Abstract
The meridional extent of marine ice during the Neoproterozoic snowball Earth events is debated. Banded iron formations associated with the Sturtian glaciation are considered evidence for a completely ice-covered, ferruginous ocean (hard snowball). Here, using an ocean general circulation model with thick sea glaciers and Neoproterozoic biogeochemistry, we find that circulation in a partially ice-covered ocean (soft snowball) yields iron deposition patterns similar to the observed distribution of Sturtian banded iron formations.
Similar content being viewed by others
Main
Banded iron formations (BIFs) are iron- and silica-rich sedimentary rocks, the genesis of which is thought to require low oceanic O2 concentrations, in agreement with their occurrence before ~1,800 million years ago (Ma), when Earth’s oceans are proposed to have been anoxic1,2. An exception to this pattern of occurrence, Neoproterozoic (1,000 to 541 Ma) iron formations, were deposited when atmospheric O2 levels were 1–10% of today’s3. Neoproterozoic BIFs occur in association with the Sturtian pan-glacial, which is the earlier and longer of two low-latitude glaciations (the ‘snowball Earth’ events4,5,6,7). The ocean and continents are suggested to have been ice-covered during these pan-glacials that lasted for tens of millions of years8,9. Widespread sedimentary and geochemical evidence supports the occurrence of the Neoproterozoic pan-glacials, though the meridional extent of marine ice is still debated. The Neoproterozoic BIFs have historically been suggested to support a ‘hard’ snowball4,5, in which pole-to-pole ice cover renders the ocean anoxic, allowing the accumulation of ferrous iron (Fe2+). Although viable explanations for the survival of the photosynthesis-based marine biosphere through a hard snowball have been proposed10, such an extreme climate poses a serious challenge to life5. Alternatively, a ‘soft’ snowball7 with tropical swaths of open water does not pose a similar challenge to the biosphere, but O2 input to the ocean by air–sea gas exchange may have prevented the mobility of Fe2+ required for widespread deposition of BIFs10,11.
In this study, we tested the sensitivity of precipitation of BIFs to the extent of ice cover using an ocean general circulation model (GCM), which includes the representation of thick sea glaciers extending to a prescribed latitude and a new biogeochemical model tailored to the Neoproterozoic (Fig. 1; see Methods for details). In the model iron cycle, a hydrothermal source of Fe2+ at mid-oceanic ridges is balanced by oxidation and deposition of ferric iron (Fe3+) oxides, which occurs where Fe2+-bearing waters meet oxygenated waters. The transport of Fe2+ and O2 from their respective hydrothermal and photosynthetic sources determines the location of such reaction and deposition fronts. Note that photosynthesis and O2 production in the partially glaciated oceans that we simulate is confined to the ice-free regions and there is no primary production beneath the >100-m-thick sea glaciers. Patterns of phosphate concentration ([PO43−]) and [O2] obtained in an idealized model configuration (Supplementary Section 1) with a 100% modern [PO43−] and a stoichiometry of organic matter oxidation (O2 consumed to P released) representative of modern marine organic matter are consistent with today’s ocean (Supplementary Fig. 1), and atmospheric O2 stabilizes at ~62% present atmospheric levels.
Cyanobacteria, limited by low seawater phosphate concentration ([PO43−]), produce organic matter (DOP and POP) and oxygen (O2) in ice-free regions. Aerobic respiration of the organic matter consumes some of the O2, as do the bacteria that oxidize ferrous iron (Fe2+) and produce ferric iron (Fe3+) oxides. The fraction of Fe3+ oxides that is not used by anaerobic bacteria to remineralize organic matter settles to the seabed, forming iron deposits.
Previous ocean–atmosphere–cryosphere modelling efforts have identified multiple stable climate states in which ice-free ocean exists equatorwards of latitudes between 60° and 5° (refs. 11,12). However, not many climate models can sustain a narrow equatorial band of ice-free ocean. Accordingly, we examined a completely ice-free ocean and partially glaciated oceans in which the ice-free region extended from 30° S to 30° N and 12° S to 12° N. The exact size of the marine PO43− pool during snowball Earth episodes is unknown, though several factors lead to expectations of lower-than-present seawater [PO43−]. These include low continental weathering rates, photosynthetic carbon fixation and nutrient uptake during the glacial advance and in regions of open water, limited organic matter remineralization in a partly ice-covered ocean, and PO43− adsorption onto iron oxides in an ocean with relatively high Fe2+ concentrations. Hence, we examined iron deposition patterns at 100%, 10%, 3% and 1% the mean present-day seawater [PO43−] (3 μM), the lower three of which are within the proposed range required to sustain low Proterozoic atmospheric O2 levels13,14, and consistent with low Proterozoic primary productivity15. With these parameter combinations, cases in which 90% or more of the iron deposited within the model grid points corresponding to the hydrothermal Fe2+ sources were considered less viable for BIF deposition on multiple continental margins, as suggested by the sedimentary record10.
Our simulations show that the spatial pattern of Fe3+ precipitation is sensitive to both the extent of ice cover and the mean seawater [PO43−]. To elucidate Fe3+ precipitation in a partially glaciated ocean, we consider the 12° S to 12° N ice-free ocean snowball with a mean [PO43−] of 3% modern levels. The flux of O2 produced (via photosynthesis) in this snowball is limited due to both a low ice-free surface area and low PO43− availability. This small amount of O2 is distributed within the ocean by the circulation. However, the circulation in the juvenile, narrow ocean basins that were formed by the break-up of the supercontinent Rodinia is much weaker than in other regions (Supplementary Fig. 2). Since a hydrothermal source is also located within these poorly ventilated basins, they are a suitable site for accumulation and mobility of Fe2+, which is oxidized at the ‘chemical reaction and deposition fronts’ in this region (Fig. 2a–f). The intersection of Fe2+-bearing water and O2-bearing water at these fronts produces Fe3+, which is insoluble in seawater. The seawater insoluble Fe3+ sinks through the water column and a fraction of it is used by anaerobic bacteria to remineralize organic matter to replenish the marine PO43− and Fe2+ pools, while the rest is deposited on the seafloor in the region. In contrast, we do not observe Fe2+ mobility around the hydrothermal vent located in the well-ventilated southeastern ocean and all the Fe2+ is oxidized (and precipitates) near the vent (Supplementary Fig. 3).
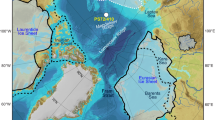
Latitude–longitude cross-section at a depth of −2.5 km (top) and latitude–depth cross-section at 166.5° E (bottom) of oxygen ([O2]) (a,d), ferrous iron ([Fe2+]) (b,e) and ferric iron (Fe3+) precipitation rate (\({{R}}_{{\mathrm{Fe}}^{3+}}\)) (c,f). The solid black contours in a–c mark the sites of hydrothermal Fe2+ injection, based on estimated locations of mid-oceanic ridges. The dashed black lines enclose the region where [O2] = 0 and the ‘chemical reaction front’ is adjacent to it. The dotted line in the top panels mark the 166.5° E longitude; the latitude–depth cross sections in d–f are along this longitude. The light-brown shading in a–c indicates the approximate continental configuration during the Sturtain pan-glacial18.
In partially ice-covered oceans with a mean [PO43−] that is 1–10% of today’s, the distribution of model iron deposition rates (Fig. 3a,b and Supplementary Figs. 4g,h and 5e,f) away from the hydrothermal sources are within the range of estimated Sturtian BIF deposition rates (between ~0.003 and ~0.4 mol m−2 yr−1; Supplementary Section 2). This model consistency with the observations is achieved at [Fe2+] approximately two orders of magnitude lower than previously thought to be required for widespread Neoproterozoic BIF deposition16,17. Thus, we suggest that Fe2+ supply and mobility, not concentration, are the actual requirements for widespread BIF deposition, in the Neoproterozoic and over Earth history in general. At modern mean seawater [PO43−], most of the ocean interior is oxygenated, and >95% of the iron precipitates near the hydrothermal Fe2+ sources.
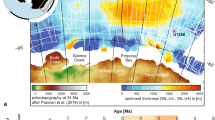
a,b, Spatial distribution of iron deposition rates (\({{R}}_{{\mathrm{Fe}}^{3+}}\)) in snowballs with an ice-free ocean between 12° S to 12° N (a) and 30° S to 30° N (b), respectively. Iron deposition rates are calculated by vertical integration of the profiles corresponding to net ferrous iron (Fe2+) oxidation. The solid black contours mark the sites of hydrothermal Fe2+ injection, based on estimated locations of mid-oceanic ridges. The dotted black horizontal lines denote the extent of the ice-free region. The dashed black lines enclose the region where iron deposition rates are within the range estimated from Neoproterozoic BIFs. The stars indicate the approximate location of BIFs associated with the Sturtian pan-glacial. BIFs denoted by the grey stars have been excluded from the estimation of BIF deposition rate (Supplementary Section 2). The light-brown shading indicates the approximate continental configuration during the Sturtain pan-glacial.
Multiple BIF deposition sites emerge in the snowball simulations with 1–10% modern [PO43−] (Fig. 3a,b and Supplementary Figs. 4g,h and 5e,f), several of which are located in the deep ocean away from the continents. BIFs deposited at these deep sites are unlikely to be preserved, in contrast with the BIFs deposited on the continental margins facing the young ocean basins that formed during the break-up of Rodinia. This is consistent with the clustering of all but one of the BIFs dated to the Sturtian pan-glacial around these young ocean basins (Supplementary Fig. 6). The palaeogeographic reconstruction18 does not resolve inland seas, which host some of the Neoproterozoic BIFs, but we postulate that iron deposition is expected also in such seas that were connected to the basins represented in the model (for example, apparent inland BIF locations in Fig. 3a,b). We assessed the agreement between iron deposition patterns obtained in different simulations with the spatial distribution of Neoproterozoic BIFs (Supplementary Section 3 and Supplementary Fig. 7). By and large, in simulations with mean seawater [PO43−] between 1% and 10% modern levels, O2-bearing and Fe2+-bearing water masses meet in the vicinity of Sturtian BIFs for the two partially ice-covered oceans considered. However, neither the ice-free ocean nor the partially ice-covered oceans show an affinity for precipitation of BIFs at modern [PO43−] levels, and all the Fe2+ is deposited close to the hydrothermal source. These results suggest that iron deposition patterns and rates consistent with the Sturtian BIFs can be obtained in partially ice-covered oceans with lower-than-present seawater [PO43−] (<~10% modern levels). Due to model uncertainties, we stop short of attempting to identify the precise [PO43−] levels and meridional ice extent that yield the most consistent iron deposition patterns.
To conclude, we contextualize our findings within the hard versus soft snowball debate. The hard snowball state19,20,21,22 is suggested to have led to an anoxic ocean interior in which dissolved Fe2+ accumulated, to be oxidized and deposited only in spatially limited regions where O2 was available, such as the interface between anoxic seawater and oxygenated meltwater plumes near the ice grounding line10,23. Under such a scenario, production of BIFs is expected on all glaciated continental margins10, in contrast with the occurrence of Neoproterozoic BIFs only on some continental margins (Fig. 3a,b). However, fortuitous combinations of ice-sheet hydrology, meltwater residence times and subglacial bedrock geology may result in a distribution akin to the observed distribution of Sturtian BIFs even in a hard snowball. Our analysis shows that a phosphate-deficient ocean is conducive to production of BIFs for different extents of marine ice cover, wherever Fe2+-bearing and oxygenated water masses meet. We find several combinations of [PO43−] and marine ice cover that predict BIF deposition at multiple sites, including on the continental margins facing the young ocean basins that formed during the fragmentation of Rodinia, consistent with the observed distribution of Sturtian BIFs (Fig. 3a,b and Supplementary Figs. 4g,h and 5e,f). The coarse resolution of our model and other limitations (for example, absence of a dynamic atmosphere–ice–ocean coupling, uncertainties in seafloor topography) prevent us from predicting the meridional extent of marine ice cover during the Neoproterozoic pan-glacials. Nevertheless, our findings prompt investigations of the plausibility of a soft snowball state in more complete climate models, which include atmosphere–ocean–cryosphere coupling and a realistic continental configuration. Lastly, our findings highlight the role of Fe2+ fluxes into, and mobility within, Earth’s oceans as the necessary conditions for widespread BIF deposition. In contrast with previous suggestions, a high Fe2+ concentration is found unnecessary.
Methods
Ocean
We use the Massachusetts Institute of Technology General Circulation Model (MITgcm)24 to perform the simulations discussed in this study. The MITgcm is a free, open-source GCM in which a finite volume method is implemented to solve the equations of fluid motion (that is, momentum equations, continuity equation and diffusion equations of temperature and salinity and equation of state). We employ the full equation of state provided in the MITgcm, MDJWF25, and make the hydrostatic and Boussinesq approximations. The model uses a spherical grid spanning 72° S to 72° N with a resolution of 3° in latitude and has 37 vertical levels spanning a depth of 3,000 m (height of vertical levels from the surface to the bottom: 10 m, 12 m, 16 m, 20 m, 25 m, 32 m, 40 m, 50 m, 60 m, 75 m, 80 m, 2 levels × 90 m, and 24 levels × 100 m). The longitudinal resolution is 3° as well, with 20 grid points in the idealized box configuration simulations and 120 grid points in the Neoproterozoic simulations. Unresolved eddies in the ocean are parameterized using the Gent-McWilliams/Redi (GM-Redi) scheme26,27, and the background diffusion coefficient is set to 1,000 m2 s−1. The lateral and vertical viscosities are set to 5 × 105 m2 s−1 and 10−3 m2 s−1, and the vertical diffusivity and implicit vertical diffusivity for convection are set to 10−4 m2 s−1 and 10 m2 s−1. We also use a non-dimensional grid-dependent biharmonic viscosity of 0.1 to suppress the grid-scale noise. The vertical diffusivity for biogeochemical variables is set to 3 × 10−5 m2 s−1. The third-order direct space-time flux limiter advection scheme (no. 33) is used for both momentum tracers and biogeochemical variables. The flat bathymetry ocean is subjected to a constant geothermal heat flux of 0.1 W m−2 (ref. 28). At the surface, the ocean is forced by a zonal wind stress that changes for the different meridional extent of the sea glaciers. The wind stress field for the idealized box configuration simulations is similar to the present-day, and for ice-free Neoproterozoic simulation is the zonally averaged wind stress obtained from fully coupled ocean–atmosphere simulation of Cambrian–Precambrian boundary29. The wind stress fields for our snowball simulation conditions (spatial grid and ice opening) are generated by setting the wind stress over the ice-covered region to zero and using zonally averaged wind stress profiles over the ice-free region that are based on results from simulations of partially glaciated climate states carried out on fully coupled GCMs30,31. The temperature at the surface (z = 0 m) is restored to temperature fields that are also generated on the basis of coupled model results11,12. Supplementary Fig. 8a,b shows the zonally averaged restoration temperature and wind stress over the ocean for different meridional extents of sea glaciers. Supplementary Fig. 8c shows the evaporation minus precipitation (E − P) fields over the ocean in the box simulations and in the Neoproterozoic ice-free simulations, these idealized E − P fields are generated by introducing a small asymmetry to the E − P field described in ref. 32. In both the snowballs, melting under the sea glaciers is balanced by evaporation in the ice-free region, which conserves the mean salinity of the ocean.
Sea glaciers
Given the uncertainties regarding the thickness of ice during the Neoproterozoic glaciations, we estimate ice thickness using a simplified energy balance. A similar calculation has been used to estimate the thickness of ice on Jupiter’s moon Europa33. We begin by assuming that a constant geothermal flux of 0.1 W m−2 is applied to the ocean bottom and that the component of tidal heating in both the ice and ocean is negligible. Moreover, the temperature within the ice varies linearly with depth. In this case, the thickness of ice is given by
where ρi is the density, \({c}_{{\mathrm{p}},{\mathrm{i}}}\) is the heat capacity and κ is the temperature diffusion constant of ice. Tf and Ti are the freezing and surface temperature of the ice, and Q is the internal heat flux (the geothermal heat flux). Tf is a function of salinity and pressure:
where S is the salinity and Pb = ρigh is the pressure at the bottom of the ice. The gradient in solar insolation yields a gradient in the surface temperature of the ice, and in turn, a gradient in ice thickness. This gradient in ice thickness causes it to flow smoothly, thereby partially relaxing the gradient34. Hence, we approximate the thickness of the ice as the meridional average of h calculated using the prescribed temperature field. Under these assumptions, the ice thickness can iteratively be estimated as:
The algorithm33, when initialized from the typical seawater freezing temperature of 271 K and the surface temperature fields such as the ones given in refs. 11,12, predicts a certain ice thickness, which implies a certain Pb value and a modified freezing temperature. For the temperature fields shown in Supplementary Fig. 8a, the ice thickness converges to 490 m and 253 m below the sea surface within a few iterations for snowballs with 12° S to 12° N ice-free ocean and 30° S to 30° N ice-free ocean, respectively. To avoid any numerical artefacts that may arise from partially filled grid cells, we use a thickness of 510 m and 265 m below the sea surface for the snowballs with 12° S to 12° N ocean and 30° S to 30° N ocean, respectively. The effect of this sea glacier is simulated using the SHELFICE package35. Unlike the SEAICE package, the SHELFICE package in the MITgcm can handle a thick sea glacier spanning over multiple vertical levels, which makes it better suited to snowball Earth modelling.
Biogeochemical cycle
The biogeochemical model presented here is based on refs. 36,37, implemented in the MITgcm using a combination of the GCHEM and DIC packages38. MITgcm’s biogeochemical setup (GCHEM + DIC), in its default configuration, considers coupled cycles of carbon, oxygen, phosphorus and alkalinity. These cycles are expressed using five variables that do not affect the physical circulation: dissolved inorganic carbon (DIC), alkalinity (Alk), phosphate (\({{\rm{PO}}}_{4}^{3-}\)), dissolved organic phosphorus (DOP) and oxygen (O2). The velocity fields and eddy diffusivities calculated by the physical model are used to transport these compounds, which are additionally produced and consumed locally by biogeochemical reactions, as described below.
For this study, we focus on the phosphorus and oxygen cycles. Additionally, we incorporate an iron (Fe2+) cycle and a well-mixed atmospheric box to the pre-existing setup. The atmospheric box only keeps track of the total O2 in the atmosphere. The values of all parameters described below are provided in Supplementary Table 1. The sources and sinks for each tracer are summarized below:
where square brackets denote concentration, Jproduction is primary production. During production, a fraction of \({{\rm{PO}}}_{4}^{3-}\) utilized by phytoplankton is converted to DOP (fDOP) and the rest (1 − fDOP) is converted to particulate organic phosphorus (POP); κremin is the rate constant of remineralization of DOP to \({{\rm{PO}}}_{4}^{3-}\) and \(\frac{\partial }{\partial z}{F}_{{\mathrm{P}}}\) is the partial derivative with depth of the POP sinking flux that is remineralized at different rates during aerobic and anaerobic respiration (see below). The gas transfer velocity for O2 is given by kw, and Δzsurf is the thickness of the surface layer. The moles of O2 in the model atmosphere and the present-day atmosphere are given by \({n}_{{{\mathrm{O}}}_{2}}^{{{{\mathrm{model} \; {\mathrm{atmosphere}}}}}}\) and \({n}_{{{\mathrm{O}}}_{2}}^{{{{\mathrm{present}}}}\;{{{\mathrm{day}}}}}\) respectively, and \({\left[{{\mathrm{O}}}_{2}\right]}_{{{{\mathrm{sat}}}}}\) and \({\left[{{\mathrm{O}}}_{2}\right]}_{{{{\mathrm{surf}}}}}\) are the saturation and surface concentration of O2, respectively. The function \(f\left(\left[{{\mathrm{O}}}_{2}\right]\right)=1\) for \(\left[{{\rm{O}}}_{2}\right] > 0\) and 0 otherwise. The number of moles of O2 consumed to remineralize 1 mole of the typical Neoproterozoic marine biomass is given by \(-{r}_{{{\mathrm{O}}}_{2}:{\mathrm{P}}}\) (see below).
\({F}_{\mathrm{Fe}}^{{{{\mathrm{hydrothermal}}}}}\) is the hydrothermal influx of Fe2+ to the ocean, kFe is the oxidation rate of Fe2+ to Fe3+, which accounts for both abiotic and microbial pathways (described below), and \(\frac{\partial }{\partial z}{F}_{{{\mathrm{Fe}}}}^{{{{\mathrm{recycle}}}}}\) is the partial derivative with depth of the particulate Fe3+ sinking flux, which gives the rate at which particulate Fe3+ is recycled back to Fe2+ by anaerobic remineralization of organic matter. The tendency term for the number of moles of O2 in the model atmosphere is
where \(\xi ={10}^{4}\) is an arbitrary constant introduced to accelerate the convergence of the atmospheric box to a steady state, Asurf is the area of the ocean surface, Φred is the (mole equivalent) rate of consumption of O2 required to balance the volcanic flux of reduced gases into the atmosphere (for example, SO2, H2S, CO, CH4 and H2). Other oxygen sinks relevant to the present-day Earth system, like oxidative weathering of the continents and respiration by the terrestrial biosphere, are considered negligible during a global glaciation.
Jproduction, which is a function of the light (L) and \(\left[{{\rm{PO}}}_{4}^{3-}\right]\), is given by
where κL and \({\kappa }_{{{{\mathrm{PO}}}}_{4}^{3-}}\) are Michaelis–Menten-type half-saturation constants typical of ocean biology, and α is the maximum community production. The expression for L is
where fPAR is the fraction of shortwave radiation (SW) available for photosynthesis and k is the light attenuation constant. Since the light available for photosynthesis declines exponentially with depth, most of the biological activity in the ocean is close to the surface. Recall that primary production converts a fraction of \({{\rm{PO}}}_{4}^{3-}\) to DOP (fDOP), which can be advected by the physical circulation in the ocean, while the rest is POP, which sinks39. The downwards sinking flux of POP is given by
where zi is a reference depth below which the downwards flux of POP is calculated and zc is the compensation depth. In present-day ocean models, POP is remineralized according to a power law relation related almost entirely to aerobic respiration40. Additionally, our model accounts for remineralization of POP by anaerobic microbes that reduce Fe3+ particles (see below).
As was previously mentioned, Fe2+ in the ocean can be oxidized by abiotic and microbial pathways, both O2-related and light driven. The Fe2+ oxidation rate accounting for both abiotic and microbial processes is
where \({k}_{{{\mathrm{Fe}}}}^{{{{\mathrm{ab}}}}}\) is the abiotic rate constant of oxidation and fbio is the fraction of microbial Fe2+ oxidation out of the total oxidation as a function of available [O2] (ref. 41); \({{k}}_{{\rm{Fe}}}^{{{\mathrm{ab}}}}\) is given by
where T is the temperature (in kelvin), \(I=\frac{19.992{S}}{{10}^{3}-1.005{S}}\) and S is the salinity of seawater42. The fraction of microbial Fe2+ oxidation is given by
where KH is Henry’s law constant for O2:
fbio is capped at 0.95, an upper bound based on experimental results41. To allow model timesteps that are long enough so that convergence is reached over reasonable runtimes, chemical rate constants could not be too large. Thus, we prescribed an upper limit on kFe of 10−5 s−1. This upper limit was applied wherever O2 concentrations were high enough to result in kFe that was too large to allow efficient model timesteps, typically in the well-oxygenated regions in the ocean (for example, near the surface in the snowball with 12° S to 12° N ocean). The upper limit of kFe was prescribed in ~68% of the ocean volume in the snowball simulation with 12° S to 12° N ocean with 1% modern [\({{\rm{PO}}}_{4}^{3-}\)], in >90% of the ocean volume for a lower ice cover or higher [\({{\rm{PO}}}_{4}^{3-}\)]. The model results were relatively insensitive to this choice of an upper limit on kFe (Supplementary Fig. 9).
The downwards flux of Fe3+ produced from oxidation of Fe2+ is given by
where zu is the reference up to which the downwards flux of Fe3+ is calculated. We assume that a fraction of sinking Fe3+ is preserved (fpreserved) while the rest is remineralized by anaerobic microbes. This process restores some Fe2+ and is summarized by the following reaction:
The anaerobic reduction of Fe3+ by bacteria follows Michaelis–Menten-type kinetics with respect to the concentrations of both organic matter and Fe3+:
where μ is the typical respiration rate, and [Fe3+] and [C] are the concentrations of Fe3+ and organic matter, respectively. The relevant half-saturation constants for Fe3+ and C are κFe and κC, respectively, and the smaller of the saturation terms is considered to limit the Fe3+ reduction rate. Assuming a typical C:P ratio of 100:1 yields
and
The factor of 400 comes from the Fe:C:P stoichiometry of the reaction. To calculate the flux of recycled Fe3+ (\({F}_{{{\mathrm{Fe}}}}^{{{{\mathrm{recycle}}}}}\)) and the effect of Fe3+ reduction on the vertical POP remineralization profile, we further assume that [POP] and \([{\rm{F}}{{\rm{e}}}^{3+}]\) are proportional to FP and \({F}_{{{\mathrm{Fe}}}}^{{{{\mathrm{down}}}}}\) respectively. If \({F}_{{{\mathrm{Fe}}}}^{{{\mathrm{down}}}} >\left(\right.{F}_{{\mathrm{P}}}\)/400), the fluxes of recycled Fe3+ and POP are given by:
and
Similarly, when \({F}_{\mathrm{Fe}}^{{\mathrm{down}}} < ({F}_{\mathrm{P}}/400)\), the fluxes of recycled Fe3+ and POP are given by
and
Here zi is a reference depth below which the downwards flux of POP is calculated (note that zi = zc when z > zc), a is the vertical gradient of particle sinking velocity such that w(z) = az yields the speed of the sinking particles at depth z, and aremin is the exponent in Martin’s curve for POP remineralization in the modern ocean. Note that even though FP and FPOP have the same units, the quantities are not the same. FPOP is the depth-dependent downwards sinking flux of POP. On the other hand, FP is the depth-dependent POP remineralization flux, which can change based on whether remineralization of organic matter is anaerobic or aerobic.
The Fe2+ influx to the ocean from axial and off-axis hydrothermal circulation depends on the Fe2+ concentration in the fluid and the total water fluxes. The concentration of Fe2+ in axial and off-axis hydrothermal fluids is estimated to be in the range (0.75–6.5) × 10−3 mol kg−1 and (0.4–6.0) × 10−6) mol kg−1, respectively43,44,45. Assuming that the Fe2+ concentration is uniformly distributed in these ranges and the axial and off-axis water fluxes follow the distributions shown in ref. 46, we generated probability distributions corresponding to the axial and off-axis influx of hydrothermal Fe2+ to the ocean (Supplementary Fig. 10a,b). Separate from hydrothermal Fe2+ fluxes, weathering of seafloor basalt can deliver Fe2+ to the ocean47,48. The Fe2+ flux from seafloor weathering is rather difficult to constrain in the well-oxygenated modern ocean but it is estimated to be ~0.1–0.3 Tmol yr−1 and as high as 1.0 Tmol yr−1 (ref. 49), though it is unclear that all the Fe2+ lost from the basalt actually reaches the ocean. Given the existing estimates, we adopt a weathering Fe2+ flux uniformly distributed between 0.1 and 0.3 Tmol yr−1 (Supplementary Fig. 10c).
Convolving the distributions of axial and off-axis hydrothermal fluxes and seafloor weathering flux yields a range of Fe2+ influxes between 0.2 and 0.67 Tmol yr−1 (95% confidence interval) with a mode of 0.36 Tmol yr−1 (Supplementary Fig. 10d). Though some have suggested higher hydrothermal fluxes in Earth’s deep past due to higher radiogenic heat production in the mantle50, it remains unclear whether the higher heat production led instead to more sluggish seafloor spreading51 and a hydrothermal flux like the Phanerozoic. We do not upscale the hydrothermal water flux, leading to conservatively low Fe2+ influx estimates. Following the above analysis, we distribute the mode Fe2+ influx of 0.36 Tmol yr−1 on the model grid points corresponding to the suggested locations of seafloor spreading centres during the Sturtian glaciation. Moreover, we do not taper the Fe2+ influx with increasing distance from the spreading centres, since both near-axis and off-axis hydrothermal inputs occur close to the spreading centres (considering the spatial resolution of the ocean model), and the dissolved products of seafloor weathering are expected to reach the ocean mostly where the sedimentary cover on the basaltic crust is thin to absent (that is, near the spreading centres).
The marine phytoplankton community during the Neoproterozoic primarily composed of cyanobacteria. Previous studies52,53,54,55 show that the metabolite content of typical cyanobacterial biomass is 51% protein, 16% carbohydrates, 23% lipid and 10% nucleic acid, which is slightly different from the average composition of modern phytoplankton (which includes diatoms, coccolithophores, dinoflagellates and cyanobacteria): 54% protein, 26% carbohydrates, 16% lipid and 4% nucleic acid56. Despite these differences in the metabolite content, the oxidation state of cyanobacterial biomass is not substantially different from the gross modern assemblage (we estimated <1% variation in H:Corg and <10% variation in O:Corg ratios), which suggests that the difference between the Neoproterozoic and present-day O2:P ratio is expected to arise from a difference between the C:P ratios of cyanobacteria and the modern assemblage and not from the difference in the carbon oxidation state. Using the mean C:N:P ratio of 152:25:1 for cyanobacteria57, we calculate the following elemental composition of typical Neoproterozoic plankton: C152H247O61N25P. As per this mean composition, the \({{r}}_{{{\rm{O}}}_{2}:{\rm{P}}}\) ratio is estimated to be −216, which is ~45% more negative than its present-day value of −150 (ref. 56). In other words, 45% more O2 is consumed per mole of organic phosphorus liberated during organic matter remineralization.
As mentioned above, the majority of O2 sinks on the present-day Earth were negligible during the Neoproterozoic snowball Earth events. However, reducing volcanic emissions (SO2, H2S, CO, CH4 and H2) can be an important sink for O2 in a globally glaciated climate (Supplementary Table 2). All listed fluxes are approximate but can be used to reasonably estimate the equivalent moles of O2 consumed annually by oxidation of the reduced gases.
Data availability
The model data and the Python script necessary to reproduce the figures presented in this study are available from the figshare repository at https://doi.org/10.6084/m9.figshare.25130783.v1 (ref. 58). The other data that have been analysed can be accessed through the links provided in the studies that have been cited.
Code availability
The simulations are carried out using the MITgcm, an open-source ocean model that can be downloaded from https://mitgcm.readthedocs.io/en/latest/overview/overview.html. The specific model configuration is available upon request.
References
Bekker, A. et al. Iron formation: the sedimentary product of a complex interplay among mantle, tectonic, oceanic, and biospheric processes. Econ. Geol. 105, 467–508 (2010).
Konhauser, K. O. et al. Iron formations: a global record of Neoarchaean to Palaeoproterozoic environmental history. Earth Sci. Rev. 172, 140–177 (2017).
Lyons, T. W., Reinhard, C. T. & Planavsky, N. J. The rise of oxygen in Earth’s early ocean and atmosphere. Nature 506, 307–315 (2014).
Kirschvink, J. L. Late Proterozoic low-latitude global glaciation: the snowball Earth. Proterozoic Biosphere 52, 51–52 (1992).
Hoffman, P. F., Kaufman, A. J., Halverson, G. P. & Schrag, D. P. A Neoproterozoic snowball Earth. Science 281, 1342–1346 (1998).
Evans, D. A. D. Stratigraphic, geochronological, and paleomagnetic constraints upon the Neoproterozoic climatic paradox. Am. J. Sci. 300, 347–433 (2000).
Hyde, W. T., Crowley, T. J., Baum, S. K. & Peltier, W. R. Neoproterozoic ‘snowball Earth’ simulations with a coupled climate/ice-sheet model. Nature 405, 425–429 (2000).
Nelson, L. L. et al. Geochronological constraints on Neoproterozoic rifting and onset of the Marinoan glaciation from the Kingston Peak Formation in Death Valley, California (USA). Geology 48, 1083–1087 (2020).
Rooney, A. D., Yang, C., Condon, D. J., Zhu, M. & Macdonald, F. A. U–Pb and Re–Os geochronology tracks stratigraphic condensation in the Sturtian snowball Earth aftermath. Geology 48, 625–629 (2020).
Hoffman, P. F. et al. Snowball Earth climate dynamics and Cryogenian geology–geobiology. Sci. Adv. 3, e1600983 (2017).
Abbot, D. S., Voigt, A. & Koll, D. The Jormungand global climate state and implications for Neoproterozoic glaciations. J. Geophys. Res. 116, D18103 (2011).
Yang, J., Peltier, W. R. & Hu, Y. The Initiation of modern “soft snowball” and “hard snowball” climates in CCSM3. Part II: climate dynamic feedbacks. J. Clim. 25, 2737–2754 (2012).
Laakso, T. A. & Schrag, D. P. Regulation of atmospheric oxygen during the Proterozoic. Earth Planet. Sci. Lett. 388, 81–91 (2014).
Laakso, T. A. & Schrag, D. P. A theory of atmospheric oxygen. Geobiology 15, 366–384 (2017).
Crockford, P. W. et al. Triple oxygen isotope evidence for limited mid-Proterozoic primary productivity. Nature 559, 613–616 (2018).
Chan, C. S., Emerson, D. & Luther, G. W. The role of microaerophilic Fe‐oxidizing micro‐organisms in producing banded iron formations. Geobiology 14, 509–528 (2016).
Song, H. et al. The onset of widespread marine red beds and the evolution of ferruginous oceans. Nat. Commun. 8, 399 (2017).
Li, Z. X. et al. Assembly, configuration, and break-up history of Rodinia: a synthesis. Precambrian Res. 160, 179–210 (2008).
Yang, J., Peltier, W. R. & Hu, Y. The initiation of modern soft and hard snowball Earth climates in CCSM4. Climate 8, 907–918 (2012).
Ashkenazy, Y. et al. Dynamics of a snowball Earth ocean. Nature 495, 90–93 (2013).
Ashkenazy, Y., Gildor, H., Losch, M. & Tziperman, E. Ocean circulation under globally glaciated snowball earth conditions: steady-state solutions. J. Phys. Oceanogr. 44, 24–43 (2014).
Ashkenazy, Y. & Tziperman, E. Variability, instabilities, and eddies in a snowball ocean. J. Clim. 29, 869–888 (2016).
Lechte, M. A. et al. Subglacial meltwater supported aerobic marine habitats during snowball Earth. Proc. Natl Acad. Sci. USA 116, 25478–25483 (2019).
Marshall, J., Adcroft, A., Hill, C., Perelman, L. & Heisey, C. A finite‐volume, incompressible Navier Stokes model for studies of the ocean on parallel computers. J. Geophys. Res. Oceans 102, 5753–5766 (1997).
McDougall, T. J., Jackett, D. R., Wright, D. G. & Feistel, R. Accurate and computationally efficient algorithms for potential temperature and density of seawater. J. Atmos. Ocean Technol. 20, 730–741 (2003).
Redi, M. H. Oceanic isopycnal mixing by coordinate rotation. J. Phys. Oceanogr. 12, 1154–1158 (1982).
Gent, P. R. & Mcwilliams, J. C. Isopycnal mixing in ocean circulation models. J. Phys. Oceanogr. 20, 150–155 (1990).
Pollack, H. N., Hurter, S. J. & Johnson, J. R. Heat flow from the Earth’s interior: analysis of the global data set. Rev. Geophys. 31, 267–280 (1993).
Valdes, P. J., Scotese, C. R. & Lunt, D. J. Deep ocean temperatures through time. Climate 17, 1483–1506 (2021).
Liu, Y., Peltier, W. R., Yang, J. & Vettoretti, G. The initiation of Neoproterozoic "snowball" climates in CCSM3: the influence of paleocontinental configuration. Climate 9, 2555–2577 (2013).
Zhao, Z., Liu, Y. & Dai, H. Sea-glacier retreating rate and climate evolution during the marine deglaciation of a snowball Earth. Glob. Planet. Change 215, 103877 (2022).
Ashkenazy, Y. & Tziperman, E. A wind-induced thermohaline circulation hysteresis and millennial variability regimes. J. Phys. Oceanogr. 37, 2446–2457 (2007).
Ashkenazy, Y. The surface temperature of Europa. Heliyon 5, e01908 (2019).
Ashkenazy, Y., Sayag, R. & Tziperman, E. Dynamics of the global meridional ice flow of Europa’s icy shell. Nat. Astron. 2, 43–49 (2017).
Losch, M. Modeling ice shelf cavities in a z coordinate ocean general circulation model. J. Geophys. Res. Oceans 113, C08043 (2008).
McKinley, G. A., Follows, M. J. & Marshall, J. Mechanisms of air–sea CO2 flux variability in the equatorial Pacific and the North Atlantic. Glob. Biogeochem. Cycles 18, 1–14 (2004).
Dutkiewicz, S., Sokolov, A. P., Scott, J. & Stone, P. H. A Three-Dimensional Ocean-Seaice-Carbon Cycle Model and Its Coupling to a Two-Dimensional Atmospheric Model: Uses in Climate Change Studies. Joint Program Report Series Report (Massachusetts Institute of Technology, 2005).
MITgcm contributors. Biogeochemistry packages. MITgcm https://mitgcm.readthedocs.io/en/latest/phys_pkgs/phys_pkgs.html#biogeochemistry-packages (2019).
Yamanaka, Y. & Tajika, E. Role of dissolved organic matter in the marine biogeochemical cycle: studies using an ocean biogeochemical general circulation model. Glob. Biogeochem. Cycles 11, 599–612 (1997).
Martin, J. H., Knauer, G. A., Karl, D. M. & Broenkow, W. W. VERTEX: carbon cycling in the northeast Pacific. Deep Sea Res. Part A 34, 267–285 (1987).
Halevy, I., Alesker, M., Schuster, E. M., Popovitz-Biro, R. & Feldman, Y. A key role for green rust in the Precambrian oceans and the genesis of iron formations. Nat. Geosci. 10, 135–139 (2017).
Millero, F. J., Sotolongo, S. & Izaguirre, M. The oxidation kinetics of Fe(II) in seawater. Geochim. Cosmochim. Acta 51, 793–801 (1987).
Komada, T. et al. Dissolved organic carbon dynamics in anaerobic sediments of the Santa Monica Basin. Geochim. Cosmochim. Acta 110, 253–273 (2013).
Mottl, M. J. et al. Warm springs discovered on 3.5 Ma oceanic crust, eastern flank of the Juan de Fuca Ridge. Geology 26, 51 (1998).
Elderfield, H. & Schultz, A. Mid-ocean ridge hydrothermal fluxes and the chemical composition of the ocean. Annu Rev. Earth Planet Sci. 24, 191–224 (1996).
Halevy, I. & Bachan, A. The geologic history of seawater pH. Science 355, 1069–1071 (2017).
Poulton, S. W. & Raiswell, R. The low-temperature geochemical cycle of iron: from continental fluxes to marine sediment deposition. Am. J. Sci. 302, 774–805 (2002).
Wolery, T. J. & Sleep, N. H. Hydrothermal circulation and geochemical flux at mid-ocean ridges. J. Geol. 84, 249–275 (1976).
Hart, R. A. A model for chemical exchange in the basalt–seawater system of oceanic layer II. Can. J. Earth Sci. 10, 799–816 (1973).
Thompson, K. J. et al. Photoferrotrophy, deposition of banded iron formations, and methane production in Archean oceans. Sci. Adv. 5, eaav2869 (2019).
Korenaga, J. in Archean Geodynamics and Environments (eds Benn, K. Mareschal, J.-C. & Condie, K. C.) 7–32 (American Geophysical Union, 2006).
Molina, E., Martínez, M. E., Sánchez, S., García, F. & Contreras, A. Growth and biochemical composition with emphasis on the fatty acids of Tetraselmis sp. Appl. Microbiol. Biotechnol. 36, 21–25 (1991).
Fábregas, J., Patiño, M., Vecino, E., Cházaro, F. & Otero, A. Productivity and biochemical composition of cyclostat cultures of the marine microalga Tetraselmis suecica. Appl. Microbiol. Biotechnol. 43, 617–621 (1995).
Tahiri, M., Benider, A., Belkoura, M. & Dauta, A. Caractérisation biochimique de l’algue verte Scenedesmus abundans: influence des conditions de culture. Ann. Limnol. 36, 3–12 (2000).
Viegas, C. V. et al. Algal products beyond lipids: comprehensive characterization of different products in direct saponification of green alga Chlorella sp. Algal Res. 11, 156–164 (2015).
Anderson, L. A. On the hydrogen and oxygen content of marine phytoplankton. Deep Sea Res. Part I 42, 1675–1680 (1995).
Sharoni, S. & Halevy, I. Geologic controls on phytoplankton elemental composition. Proc. Natl Acad. Sci. USA 119, e2113263118 (2022).
Gianchandani, K. Dataset for “Production of Neoproterozoic banded iron formations in a partially ice-covered ocean” by Gianchandani et al. figshare https://doi.org/10.6084/m9.figshare.25130783.v1 (2024).
Acknowledgements
K.G., H.G. and Y.A. acknowledge the Joint National Natural Science Foundation of China, Israel Science Foundation Research Grant No. 2547/17 and the US-Israel Binational Science Foundation (BSF) Grant No. 2018152. I.H. acknowledges a Starting Grant from the European Research Council (OOID No. 755053). E.T. acknowledges NSF grant 2303486 from the P4CLIMATE programme and DOE grant DE-SC0023134. E.T. also thanks the Weizmann Institute for its hospitality during parts of this work.
Author information
Authors and Affiliations
Contributions
K.G. led the conceptualization, method development, data curation and analysis, and code development and performed the numerical simulations presented in this study. I.H., H.G., Y.A. and E.T. contributed to the conceptualization, method development, supervision and validation of the study. H.G. and Y.A. additionally contributed to project administration, resource management and funding acquisition. Y.A. additionally was involved in software development. K.G. drafted the initial manuscript, and all the authors contributed to the text and participated in the review and approval of the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Geoscience thanks Maxwell Lechte and Lennart Ramme for their contribution to the peer review of this work. Primary Handling Editor(s): James Super, in collaboration with the Nature Geoscience team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Sections 1–4, Figs. 1–11 and Tables 1–3.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gianchandani, K., Halevy, I., Gildor, H. et al. Production of Neoproterozoic banded iron formations in a partially ice-covered ocean. Nat. Geosci. 17, 298–301 (2024). https://doi.org/10.1038/s41561-024-01406-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41561-024-01406-4