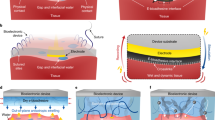
Abstract
Flexible electronic/optoelectronic systems that can intimately integrate onto the surfaces of vital organ systems have the potential to offer revolutionary diagnostic and therapeutic capabilities relevant to a wide spectrum of diseases and disorders. The critical interfaces between such technologies and living tissues must provide soft mechanical coupling and efficient optical/electrical/chemical exchange. Here, we introduce a functional adhesive bioelectronic–tissue interface material, in the forms of mechanically compliant, electrically conductive, and optically transparent encapsulating coatings, interfacial layers or supporting matrices. These materials strongly bond both to the surfaces of the devices and to those of different internal organs, with stable adhesion for several days to months, in chemistries that can be tailored to bioresorb at controlled rates. Experimental demonstrations in live animal models include device applications that range from battery-free optoelectronic systems for deep-brain optogenetics and subdermal phototherapy to wireless millimetre-scale pacemakers and flexible multielectrode epicardial arrays. These advances have immediate applicability across nearly all types of bioelectronic/optoelectronic system currently used in animal model studies, and they also have the potential for future treatment of life-threatening diseases and disorders in humans.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Source data are provided with this paper. All other data that support the results in this study are available from the corresponding author upon reasonable request.
Code availability
Software for the analysis of optical mapping data, RHYTHM, is openly available for free download at https://github.com/optocardiography. Other custom codes used in this study are available from the corresponding author upon reasonable request.
References
Kalantar-Zadeh, K. et al. A human pilot trial of ingestible electronic capsules capable of sensing different gases in the gut. Nat. Electron. 1, 79–87 (2018).
Dagdeviren, C. et al. Flexible piezoelectric devices for gastrointestinal motility sensing. Nat. Biomed. Eng. 1, 807–817 (2017).
Feiner, R. et al. Engineered hybrid cardiac patches with multifunctional electronics for online monitoring and regulation of tissue function. Nat. Mater. 15, 679–685 (2016).
Jun, J. J. et al. Fully integrated silicon probes for high-density recording of neural activity. Nature 551, 232–236 (2017).
Mickle, A. D. et al. A wireless closed-loop system for optogenetic peripheral neuromodulation. Nature 565, 361–365 (2019).
Feiner, R. & Dvir, T. Tissue–electronics interfaces: from implantable devices to engineered tissues. Nat. Rev. Mater. 3, 17076 (2018).
Yang, C. & Suo, Z. Hydrogel ionotronics. Nat. Rev. Mater. 3, 125–142 (2018).
Yuk, H., Lu, B. & Zhao, X. Hydrogel bioelectronics. Chem. Soc. Rev. 48, 1642–1667 (2019).
Wound Closure Products Market By Product type Analysis (Sutures, Adhesives and Tissue Sealants, Hemostats, Surgical Staples, Wound Closure Strips) and By Regional Analysis – Global Forecast by 2021–2026. Report MDWCM417(Market Research Engine, 2019); https://www.marketresearchengine.com/wound-closure-products-market
Potvin, R., Matossian, C. & Makari, S. Cataract surgery and methods of wound closure: a review. Clin. Ophthalmol. 2015(9), 921–928 (2015).
Edmiston, C. E. et al. Microbiology of explanted suture segments from infected and noninfected surgical patients. J. Clin. Microbiol. 51, 417–421 (2013).
Owens, C. D. & Stoessel, K. Surgical site infections: epidemiology, microbiology and prevention. J. Hospital Infect. 70, 3–10 (2008).
Mond, H. G., Helland, J. R., Stokes, K., Bornzin, G. A. & McVenes, R. The electrode–tissue interface: the revolutionary role of steroid-elution. Pacing Clin. Electrophysiol. 37, 1232–1249 (2014).
Yuk, H. et al. Dry double-sided tape for adhesion of wet tissues and devices. Nature 575, 169–174 (2019).
Li, J. et al. Tough adhesives for diverse wet surfaces. Science 357, 378–381 (2017).
Lin, X. et al. A viscoelastic adhesive epicardial patch for treating myocardial infarction. Nat. Biomed. Eng. 3, 632–643 (2019).
Liang, S. et al. Paintable and rapidly bondable conductive hydrogels as therapeutic cardiac patches. Adv. Mater. 30, 1704235 (2018).
Gan, D. et al. Plant-inspired adhesive and tough hydrogel based on Ag–lignin nanoparticles-triggered dynamic redox catechol chemistry. Nat. Commun. 10, 1487 (2019).
Blacklow, S. O. et al. Bioinspired mechanically active adhesive dressings to accelerate wound closure. Sci. Adv. 5, eaaw3963 (2019).
Wang, X. et al. Three-dimensional electronic scaffolds for monitoring and regulation of multifunctional hybrid tissues. Extreme Mech. Lett. 35, 100634 (2020).
Shin, G. et al. Flexible near-field wireless optoelectronics as subdermal implants for broad applications in optogenetics. Neuron 93, 509–521.e3 (2017).
Koo, J. et al. Wireless bioresorbable electronic system enables sustained nonpharmacological neuroregenerative therapy. Nat. Med. 24, 1830–1836 (2018).
Yu, K. J. et al. Bioresorbable silicon electronics for transient spatiotemporal mapping of electrical activity from the cerebral cortex. Nat. Mater. 15, 782–791 (2016).
Mathur, A. B., Collinsworth, A. M., Reichert, W. M., Kraus, W. E. & Truskey, G. A. Endothelial, cardiac muscle and skeletal muscle exhibit different viscous and elastic properties as determined by atomic force microscopy. J. Biomech. 34, 1545–1553 (2001).
Arda, K., Ciledag, N., Aktas, E., Arıbas, B. K. & Köse, K. Quantitative assessment of normal soft-tissue elasticity using shear-wave ultrasound elastography. Am. J. Roentgenol. 197, 532–536 (2011).
Yanniotis, S., Skaltsi, S. & Karaburnioti, S. Effect of moisture content on the viscosity of honey at different temperatures. J. Food Eng. 72, 372–377 (2006).
Kang, S.-K. et al. Bioresorbable silicon electronic sensors for the brain. Nature 530, 71–76 (2016).
Yamagishi, K. et al. Tissue-adhesive wirelessly powered optoelectronic device for metronomic photodynamic cancer therapy. Nat. Biomed. Eng. 3, 27–36 (2019).
Zhang, Y. et al. Battery-free, lightweight, injectable microsystem for in vivo wireless pharmacology and optogenetics. Proc. Natl Acad. Sci. USA 116, 21427–21437 (2019).
Gutruf, P. et al. Fully implantable optoelectronic systems for battery-free, multimodal operation in neuroscience research. Nat. Electron. 1, 652–660 (2018).
Burton, A. et al. Wireless, battery-free subdermally implantable photometry systems for chronic recording of neural dynamics. Proc. Natl Acad. Sci. USA 117, 2835–2845 (2020).
Bai, W. et al. Bioresorbable photonic devices for the spectroscopic characterization of physiological status and neural activity. Nat. Biomed. Eng. 3, 644–654 (2019).
Tharanathan, R. N. Biodegradable films and composite coatings: past, present and future. Trends Food Sci. Technol. 14, 71–78 (2003).
Boutry, C. M. et al. Towards biodegradable wireless implants. Phil. Trans. R. Soc. A 370, 2418–2432 (2012).
Chen, T.-W. et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013).
Yang, Y. et al. Wireless multilateral devices for optogenetic studies of individual and social behaviors. Nat. Neurosci. 24, 1035–1045 (2021).
Fang, H. et al. Capacitively coupled arrays of multiplexed flexible silicon transistors for long-term cardiac electrophysiology. Nat. Biomed. Eng. 1, 0038 (2017).
Lee, W. et al. Nonthrombogenic, stretchable, active multielectrode array for electroanatomical mapping. Sci. Adv. 4, eaau2426 (2018).
Sawhney, A. S., Pathak, C. P. & Hubbell, J. A. Bioerodible hydrogels based on photopolymerized poly(ethylene glycol)-co-poly(α-hydroxy acid) diacrylate macromers. Macromolecules 26, 581–587 (1993).
Park, y. et al. Three-dimensional, multifunctional neural interfaces for cortical spheroids and engineered assembloids. Sci. Adv . 7, eabf9153 (2021).
Sun, J.-Y. et al. Highly stretchable and tough hydrogels. Nature 489, 133–136 (2012).
Gong, J. P. et al. Synthesis of hydrogels with extremely low surface friction. J. Am. Chem. Soc. 123, 5582–5583 (2001).
Rudy, A. et al. Lubricous hydrogel surface coatings on polydimethylsiloxane (PDMS). Tribol. Lett. 65, 3 (2017).
Rodriguez, A. et al. ToxTrac: a fast and robust software for tracking organisms. Methods Ecol. Evol. 9, 460–464 (2018).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Wu, M. et al. Attenuated dopamine signaling after aversive learning is restored by ketamine to rescue escape actions. eLife 10, e64041 (2021).
Wu, M., Minkowicz, S., Dumrongprechachan, V., Hamilton, P. & Kozorovitskiy, Y. Ketamine rapidly enhances glutamate-evoked dendritic spinogenesis in medial prefrontal cortex through dopaminergic mechanisms. Biol. Psychiat. 89, 1096–1105 (2021).
Acknowledgements
This work was generously funded by the Leducq Foundation project RHYTHM and the National Institutes of Health (R01-HL141470 to I.R.E. and J.A.R.). This work made use of the NUFAB facility of Northwestern University’s NUANCE Center, which received support from the Soft and Hybrid Nanotechnology Experimental (SHyNE) Resource (NSF ECCS-2025633); the MRSEC programme (NSF DMR-1720139) at the Materials Research Center; the International Institute for Nanotechnology (IIN); the Keck Foundation; the Querrey Simpson Institute for Bioelectronics; the Keck Biophysics Facility, a shared resource of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University, which received support in part by the NCI Cancer Center Support (P30 CA060553); the Center for Advanced Molecular Imaging (RRID:SCR_021192); Northwestern University; and the State of Illinois, through the IIN. R.T.Y. acknowledges support from the American Heart Association (19PRE34380781). M.W. acknowledges support from the National Institutes of Health (T32 AG20506). Z.X. acknowledges support from the National Natural Science Foundation of China (12072057), LiaoNing Revitalization Talents Program (XLYC2007196) and Fundamental Research Funds for the Central Universities (DUT20RC(3)032). K.A. acknowledges support from the National Institutes of Health (5K99-HL148523-02). Y.H. acknowledges support from the National Foundation of Science (CMMI1635443). Y.K. acknowledges support from the National Institutes of Health (R01NS107539 and R01MH117111), Beckman Young Investigator Award, Rita Allen Foundation Scholar Award and Searle Scholar Award. The diagrams of the mouse body with organs in Fig. 1f were created with BioRender.com.
Author information
Authors and Affiliations
Contributions
Q.Y., T. Wei, Y.K., I.R.E. and J.A.R. conceived the ideas and designed the research. Q.Y., T. Wei, Y.X. and T.-L.L. synthesized and characterized the materials. Q.Y., Y.X., J.K., Y.S.C., W.B., Y.Y., M.H., Q.Z., T. Wang, M.-H.S., H.L., S.M.L. and A. Banks designed and fabricated the devices. R.T.Y., M.W., S.W.C., I.K., S.Y., C.R.H., K.B.L., K.A., A. Brikha, I.S., F.A., E.A.W. and G.D.T. performed the in vitro, ex vivo and in vivo studies. Q.Y., R.T.Y., Y.X., M.W., C.R.H., A. Banks and E.A.W. performed the data analysis. Z.X., R.A. and Y.D. performed the mechanical and electrical modelling. Q.Y., Y.X., R.T.Y., T.W., M.W, J.M.T., Y.H., Y.K., I.R.E. and J.A.R. wrote the manuscript with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Materials thanks John Ho, Yuhan Lee, Tsuyoshi Sekitani and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Supporting matrices for electrode-embedded 3D electronic systems.
a, Schematic illustration of procedures to integrate 3D electronics into a matrix of the BTIM. The matrix allows manual manipulation without damaging fragile features, with the ability to bond to dynamic, curved tissue surfaces. b, Image of a 3D device formed by compressive buckling on an elastomer substrate. c, Micrograph of a 3D device in a BTIM supporting matrix. d, Schematic illustrations of the 2D precursor before compressive buckling (first panel) and the 3D structure after compressive buckling (second panel), with the measuring electrode in the central area. e, Schematic illustration of the original and deformed status of the non-encapsulated (first row) and the BTIM-encapsulated (second row) 3D devices under compressive pressure. f, g, Curves for impedance and phase angle over the frequency sweep without the BTIM before and after compressive pressure. h, i, Impedance and phase angle over the frequency sweep with the BTIM under static compression fatigue tests. j, k, Impedance and phase angle over the frequency sweep with the BTIM under dynamic compression fatigue tests (pressure: 2 kPa; cycle number: 104).
Extended Data Fig. 2 Influence of APTES-functionalization on bioelectronics surfaces.
a, ToF-SIMS analysis of a representative pristine bioelectronic surface (PLA). b, Steps for selective functionalization of this surface through a patterned polyimide adhesive-coated shadow mask. c, ToF-SIMS analysis on the shadowed area of the surface shows results that are similar to those from the pristine surface. d, ToF-SIMS analysis of the exposed area of the bioelectronic surface. To normalize the intensity, the intensity I28, where the mass is 28, was set as 100 and calculate the relative intensity of each case. e, Thickness of an APTES layer formed on a silicon wafer exposed in air and submerged in water as a function of time after formation. Spectroscopic ellipsometry was used for these measurements. f, Impedance of MEA electrodes at 1 kHz in 0.1 M PBS at room temperature before APTES functionalization and immediately and 7 days after APTES functionalization (F(2,123) = 0.0201, P = 0. 9801). n = 6 independent samples in e and n = 40 independent electrodes from 10 independent devices and 3 independent batches in f. Values in e and f represent the mean ± s.d. Statistical significance and P values are determined by one-way ANOVA at a significance level of 0.05. ns indicates no statistically significant differences.
Extended Data Fig. 3 Analysis of the electrical distributions associated with electrical stimulation.
a, Influence of the conductivity of the interface material on electrical stimulation. b, Current density distributions in the myocardium layer and the interface material with different conductivities. c, Potential distribution in the myocardium layer and the interface material with different conductivities. d, Corresponding potential distributions on the top of the myocardium layer as a function of the interface material conductivity. e, Ratio of the potential difference on the tissues (V3 – V4) to the corresponding potential difference on the electrodes (V1 – V2, 3 V) as a function of the interface material conductivity.
Extended Data Fig. 4 Analysis of the electrical distributions associated with biopotential mapping.
a, Influence of the conductivity of the BTIM on biopotential mapping. b, Biopotential distribution in the myocardium layer and the interface material layer with various conductivities. c, Calculated potential at all of the electrodes for different conductivities. d, Calculated potential on all the electrodes in the presence of the interface material layer with various conductivities (blue solid line) and in the absence of the interface material layer (red dash line).
Extended Data Fig. 5 Mechanical stability of BTIM-encapsulated wireless optoelectronics.
a, Original device position determined by photographs, and positions of BTIM-encapsulated devices (first row) and non-adhesive (second row) devices on day 1 (white), 4 (blue), and 7 (purple) post-surgery determined by MicroCT. b, c, Statistical analyses of the net translations (b) and rotations (c) of the µ-ILED in the BTIM-encapsulated (blue) and non-adhesive devices (red) on day 4 and 7 compared with those on day 1 post-surgery. n = 3 biologically independent animals. Values in b–c represent the mean ± s.d.
Extended Data Fig. 6 Applying BTIM on brain-integrated bioelectronics/optoelectronics.
a, Image of the brain areas exposed to the BTIM. b, c, Astrocyte area percentages and microglia area percentages for the sham group and the BTIM group. Black dots: animal average. Black dots: animal average, 4 animals in total. Grey dots: individual ROIs. 10–12 ROIs from 4 animals in total, 2–3 ROIs from each animal. P = 0.3546 in b and P = 0.8049 in c. d, The image of the BTIM-encapsulated b-MEA on a mouse brain ex vivo. e, Robust adhesion even when the b-MEA is lifted or shaken. n = 12 independent measurements from 4 biologically independent animals in b–c. Values in b–c represent the mean ± s.d. Statistical significance and P values are determined by two-sided Student’s t-test at a significance level of 0.05. ns indicates no statistically significant differences.
Extended Data Fig. 7 Electrogram signals of ventricular fibrillation from an MEA with BTIM encapsulation.
a, Representative electrogram signal collected from a Langendorff-perfused rabbit heart during ventricular fibrillation (VF). b, c, Phase (b) and voltage (c) map of VF over 2500 ms.
Supplementary information
Supplementary Information
Supplementary Notes 1–11, Tables 1 and 2 and Figs. 1–33.
Supplementary Video 1
Optoelectronic device remains in the bonded location and exhibits stable operation during natural movements of animals. The device can be wirelessly activated to emit red light (peak wavelength 630 nm), designed for its relevance in tumour irradiation.
Supplementary Video 2
BTIM firmly secures an optoelectronic device to the dorsal subcutaneous area throughout 2 months after surgery. The BTIM severs as an encapsulating coating in this situation.
Supplementary Video 3
Optoelectronic device encapsulated by the BTIM remains functional for 2 months after surgery.
Supplementary Video 4
Instability of the device coil on the mouse skull without the BTIM.
Supplementary Video 5
Stability of the device coil on the mouse skull with the BTIM encapsulating coating.
Supplementary Video 6
Battery-free devices designed for optogenetics remain functional for 2 weeks after surgery. The BTIM serves as an encapsulating coating in this situation.
Supplementary Video 7
Robust adhesion of the BTIM to a mouse cerebrum.
Supplementary Video 8
Adhesive conduit supports and protects an interconnect/cable under compression in vivo.
Supplementary Video 9
BTIM in the encapsulation strategy establishes conformal contact between electrodes of wireless cardiac pacemakers and the myocardium in vivo.
Supplementary Video 10
In vivo characterization of adhesion between electrodes and the epicardium. The heart can be dragged out of the chest due to the robust adhesion provided by the BTIM.
Supplementary Video 11
Characterization of adhesion between electrodes and the epicardium on day 10 after surgery. The robust adhesion remains in vivo 10 d after surgery.
Supplementary Video 12
In vivo representative ECG traces during rapid ventricular pacing at ~1,000 bpm.
Supplementary Video 13
Accurate mapping of ventricular fibrillation on a Langendorff-perfused rabbit heart. The BTIM serves as the interfacial layer in this situation to bridge the MEA and the myocardium.
Source data
Source Data Fig. 2
Raw data and statistical source data for Fig. 2.
Source Data Fig. 3
Raw data and statistical source data for Fig. 3.
Source Data Fig. 4
Raw data for Fig. 4.
Source Data Fig. 5
Raw data and statistical source data for Fig. 5.
Source Data Fig. 6
Raw data and statistical source data for Fig. 6.
Source Data Extended Data Fig. 1
Raw data for Extended Data Fig. 1.
Source Data Extended Data Fig. 2
Raw data and statistical source data for Extended Data Fig. 2.
Source Data Extended Data Fig. 3
Raw data for Extended Data Fig. 3.
Source Data Extended Data Fig. 4
Raw data for Extended Data Fig. 4.
Source Data Extended Data Fig. 5
Raw data and statistical source data for Extended Data Fig. 5.
Source Data Extended Data Fig. 6
Raw data and statistical source data for Extended Data Fig. 6.
Source Data Extended Data Fig. 7
Raw data for Extended Data Fig. 7.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, Q., Wei, T., Yin, R.T. et al. Photocurable bioresorbable adhesives as functional interfaces between flexible bioelectronic devices and soft biological tissues. Nat. Mater. 20, 1559–1570 (2021). https://doi.org/10.1038/s41563-021-01051-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-021-01051-x
This article is cited by
-
Recent advances in two-dimensional nanomaterials for sustainable wearable electronic devices
Journal of Nanobiotechnology (2024)
-
Motion artefact management for soft bioelectronics
Nature Reviews Bioengineering (2024)
-
Adhesive anti-fibrotic interfaces on diverse organs
Nature (2024)
-
Sticky gels designed for tissue-healing therapies and diagnostics
Nature (2024)
-
Miniaturized implantable temperature sensors for the long-term monitoring of chronic intestinal inflammation
Nature Biomedical Engineering (2024)