Abstract
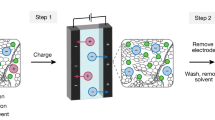
With the global trend towards carbon neutrality, the demand for lithium-ion batteries (LIBs) is continuously increasing. However, current recycling methods for spent LIBs need urgent improvement in terms of eco-friendliness, cost and efficiency. Here we propose a mechano-catalytic method, dubbed contact-electro-catalysis, utilizing radicals generated by contact electrification to promote the metal leaching under the ultrasonic wave. We also use SiO2 as a recyclable catalyst in the process. For lithium cobalt (III) oxide batteries, the leaching efficiency reached 100% for lithium and 92.19% for cobalt at 90 °C within 6 hours. For ternary lithium batteries, the leaching efficiencies of lithium, nickel, manganese and cobalt reached 94.56%, 96.62%, 96.54% and 98.39% at 70 °C, respectively, within 6 hours. We anticipate that this method can provide a green, high efficiency and economic approach for LIB recycling, meeting the exponentially growing demand for LIB productions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the paper and its Supplementary Information files. Source data are provided with this paper.
References
Lv, H. et al. Electric field driven de-lithiation: a strategy towards comprehensive and efficient recycling of electrode materials from spent lithium ion batteries. Applied Catal. B 283, 119634 (2021).
Kang, D. H., Chen, M. & Ogunseitan, O. A. Potential environmental and human health impacts of rechargeable lithium batteries in electronic waste. Environ. Sci. Technol. 47, 5495–5503 (2013).
Ciez, R. E. & Whitacre, J. F. Examining different recycling processes for lithium-ion batteries. Nat. Sustain. 2, 148–156 (2019).
Global Supply Chains of EV Batteries (IEA, 2022); https://www.iea.org/reports/global-supply-chains-of-ev-batteries
Chernyaev, A. et al. The efficiency of scrap Cu and Al current collector materials as reductants in LIB waste leaching. Hydrometallurgy 203, 105608 (2021).
Wang, S., Zhang, Z., Lu, Z. & Xu, Z. A novel method for screening deep eutectic solvent to recycle the cathode of Li-ion batteries. Green Chem. 22, 4473–4482 (2020).
Yang, Y. et al. Selective recovery of lithium from spent lithium iron phosphate batteries: a sustainable process. Green Chem. 20, 3121–3133 (2018).
Zeng, A. et al. Battery technology and recycling alone will not save the electric mobility transition from future cobalt shortages. Nat. Commun. 13, 1341 (2022).
Or, T., Gourley, S. W. D., Kaliyappan, K., Yu, A. & Chen, Z. Recycling of mixed cathode lithium‐ion batteries for electric vehicles: current status and future outlook. Carbon Energy 2, 6–43 (2020).
Wang, Y. et al. Recent progress on the recycling technology of Li-ion batteries. J. Energy Chem. 55, 391–419 (2021).
Raj, T. et al. Recycling of cathode material from spent lithium-ion batteries: challenges and future perspectives. J. Hazard. Mater. 429, 128312 (2022).
Baum, Z. J., Bird, R. E., Yu, X. & Ma, J. Lithium-ion battery recycling─overview of techniques and trends. ACS Energy Lett. 7, 712–719 (2022).
Garole, D. J. et al. Recycle, recover and repurpose strategy of spent Li-ion batteries and catalysts: current status and future opportunities. ChemSusChem 13, 3079–3100 (2020).
Barik, S. P., Prabaharan, G. & Kumar, L. Leaching and separation of Co and Mn from electrode materials of spent lithium-ion batteries using hydrochloric acid: laboratory and pilot-scale study. J. Clean. Prod. 147, 37–43 (2017).
Chen, X., Ma, H., Luo, C. & Zhou, T. Recovery of valuable metals from waste cathode materials of spent lithium-ion batteries using mild phosphoric acid. J. Hazard. Mater. 326, 77–86 (2017).
Zhou, S. et al. Recycling of LiCoO2 cathode material from spent lithium ion batteries by ultrasonic enhanced leaching and one-step regeneration. J. Environ. Manage. 277, 111426 (2021).
Bai, Y. et al. Energy and environmental aspects in recycling lithium-ion batteries: concept of battery identity global passport. Mater. Today 41, 304–315 (2020).
Li, L. et al. Succinic acid-based leaching system: a sustainable process for recovery of valuable metals from spent Li-ion batteries. J. Power Sources 282, 544–551 (2015).
Gao, W. et al. Comprehensive evaluation on effective leaching of critical metals from spent lithium-ion batteries. Waste Manage. 75, 477–485 (2018).
Li, L. et al. Recovery of metals from spent lithium-ion batteries with organic acids as leaching reagents and environmental assessment. J. Power Sources 233, 180–189 (2013).
Fan, E. et al. Sustainable recycling technology for Li-ion batteries and beyond: challenges and future prospects. Chem. Rev. 120, 7020–7063 (2020).
Tran, M. K., Rodrigues, M.-T. F., Kato, K., Babu, G. & Ajayan, P. M. Deep eutectic solvents for cathode recycling of Li-ion batteries. Nat. Energy 4, 339–345 (2019).
Wang, Z. et al. Contact-electro-catalysis for the degradation of organic pollutants using pristine dielectric powders. Nat. Commun. 13, 130 (2022).
Chen, B. et al. Water-solid contact electrification causes hydrogen peroxide production from hydroxyl radical recombination in sprayed microdroplets. Proc. Natl Acad. Sci. USA 119, e2209056119 (2022).
Lin, S., Xu, L., Chi Wang, A. & Wang, Z. L. Quantifying electron-transfer in liquid–solid contact electrification and the formation of electric double-layer. Nat. Commun. 11, 399 (2020).
Sun, M., Lu, Q., Wang, Z. L. & Huang, B. Understanding contact electrification at liquid–solid interfaces from surface electronic structure. Nat. Commun. 12, 1752 (2021).
Lin, M.-F. et al. Imaging the short-lived hydroxyl-hydronium pair in ionized liquid water. Science 374, 92–95 (2021).
Wang, J. et al. Sonocatalytic damage of bovine serum albumin (BSA) under ultrasonic irradiation by mixed TiO2/SiO2 powder. J. Chem. Technol. Biotechnol. 84, 538–546 (2009).
Deveci, I. & Mercimek, B. Performance of SiO2/Ag core/shell particles in sonocatalalytic degradation of rhodamine B. Ultrason. Sonochem. 51, 197–205 (2019).
Zhan, F. et al. Electron transfer as a liquid droplet contacting a polymer surface. ACS Nano. 14, 17565–17573 (2020).
Behera, S. S. & Parhi, P. K. Leaching kinetics study of neodymium from the scrap magnet using acetic acid. Sep. Purif. Technol. 160, 59–66 (2016).
Hu, W. et al. Photocatalytic reduction of Cr(VI) using a wurtzite/natural sphalerite heterostructure: synergistic effects of exposed active facets, vacancies and a heterophase junction. Appl. Surf. Sci. 550, 149267 (2021).
Liang, H. et al. 3-D hierarchical Ag/ZnO@CF for synergistically removing phenol and Cr(VI): heterogeneous vs homogeneous photocatalysis. J. Colloid Interface Sci. 558, 85–94 (2020).
Nosaka, Y. & Nosaka, A. Y. Generation and detection of reactive oxygen species in photocatalysis. Chem. Rev. 117, 11302–11336 (2017).
Xiao, R. et al. In situ fabrication of 1D CdS nanorod/2D Ti3C2 MXene nanosheet Schottky heterojunction toward enhanced photocatalytic hydrogen evolution. Appl. Catal. B 268, 118382 (2020).
Xu, C. et al. Raising the working temperature of a triboelectric nanogenerator by quenching down electron thermionic emission in contact-electrification. Adv. Mater. 30, e1803968 (2018).
Shen, F. et al. Influence of temperature difference on performance of solid–liquid triboelectric nanogenerators. Nano Energy 99, 107431 (2022).
Huang, H. et al. Macroscopic polarization enhancement promoting photo- and piezoelectric-induced charge separation and molecular oxygen activation. Angew. Chem. Int. Ed. 56, 11860–11864 (2017).
Geng, M. & Duan, Z. Prediction of oxygen solubility in pure water and brines up to high temperatures and pressures. Geochim. Cosmochim. Acta 74, 5631–5640 (2010).
Rodrigues, C. et al. Emerging triboelectric nanogenerators for ocean wave energy harvesting: state of the art and future perspectives. Energy Environ. Sci. 13, 2657–2683 (2020).
Lin, S., Chen, X. & Wang, Z. L. Contact electrification at the liquid–solid interface. Chem. Rev. 122, 5209–5232 (2022).
Huang, Z. et al. Solution inheritance of CoC2O4·2H2O rods to nanoparticle-assembled Co3O4 rods. Colloids Surf. A 490, 307–317 (2016).
Qiu, X. et al. Enabling the sustainable recycling of LiFePO4 from spent lithium-ion batteries. Green Chem. 24, 2506–2515 (2022).
Cai, W., Chen, R., Yang, Y., Yi, M. & Xiang, L. Removal of SO42− from Li2CO3 by recrystallization in Na2CO3 solution. Crystals 8, 19 (2018).
Chen, X., Kang, D., Li, J., Zhou, T. & Ma, H. Gradient and facile extraction of valuable metals from spent lithium ion batteries for new cathode materials re-fabrication. J. Hazard. Mater. 389, 121887 (2020).
Wang, K. et al. Hexadecane-containing sandwich structure based triboelectric nanogenerator with remarkable performance enhancement. Nano Energy 87, 106198 (2021).
Acknowledgements
We appreciate C. Li, S. Shu and Y. Su for their assistance with collecting and organizing data. This research was supported by the National Key R&D Project from the Minister of Science and Technology (2021YFA1201601) and National Natural Science Foundation of China (grant number 52192610), Youth Innovation Promotion Association (W.T.) and CAS-TWAS President’s Fellowship (A.B.).
Author information
Authors and Affiliations
Contributions
W.T. and Z.L.W. conceived the idea and supervised the experiment. H.L., A.B. and X.Z. performed the experiments, H.L. and W.T. analysed the data. Z.W. assisted in data measurements. H.L., A.B. and W.T. prepared the manuscript. Z.L.W. revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Energy thanks Gavin Harper, Jia Li and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–8, Note 1 and Tables 1 and 2.
Supplementary Table 1
Statistical source data.
Supplementary Data
Statistical source data.
Supplementary Data
Statistical source data.
Supplementary Data
Statistical source data.
Supplementary Data
Statistical source data.
Supplementary Data
Statistical source data.
Supplementary Data
Statistical source data.
Supplementary Data
Statistical source d.ata
Source data
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, H., Berbille, A., Zhao, X. et al. A contact-electro-catalytic cathode recycling method for spent lithium-ion batteries. Nat Energy 8, 1137–1144 (2023). https://doi.org/10.1038/s41560-023-01348-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-023-01348-y
This article is cited by
-
Reduction of precious metal ions in aqueous solutions by contact-electro-catalysis
Nature Communications (2024)
-
A contact-electro-catalysis process for producing reactive oxygen species by ball milling of triboelectric materials
Nature Communications (2024)
-
Spontaneously established reverse electric field to enhance the performance of triboelectric nanogenerators via improving Coulombic efficiency
Nature Communications (2024)