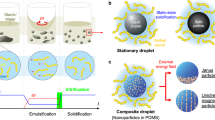
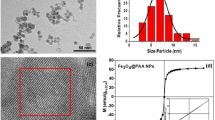
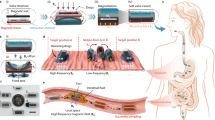
Abstract
Brownian motion allows microscopically dispersed nanoparticles to be stable in ferrofluids, as well as causes magnetization relaxation and prohibits permanent magnetism. Here we decoupled the particle Brownian motion from colloidal stability to achieve a permanent fluidic magnet with high magnetization, flowability and reconfigurability. The key to create such permanent fluidic magnets is to maintain a stable magnetic colloidal fluid by using non-Brownian magnetic particles to self-assemble a three-dimensional oriented and ramified magnetic network structure in the carrier fluid. This structure has high coercivity and permanent magnetization, with long-term magnetization stability. We establish a scaling theory model to decipher the permanent fluid magnet formation criteria and formulate a general assembly guideline. Further, we develop injectable and retrievable permanent-fluidic-magnet-based liquid bioelectronics for highly sensitive, self-powered wireless cardiovascular monitoring. Overall, our findings highlight the potential of permanent fluidic magnets as an ultrasoft material for liquid devices and systems, from bioelectronics to robotics.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Source data are provided with this paper. Other relevant information in this study is included in Supplementary Information. Further data are available from the corresponding author upon request.
References
Yu, W. et al. A ferrobotic system for automated microfluidic logistics. Sci. Robot. 5, eaba4411 (2020).
Zhang, J. et al. Wetting ridge assisted programmed magnetic actuation of droplets on ferrofluid-infused surface. Nat. Commun. 12, 7136 (2021).
Liu, X. et al. Reconfigurable ferromagnetic liquid droplets. Science 365, 264–267 (2019).
Lee, J. H. et al. Exchange-coupled magnetic nanoparticles for efficient heat induction. Nat. Nanotechnol. 6, 418–422 (2011).
Kim, C. et al. Gravitational stability of suspensions of attractive colloidal particles. Phys. Rev. Lett. 99, 028303 (2007).
Buscall, R. The sedimentation of concentrated colloidal suspensions. Colloids Surf. 43, 33–53 (1990).
Manoharan Vinothan, N. Colloidal matter: packing, geometry, and entropy. Science 349, 1253751 (2015).
Perrin, J. Atoms. Authorised translation by D.L. Hammick (Constable, 1916).
Wang, C. et al. Continuous monitoring of deep-tissue haemodynamics with stretchable ultrasonic phased arrays. Nat. Biomed. Eng. 5, 749–758 (2021).
Boutry, C. M. et al. Biodegradable and flexible arterial-pulse sensor for the wireless monitoring of blood flow. Nat. Biomed. Eng. 3, 47–57 (2019).
Kim, Y. et al. Chip-less wireless electronic skins by remote epitaxial freestanding compound semiconductors. Science 377, 859–864 (2022).
Weitz, D. A. Soft materials evolution and revolution. Nat. Mater. 21, 986–988 (2022).
Yan, Z. et al. Highly stretchable van der Waals thin films for adaptable and breathable electronic membranes. Science 375, 852–859 (2022).
Wang, C. et al. Bioadhesive ultrasound for long-term continuous imaging of diverse organs. Science 377, 517–523 (2022).
Kim, D. H. et al. Biofunctionalized magnetic-vortex microdiscs for targeted cancer-cell destruction. Nat. Mater. 9, 165–171 (2010).
Fan, X., Dong, X., Karacakol, A. C., Xie, H. & Sitti, M. Reconfigurable multifunctional ferrofluid droplet robots. Proc. Natl Acad. Sci. USA 117, 27916–27926 (2020).
Mertelj, A., Lisjak, D., Drofenik, M. & Copic, M. Ferromagnetism in suspensions of magnetic platelets in liquid crystal. Nature 504, 237–241 (2013).
Zhou, Y. et al. Giant magnetoelastic effect in soft systems for bioelectronics. Nat. Mater. 20, 1670–1676 (2021).
Vecchio, D. A., Mahler, S. H., Hammig, M. D. & Kotov, N. A. Structural analysis of nanoscale network materials using graph theory. ACS Nano 15, 12847–12859 (2021).
Viveros, R. D. et al. Advanced one- and two-dimensional mesh designs for injectable electronics. Nano Lett. 19, 4180–4187 (2019).
Hong, G. et al. Syringe injectable electronics: precise targeted delivery with quantitative input/output connectivity. Nano Lett. 15, 6979–6984 (2015).
Liu, J. et al. Syringe-injectable electronics. Nat. Nanotechnol. 10, 629–636 (2015).
Liu, Z. et al. Transcatheter self-powered ultrasensitive endocardial pressure sensor. Adv. Funct. Mater. 29, 1807560 (2019).
Hwang, J. C. et al. In situ diagnosis and simultaneous treatment of cardiac diseases using a single-device platform. Sci. Adv. 8, eabq0897 (2022).
About Multiple Cause of Death, 1999–2020 (National Center for Health Statistics, Centers for Disease Control and Prevention, 2022).
Fernández-Ruiz, I. Atrial fibrillation induces functional remodelling of the left ventricle. Nat. Rev. Cardiol. 19, 285 (2022).
Witkowski, F. X. et al. Spatiotemporal evolution of ventricular fibrillation. Nature 392, 78–82 (1998).
Schiavone, G. & Lacour Stéphanie, P. Conformable bioelectronic interfaces: mapping the road ahead. Sci. Transl. Med. 11, eaaw5858 (2019).
Fernández-Ruiz, I. A robotic heart sleeve to keep the beat. Nat. Rev. Cardiol. 14, 129 (2017).
Bannerman, D., Pascual-Gil, S. & Radisic, M. An optimal gel patch for the injured heart. Nat. Biomed. Eng. 3, 592–593 (2019).
Implants that vanish. Nat. Biomed. Eng. 3, 585 (2019).
Deng, J. et al. Electrical bioadhesive interface for bioelectronics. Nat. Mater. 20, 229–236 (2021).
Dagdeviren, C. et al. Conformal piezoelectric systems for clinical and experimental characterization of soft tissue biomechanics. Nat. Mater. 14, 728–736 (2015).
Ryu, H. et al. Self-rechargeable cardiac pacemaker system with triboelectric nanogenerators. Nat. Commun. 12, 4374 (2021).
Choi, Y. S. et al. Fully implantable and bioresorbable cardiac pacemakers without leads or batteries. Nat. Biotechnol. 39, 1228–1238 (2021).
Wu, X. et al. Ferromagnetic liquid droplets with adjustable magnetic properties. Proc. Natl Acad. Sci. USA 118, e2017355118 (2021).
Durable miniaturized bioelectronics. Nat. Biomed. Eng. 1, 0053 (2017).
Acknowledgements
We acknowledge the Henry Samueli School of Engineering & Applied Science and the Department of Bioengineering at the University of California, Los Angeles, for the startup support. J.C. acknowledges the Vernroy Makoto Watanabe Excellence in Research Award at the UCLA Samueli School of Engineering, the Office of Naval Research Young Investigator Award (award ID N00014-24-1-2065), NIH Grant (award ID R01 CA287326), the American Heart Association Innovative Project Award (award ID 23IPA1054908), the American Heart Association Transformational Project Award (award ID 23TPA1141360), the American Heart Association’s Second Century Early Faculty Independence Award (award ID 23SCEFIA1157587), the Brain & Behavior Research Foundation Young Investigator Grant (grant number 30944), and the NIH National Center for Advancing Translational Science UCLA CTSI (grant number KL2TR001882). J.C. and T.T. acknowledge the support from Caltech/UCLA joint NIH T32 Training Grant (award ID T32EB027629). S.L. acknowledges support from an NIH Grant (award ID R01 NS126918). We also acknowledge the insightful comments and careful editing from D. Di Carlo and the UCLA Writing Center for a one-on-one personalized writing consultation.
Author information
Authors and Affiliations
Contributions
J.C. guided the whole research project. X.Z., Y.Z. and J.C. conceived the idea, designed the experiment, analysed the data, drew the figures and composed the paper. J.X., J.L., T.T. and G.C. assisted in device fabrication and testing. S.L., Y.S. and X.Z. performed the in vivo animal study. All authors have read the paper, agreed to its content and approved the submission.
Corresponding author
Ethics declarations
Competing interests
J.C., X.Z. and Y.Z. have filed a patent related to this work under the US provisional patent application no. 63/596,815 from the University of California, Los Angeles. The authors declare no competing interests.
Peer review
Peer review information
Nature Materials thanks Wei Gao and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–27, Table 1, Notes 1–10 and References.
Supplementary Video 1
The 3D ORM network structure in the carrier fluid.
Supplementary Video 2
A continuous magnetic network structure forming in the carrier fluid.
Supplementary Video 3
ORM networks dynamically self-assemble, disassemble and reassemble.
Supplementary Video 4
Separation of PFM droplets in response to an external rotating magnetic field.
Supplementary Video 5
PFM droplet merging in response to the external rotating magnetic field.
Supplementary Video 6
PFM droplet moving under an external magnetic field.
Supplementary Video 7
PFM droplet buoyant in the water responding to the external rotating magnetic field.
Supplementary Video 8
Ferrofluid droplet buoyant in the water responding to the external rotating magnetic field.
Source data
Source Data Fig. 1
Source data for Fig. 1.
Source Data Fig. 2
Source data for Fig. 2.
Source Data Fig. 3
Source data for Fig. 3.
Source Data Fig. 4
Source data for Fig. 4.
Source Data Fig. 5
Source data for Fig. 5.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, X., Zhou, Y., Song, Y. et al. Permanent fluidic magnets for liquid bioelectronics. Nat. Mater. 23, 703–710 (2024). https://doi.org/10.1038/s41563-024-01802-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-024-01802-6
This article is cited by
-
Injectable and retrievable soft electronics
Nature Materials (2024)
-
A magnetic liquid makes for an injectable sensor in living tissue
Nature (2024)