-HTL. bc Z rings in cells missing synthetically lethal combinations of ZBPs are wider than control cells and cells missing individual ZBPs. Average intensity projections (b) and widths (c) of Z rings in each condition. Z rings were visualized using epifluorescence images of cells expressing FtsZ-mNeonGreen, induced with 20 µM IPTG for 2 hours. Z ring projections were created by averaging >100 Z ring images for each strain. Because ZBPs can be removed by either knockout or depletion, for each strain we compare to the equivalent single mutant knockouts and depletions.
Extended Data Fig. 7 Effects of a ∆sepF ∆zapA dual knockout.
top Each pair of images shows cell morphology (phase-contrast imaging, left), and Z ring morphology (epifluorescence images of cells expressing FtsZ-mNeonGreen induced with 20 µM IPTG for 2 hours, right), in control cells and cells with both sepF and zapA knocked out: ∆sepF ∆zapA is the only combination of ZBP deletions that is not synthetically lethal and their Z ring morphology is normal. Representative images from at least two replicates of each condition. Scale bars: 2 µm. bottom ∆sepF ∆zapA cells have similar FtsZ treadmilling velocities (left), subunit lifetimes (centre), and Z ring widths (right) to control cells. For velocity measurements, FtsZ-mNeonGreen was induced with 20 µM IPTG for 2 hours, imaged by TIRFM, and analysed from kymographs. For lifetime measurements, FtsZ-HaloTag was induced with 20 µM IPTG for 2 hours and labelled with 40 pM JF549-HTL. For Z ring width measurements, FtsZ-mNeonGreen was induced with 20 µM IPTG for 2 hours and imaged by epifluorescence.
Extended Data Fig. 8 Characterization of the FtsZ(K86E) suppressor mutant.
a Each pair of images shows cell morphology (phase-contrast imaging, left), and Z ring morphology (epifluorescence images of cells expressing FtsZ-mNeonGreen induced with 20 µM IPTG for 2 hours, right) in FtsZ(K86E) and FtsZ(K86E) ∆ezrA ∆zapA cells. FtsZ(K86E) Z rings look similar to the control. Z rings in FtsZ(K86E) ∆ezrA ∆zapA are somewhat perturbed, but less so than typical cells missing synthetically lethal combinations of ZBPs; they also have polar Z rings, as is typical for ∆ezrA strains. Representative images from at least two replicates of each condition. Scale bars: 2 µm. b FtsZ(K86E) and FtsZ(K86E) ∆ezrA ∆zapA have similar FtsZ treadmilling velocities to control (left), and FtsZ(K86E) Z rings are identical in width to control, while FtsZ(K86E) ∆ezrA ∆zapA are wider (right). For velocity measurements in each strain, FtsZ-mNeonGreen was induced with 20 µM IPTG for 2 hours, imaged by TIRFM, and analysed from kymographs. For Z ring width measurements, FtsZ-mNeonGreen was induced with 20 µM IPTG for 2 hours and imaged by epifluorescence. c Pbp2B intensity at midcell in FtsZ(K86E) mutant cells. Left: Representative images of Pbp2B in the indicated strains, visualized by epifluorescence imaging of cells expressing Pbp2B-mNeonGreen, from at least 4 replicates of each condition. Right: Pbp2B intensity at the division site in each strain. Although the FtsZ(K86E) restores viability in a ∆ezrA ∆zapA strain, it does so without rescuing Pbp2B recruitment to midcell. For each box plot, the white line indicates the median, the box extends to the 25th and 75th percentiles, and the whiskers indicate 1.5x interquartile range. P-values were obtained from a two-sided t-test; ns indicates p > 0.5, **** indicates p<0.0001, and p-values are included in parenthesis. N>5000 division sites for each condition. Scale bars: 2 µm.
Extended Data Fig. 9 Pbp2B localization and FDAA incorporation in ∆ZBPs cells.
a Z rings (left) and Pbp2B localization (right) (epifluorescence images of cells expressing both Pbp2B-mNeonGreen and FtsZ-HaloTag induced with 20 µM IPTG for 2 hours and labelled with 5 nM JF549-HTL) in control and ∆ZBPs cells. White arrows indicate the Z ring positions in each image. Representative images from at least two replicates of each condition. Scale bars: 2 µm. b Z rings and fluorescent D-amino acid (FDAA) incorporation (epifluorescence images of cells labelled with 1 mM fluorescent D-lysine (FDL) for 30 seconds, right) in control and ∆ZBPs cells. White arrows indicate the Z ring positions in each image. Representative images from at least 4 replicates of each condition. Scale bars: 2 µm. c Z ring width versus Pbp2B recruitment. Pbp2B intensity at midcell is higher when Z rings are more condensed; this is expected given that Pbp2B recruitment and Z ring condensation both increase over time. N = 2761 Z rings. d Pbp2B directional motion is seen at Z rings of all widths. Z ring width distributions of all Z rings (solid lines) and the Z rings at which Pbp2B moves directionally (dashed lines) for either control cells (left) or ∆ZBPs cells (right) are similar. This confirms that the Pbp2B motion seen in ∆ZBPs is present at decondensed rings. N>100 Z rings in each distribution. Shaded area: SEM.
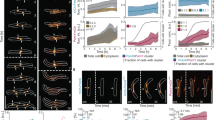
Extended Data Fig. 10 FtsA modulates FtsZ dynamics and Z ring formation.
a Z ring morphology (epifluorescence images of cells expressing FtsZ-mNeonGreen induced with 30 mM xylose for 2 hours) in control cells and ∆ftsA cells. ∆ftsA cells have highly altered Z rings. In ∆ftsA cells, FtsZ is expressed with 10 µM IPTG from the pHyperSpank promoter; higher or lower expression levels do not allow for cell survival. Representative images from three replicates of each condition. Scale bars: 2 µm. b Distributions of α values for FtsZ motion in control and ∆ftsA cells, obtained by tracking FtsZ filament motion and fitting each track to MSD(∆t) = D*∆tα. α > 1 indicates directional motion, so FtsZ filaments in ∆ftsA cells exhibit less directional treadmilling compared to control cells. N>6000 tracks for each condition. c Tracks of FtsZ filament motion in control and ∆ftsA cells. Tracks with α > 1 are cyan, tracks with α ≤ 1 are magenta. In control cells, FtsZ filaments often treadmill directionally along the short axis of the cell. In ∆ftsA cells, directional motion occurs less frequently and follows the short axis of the cell less consistently. Segmented cells are shown in black on a grey background. Scale bars: 2 µm.
Supplementary information
Supplementary Information
Supplementary Tables 1–6, Discussion 1, legends for Videos 1–7 and references.
Supplementary Video 1
FtsZ filaments treadmill around the cell. Cells expressing FtsZ–mNeonGreen (strain bAB219) were induced with 20 µM IPTG for 2 h, then imaged at 1-s intervals for 100 s by TIRFM. The video is displayed at 30 frames per second (30× actual speed). Scale bar, 5 µm.
Supplementary Video 2
Single molecules of EzrA, SepF and ZapA are stationary. Each protein was expressed as a HaloTag fusion and labelled with JF549-HTL dye for single-molecule imaging using TIRFM. Videos begin with an image of the Z rings in each cell, visualized by FtsZ–mNeonGreen. Cells were imaged at 1-s intervals for 200 s; every other frame is displayed here. The video is displayed at 30 frames per second (60× actual speed). Scale bar, 5 µm.
Supplementary Video 3
Single molecules of DivIB, DivIC, FtsL, FtsW and Pbp2B move directionally around the division site. Each protein was expressed as a HaloTag fusion and labelled with JF549-HTL dye for single-molecule imaging using TIRFM. Videos begin with an image of the Z rings in each cell, visualized by FtsZ–mNeonGreen. Cells were imaged at 1-s intervals for 200 s; every other frame is displayed here. The video is displayed at 30 frames per second (60× actual speed). Scale bar, 5 µm.
Supplementary Video 4
Single-molecule imaging of FtsZ for lifetime analysis. Cells expressing FtsZ–HaloTag were imaged at 500-ms intervals for 50 s by TIRFM. The video is displayed at 30 frames per second (15× actual speed). Scale bar, 5 µm.
Supplementary Video 5
EzrA overexpression decreases FtsZ filament length, visualized by SIM–TIRF microscopy. A second copy of ezrA was expressed from a xylose-inducible promoter. Panel labels indicate the amount of xylose added in each experiment. Cells expressed FtsZ–mNeonGreen to visualize FtsZ filaments and were imaged at 1-s intervals for 100 s by SIM–TIRF microscopy. The video is displayed at 30 frames per second (30× actual speed). Scale bar, 5 µm.
Supplementary Video 6
FtsZ filament treadmilling is unaltered in the absence of ZBPs. ∆ZBPs cells have sepF and zapA knocked out, and ezrA depleted from a xylose-inducible promoter for 7 h before imaging. Cells expressing FtsZ–mNeonGreen were imaged at 1-s intervals for 100 s by TIRFM. The video is displayed at 30 frames per second (30× actual speed). Scale bar, 5 µm.
Supplementary Video 7
FtsA filaments show less directional treadmilling in a ∆ftsA strain. ∆ftsA cells express FtsZ under the pHyperSpank promoter induced with 10 µM IPTG. Cells expressing FtsZ–mNeonGreen were imaged at 1-s intervals for 100 s by TIRFM. The video is displayed at 30 frames per second (30× actual speed). Scale bar, 5 µm.
Source data
Source Data Fig. 1
Source data for Fig. 1.
Source Data Fig. 2
Source data for Fig. 2.
Source Data Fig. 3
Source data for Fig. 3.
Source Data Fig. 4
Source data for Fig. 4.
Source Data Extended Data Fig. 1
Source data for Extended Data Fig. 1.
Source Data Extended Data Fig. 2
Source data for Extended Data Fig. 2.
Source Data Extended Data Fig. 3
Source data for Extended Data Fig. 3.
Source Data Extended Data Fig. 4
Source data for Extended Data Fig. 4.
Source Data Extended Data Fig. 6
Source data for Extended Data Fig. 6.
Source Data Extended Data Fig. 7
Source data for Extended Data Fig. 7.
Source Data Extended Data Fig. 8
Source data for Extended Data Fig. 8.
Source Data Extended Data Fig. 9
Source data for Extended Data Fig. 9.
Source Data Extended Data Fig. 10
Source data for Extended Data Fig. 10.
Rights and permissions
About this article
Cite this article
Squyres, G.R., Holmes, M.J., Barger, S.R. et al. Single-molecule imaging reveals that Z-ring condensation is essential for cell division in Bacillus subtilis. Nat Microbiol 6, 553–562 (2021). https://doi.org/10.1038/s41564-021-00878-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-021-00878-z
This article is cited by
-
Insights into the assembly and regulation of the bacterial divisome
Nature Reviews Microbiology (2024)
-
Cell constriction requires processive septal peptidoglycan synthase movement independent of FtsZ treadmilling in Staphylococcus aureus
Nature Microbiology (2024)
-
Peptidoglycan synthesis drives a single population of septal cell wall synthases during division in Bacillus subtilis
Nature Microbiology (2024)
-
Single-molecule dynamics show a transient lipopolysaccharide transport bridge
Nature (2023)
-
Bacterial divisome protein FtsA forms curved antiparallel double filaments when binding to FtsN
Nature Microbiology (2022)