Abstract
Ocean deoxygenation is predicted to threaten marine ecosystems globally. However, current and future oxygen concentrations and the occurrence of hypoxic events on coral reefs remain underexplored. Here, using autonomous sensor data to explore oxygen variability and hypoxia exposure at 32 representative reef sites, we reveal that hypoxia is already pervasive on many reefs. Eighty-four percent of reefs experienced weak to moderate (≤153 µmol O2 kg−1 to ≤92 µmol O2 kg−1) hypoxia and 13% experienced severe (≤61 µmol O2 kg−1) hypoxia. Under different climate change scenarios based on four Shared Socioeconomic Pathways (SSPs), we show that projected ocean warming and deoxygenation will increase the duration, intensity and severity of hypoxia, with more than 94% and 31% of reefs experiencing weak to moderate and severe hypoxia, respectively, by 2100 under SSP5-8.5. This projected oxygen loss could have negative consequences for coral reef taxa due to the key role of oxygen in organism functioning and fitness.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
All data included in this study (for all figures and statistics56) are freely available on Dryad (https://doi.org/10.5061/dryad.41ns1rnj7). Data may be used if cited appropriately.
Code availability
All code files written and used for analyses in this study57 are freely available on GitHub (https://github.com/apezner/GlobalReefOxygen). Code may be used if cited appropriately.
References
Stramma, L., Johnson, G. C., Sprintall, J. & Mohrholz, V. Expanding oxygen-minimum zones in the tropical oceans. Science 320, 655–658 (2008).
Keeling, R. F., Körtzinger, A. & Gruber, N. Ocean deoxygenation in a warming world. Ann. Rev. Mar. Sci. 2, 199–229 (2010).
Breitburg, D. et al. Declining oxygen in the global ocean and coastal waters. Science 359, eaam7240 (2018).
Bopp, L. et al. Multiple stressors of ocean ecosystems in the 21st century: projections with CMIP5 models. Biogeosciences 10, 6225–6245 (2013).
Kwiatkowski, L. et al. Twenty-first century ocean warming, acidification, deoxygenation, and upper-ocean nutrient and primary production decline from CMIP6 model projections. Biogeosciences 17, 3439–3470 (2020).
Diaz, R. J. & Rosenberg, R. Marine benthic hypoxia: a review of its ecological effects and the behavioural responses of benthic macrofauna. Oceanogr. Mar. Biol. 33, 245–303 (1995).
Altieri, A. H. et al. Tropical dead zones and mass mortalities on coral reefs. Proc. Natl Acad. Sci. USA 114, 3660–3665 (2017).
Nelson, H. R. & Altieri, A. H. Oxygen: the universal currency on coral reefs. Coral Reefs 38, 177–198 (2019).
Hughes, D. J. et al. Coral reef survival under accelerating ocean deoxygenation. Nat. Clim. Change 10, 296–307 (2020).
Kealoha, A. K. et al. Localized hypoxia may have caused coral reef mortality at the Flower Garden Banks. Coral Reefs 39, 119–132 (2020).
Frölicher, T. L., Fischer, E. M. & Gruber, N. Marine heatwaves under global warming. Nature 560, 360–364 (2018).
Vaquer-Sunyer, R., Duarte, C. M., Jordà, G. & Ruiz-Halpern, S. Temperature dependence of oxygen dynamics and community metabolism in a shallow Mediterranean macroalgal meadow (Caulerpa prolifera). Estuaries Coast. 35, 1182–1192 (2012).
Sutherland, W. J. et al. A 2021 horizon scan of emerging global biological conservation issues. Trends Ecol. Evol. 36, 87–97 (2021).
Cyronak, T. et al. Diel temperature and pH variability scale with depth across diverse coral reef habitats. Limnol. Oceanogr. Lett. 5, 193–203 (2020).
Gray, J. S., Wu, R. S. S. & Or, Y. Y. Effects of hypoxia and organic enrichment on the coastal marine environment. Mar. Ecol. Prog. Ser. 238, 249–279 (2002).
Vaquer-Sunyer, R. & Duarte, C. M. Thresholds of hypoxia for marine biodiversity. Proc. Natl Acad. Sci. USA 105, 15452–15457 (2008).
Vaquer-Sunyer, R. & Duarte, C. M. Temperature effects on oxygen thresholds for hypoxia in marine benthic organisms. Glob. Change Biol. 17, 1788–1797 (2011).
Haas, A. F., Smith, J. E., Thompson, M. & Deheyn, D. D. Effects of reduced dissolved oxygen concentrations on physiology and fluorescence of hermatypic corals and benthic algae. PeerJ 2, e235 (2014).
Johnson, M. D., Swaminathan, S. D., Nixon, E. N., Paul, V. J. & Altieri, A. H. Differential susceptibility of reef-building corals to deoxygenation reveals remarkable hypoxia tolerance. Sci. Rep. 11, 23168 (2021).
Gravinese, P. M., Douwes, A., Eaton, K. R. & Muller, E. M. Ephemeral hypoxia reduces oxygen consumption in the Caribbean coral Orbicella faveolata. Coral Reefs 41, 13–18 (2021).
Nilsson, G. E., Östlund-Nilsson, S. & Munday, P. L. Effects of elevated temperature on coral reef fishes: loss of hypoxia tolerance and inability to acclimate. Comp. Biochem. Physiol. 156, 389–393 (2010).
DeCarlo, T. M. et al. Mass coral mortality under local amplification of 2 °C ocean warming. Sci. Rep. 7, 44586 (2017).
Hauri, C., Gruber, N., McDonnell, A. M. P. & Vogt, M. The intensity, duration, and severity of low aragonite saturation state events on the California continental shelf. Geophys. Res. Lett. 40, 3424–3428 (2013).
Guzmán, H. M., Cortés, J., Glynn, P. W. & Richmond, R. H. Coral mortality associated with dinoflagellate blooms in the Eastern Pacific (Costa Rica and Panama). Mar. Ecol. Prog. Ser. 60, 299–303 (1990).
Raj, K. D. et al. Low oxygen levels caused by Noctiluca scintillans bloom kills corals in Gulf of Mannar, India. Sci. Rep. 10, 22133 (2020).
Johnson, M. D. et al. Rapid ecosystem-scale consequences of acute deoxygenation on a Caribbean coral reef. Nat. Commun. 12, 4522 (2021).
Andréfouët, S., Dutheil, C., Menkes, C. E., Bador, M. & Lengaigne, M. Mass mortality events in atoll lagoons: environmental control and increased future vulnerability. Glob. Change Biol. 21, 195–205 (2015).
Altieri, A. H. & Gedan, K. B. Climate change and dead zones. Glob. Change Biol. 21, 1395–1406 (2015).
Murphy, J. W. A. & Richmond, R. H. Changes to coral health and metabolic activity under oxygen deprivation. PeerJ 4, e1956 (2016).
Alderdice, R. et al. Divergent expression of hypoxia response systems under deoxygenation in reef‐forming corals aligns with bleaching susceptibility. Glob. Change Biol. 27, 312–326 (2021).
Al-Horani, F. A., Tambutté, É. & Allemand, D. Dark calcification and the daily rhythm of calcification in the scleractinian coral, Galaxea fascicularis. Coral Reefs 26, 531–538 (2007).
Wijgerde, T., Jurriaans, S., Hoofd, M., Verreth, J. A. J. & Osinga, R. Oxygen and heterotrophy affect calcification of the scleractinian coral Galaxea fascicularis. PLoS ONE 7, e52702 (2012).
Wijgerde, T., Silva, C. I. F., Scherders, V., van Bleijswijk, J. & Osinga, R. Coral calcification under daily oxygen saturation and pH dynamics reveals the important role of oxygen. Biol. Open 3, 489–493 (2014).
Deleja, M. et al. Effects of hypoxia on coral photobiology and oxidative stress. Biology 11, 1068 (2022).
Alderdice, R. et al. Hypoxia as a physiological cue and pathological stress for coral larvae. Mol. Ecol. 31, 571–587 (2022).
Alderdice, R. et al. Disparate inventories of hypoxia gene sets across corals align with inferred environmental resilience. Front. Mar. Sci. 9, 834332 (2022).
Jorissen, H. & Nugues, M. M. Coral larvae avoid substratum exploration and settlement in low-oxygen environments. Coral Reefs 9, 31–39 (2021).
Villanueva, R. D., Yap, H. T. & Montaño, M. N. E. Survivorship of coral juveniles in a fish farm environment. Mar. Pollut. Bull. 10, 580–589 (2005).
Pörtner, H.-O., Bock, C. & Mark, F. C. Oxygen- and capacity-limited thermal tolerance: Bridging ecology and physiology. J. Exp. Biol. 220, 2685–2696 (2017).
Deutsch, C., Ferrel, A., Seibel, B., Pörtner, H.-O. & Huey, R. B. Climate change tightens a metabolic constraint on marine habitats. Science 348, 1132–1135 (2015).
Alderdice, R. et al. Deoxygenation lowers the thermal threshold of coral bleaching. Sci. Rep. 12, 18273 (2022).
Steckbauer, A., Klein, S. G. & Duarte, C. M. Additive impacts of deoxygenation and acidification threaten marine biota. Glob. Change Biol. 26, 5602–5612 (2020).
Cai, W. J. et al. Acidification of subsurface coastal waters enhanced by eutrophication. Nat. Geosci. 4, 766–770 (2011).
D’Angelo, C. & Wiedenmann, J. Impacts of nutrient enrichment on coral reefs: new perspectives and implications for coastal management and reef survival. Curr. Opin. Environ. Sustain. 7, 82–93 (2014).
Grégoire, M. et al. A global ocean oxygen database and atlas for assessing and predicting deoxygenation and ocean health in the open and coastal ocean. Front. Mar. Sci. 8, 724913 (2021).
Yates, K. K., Moore, C. S. & Smiley, N. A. Time Series of Autonomous Carbonate System Parameter Measurements from Crocker Reef, Florida, USA (US Geological Survey, 2019); https://doi.org/10.5066/P90NCI8T
Kekuewa, S. A. H. et al. Temporal and spatial variabilities of chemical and physical parameters on the Heron Island coral reef platform. Aquat. Geochem. 27, 241–268 (2021).
Pedersen, K. A. Spatiotemporal Variability in Seawater Carbonate Chemistry at Two Contrasting Reef Locations in Bocas del Toro, Panama. MSc thesis, Univ. California (2019).
Page, H. N. et al. Spatiotemporal variability in seawater carbon chemistry for a coral reef flat in Kāne’ohe Bay, Hawai’i. Limnol. Oceanogr. 64, 913–934 (2018).
Pezner, A. K. et al. Lateral, vertical, and temporal variability of seawater carbonate chemistry at Hog Reef, Bermuda. Front. Mar. Sci. 8, 1–18 (2021).
Ecosystem Sciences Division National Coral Reef Monitoring Program: Diel Seawater Carbonate Chemistry Observations from a Suite of Instrumentation Deployed at Coral Reef Sites at Tutuila Island, American Samoa from June 23 to July 17, 2018 NCEI Accession 0240606 (Pacific Islands Fisheries Science Center, 2021).
Ecosystem Sciences Division National Coral Reef Monitoring Program: Diel Seawater Carbonate Chemistry Observations from a Suite of Instrumentation Deployed at Coral Reef Sites at Baker Island, Jarvis Island, and Palmyra Atoll in the Pacific Remote Islands Marine National Monument Between 2018-06-12 and 2018-08-07 NCEI Accession 0240686 (Pacific Islands Fisheries Science Center, 2021).
Rintoul, M. S. et al. The effects of light intensity and flow speed on biogeochemical variability within a fringing coral reef in Onna‐son, Okinawa, Japan. J. Geophys. Res. Oceans 127, e2021JC018369 (2022).
Kelley, D. & Richards, C. gsw: Gibbs sea water functions. R package version 1.0-5 https://CRAN.R-project.org/package=gsw (2017).
RStudio Team RStudio: Integrated Development for R (RStudio, 2020).
Pezner, A. K. et al. Data for: Increasing hypoxia on global coral reefs under ocean warming. Dryad https://doi.org/10.5061/dryad.41ns1rnj7 (2023).
Pezner, A. K. et al. Global reef oxygen. GitHub https://github.com/apezner/GlobalReefOxygen (2023).
Kennedy, E. V. et al. Reef cover, a coral reef classification for global habitat mapping from remote sensing. Sci. Data 8, 196 (2021).
Dowle, M. & Srinivasan, A. data.table: extension of ‘data.frame’. R package version 1.13.6 https://CRAN.R-project.org/package=data.table (2020).
Rosenberg, R. in Fjord Oceanography: Effects of Oxygen Deficiency on Benthic Macrofauna in Fjord oceanography, H. J. Freeland, D. M. Farmer, and C. D. Levings (eds), 499–514 (Plenum Press, 1980).
Hofmann, A. F., Peltzer, E. T., Walz, P. M. & Brewer, P. G. Hypoxia by degrees: establishing definitions for a changing ocean. Deep Sea Res. I 58, 1212–1226 (2011).
Klein, S. G., Steckbauer, A. & Duarte, C. M. Defining CO2 and O2 syndromes of marine biomes in the Anthropocene. Glob. Change Biol. 26, 355–363 (2020).
Danabasoglu, G. NCAR CESM2-WACCM Model Output Prepared for CMIP6 CMIP (Earth System Grid Federation, 2019); https://doi.org/10.22033/ESGF/CMIP6.10028
Danabasoglu, G. NCAR CESM2-WACCM Model Output Prepared for CMIP6 ScenarioMIP (Earth System Grid Federation, 2019); https://doi.org/10.22033/ESGF/CMIP6.10101
Garcia, H. E. & Gordon, L. I. Oxygen solubility in seawater: better fitting equations. Limnol. Oceanogr. 37, 1307–1312 (1992).
Hochachka, P. W. & Somero, G. N. Biochemical Adaptations (Oxford Univ. Press, 2002).
Brown, J. H., Gillooly, J. F., Allen, A. P., Savage, V. M. & West, G. B. Toward a metabolic theory of ecology. Ecology 85, 1771–1789 (2004).
Clausen, C. D. & Roth, A. A. Effect of temperature and temperature adaptation on calcification rate in the hermatypic coral Pocillopora damicornis. Mar. Biol. 33, 93–100 (1975).
Howe, S. A. & Marshall, A. T. Thermal compensation of metabolism in the temperate coral, Plesiastrea versipora (Lamarck, 1816). J. Exp. Mar. Biol. Ecol. 259, 231–248 (2001).
Edmunds, P., Gates, R. & Gleason, D. The biology of larvae from the reef coral Porites astreoides, and their response to temperature disturbances. Mar. Biol. 139, 981–989 (2001).
Edmunds, P. J. Effect of elevated temperature on aerobic respiration of coral recruits. Mar. Biol. 146, 655–663 (2005).
Edmunds, P. J. Differential effects of high temperature on the respiration of juvenile Caribbean corals. Bull. Mar. Sci. 83, 453–464 (2008).
Acknowledgments
We thank K. Inoha, R.-W. Syu and all field station administrators and field assistants who were essential in collecting these datasets. Any use of trade, firm or product names is for descriptive purposes only and does not imply endorsement by the US Government. Funding was provided by the National Science Foundation: OCE-1255042 (A.J.A.), OCE-1829778 (A.J.A.), OCE-1538495 (D.I.K. and M.T.), OCE-1459255 (M.D.D) and OPP-1951294 (M.D.D); Belmont Forum/NSF ICER 2029205 (A.J.A.); UCSD Marine Sciences grant no. A105437 (A.J.A.); the National Science Foundation Graduate Research Fellowship DGE-2038238 (A.K.P.); a Philanthropic Educational Organization International Scholar Award (A.K.P.); the NOAA Coral Reef Conservation Program and NOAA Ocean Acidification Program, through the NOAA National Coral Reef Monitoring Program (H.C.B.); the US Geological Survey Coastal and Marine Hazards and Resources Program-funded data collection at Crocker Reef, Florida, USA (K.K.Y.46); internal funding from the Okinawa Institute of Science and Technology (S.M.); and the Ministry of Science and Technology of Taiwan grant no. 107-2611-M-019-001-MY3 (W.-C.C.). Funding for the long-term monitoring programme on Palmyra Atoll was provided to Smith Lab (J.E.S.) from the Bohn Family Foundation and the Bill and Kathy Scripps Family Foundation.
Author information
Authors and Affiliations
Contributions
A.K.P., T.A.C. and A.J.A. conceptualized the paper and methodology, with contributions from M.S.R. to methodology. A.K.P. performed the formal analysis and visualization, under supervision of T.A.C. and A.J.A. A.K.P. and A.J.A. wrote the original draft of the paper. All authors (A.K.P., T.A.C., H.C.B., W.-C.C., H.-C.C., S.M.C., T.C., M.D.G, S.A.H.K., D.I.K., Y.-B.L., T.R.M., S.M., H.N.P., M.S.R., J.E.S., K.S., Y.T., M.T., Y.W., K.K.Y. and A.J.A.) contributed to investigation as well as review and editing of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Climate Change thanks Rachel Alderdice, Christopher Cornwall and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
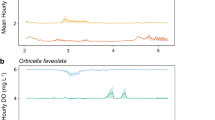
Extended Data Fig. 1 Dissolved oxygen and temperature time series for global coral reef sites.
Dissolved oxygen (µmol O2 kg−1; black, left y-axis) and temperature (°C; blue, right y-axis) as a function of time in order of sites with increasing deployment length, ranging from 3 to 309 days (Supplementary Table 1). For each location, different instrument deployment sites are represented by numbers (for example, Dongsha 1 and Dongsha 2), or a combination of letters and numbers where letters represent either different depths at the same site (for example, Bocas 1a and 1b) or different deployments at the same site over time (for example, Crocker 1a, 1b, and 1c) (see Supplementary Table 1 for specific site information).
Extended Data Fig. 2 Nonlinear regressions between dissolved oxygen metrics, depth, and flow speed categorized by reef type.
(a) Dissolved oxygen (DO) variability (mean daily range in DO; µmol O2 kg−1; ± 1 standard deviation (s.d.)) as a function of mean depth (m; ± 1 s.d.) and (b) mean flow speed (m s−1; ± 1 s.d.) at global coral reef sites categorized by reef type (colors; Supplementary Table 1). (c) Mean daily minimum DO (µmol O2 kg−1; ± 1 s.d.) as a function of mean depth (m; ± 1 s.d.) and (d) mean flow speed (m s−1; ± 1 s.d.). For DO metrics, error bars represent ± 1 s.d. (n varies by site, see Supplementary Table 2). Measurements of flow speed and depth were only made at a subset of locations (n varied by site, see Supplementary Table 3). Error bars also represent ± 1 s.d. for the sites where these data were recorded. For sites where no current meter was deployed, recorded deployment depth was used instead of a calculated mean depth (no error bars plotted). Regression lines were plotted using power model regression statistics reported in Supplementary Table 4. No regression line is plotted in B due to poor fit.
Extended Data Fig. 3 Sea surface temperature predictions for global coral reef locations.
Mean monthly Coupled Model Intercomparison Project 6 (CMIP6) ensemble member Community Earth System Model 2 Whole Atmosphere Community Climate Model (CESM2-WACCM)63,64 sea surface temperature (SST) projections at global coral reef sites for the Shared Socioeconomic Pathways (SSPs) SSP1-2.6, SSP2-4.5, SSP3-7.0, and SSP5-8.5 scenarios (blue to red) between 2015 and 2100.
Extended Data Fig. 4 Conceptual diagram of calculation approach used to estimate changes in coral reef dissolved oxygen under warming (Equations 6–13).
(a) Present-day dissolved oxygen (DO; µmol O2 kg−1; solid grey line), mean DO value across time series (purple dashed line; assumed to be close to equilibrium), approximation of drawdown of DO by respiration at night (DOoffset; yellow arrow) expressed as the difference between mean DO and mean daily minimum (DOmin; orange), and the projected increase in respiration under 3 °C warming using a Q10 relationship (∆DOQ10; pink arrow). (b) Present-day DO (solid grey line), present-day DO solubility (dashed dark blue line; DOsol present), DO solubility under 3 °C warming (dashed light blue line), and the calculated decrease in solubility under 3 °C warming (∆DOsol; green arrow). (c) Present-day DO (solid grey line) and new calculated DO under 3 °C warming (black solid line) due to increased respiration and decreased solubility (pink and green arrows, respectively).
Extended Data Fig. 5 Box model simulations and validation of calculation approach to estimate dissolved oxygen changes as a result of warming.
(a) Modeled variations in seawater dissolved oxygen (DO; µmol O2 kg−1) in a hypothetical coral reef system over 7 days under three temperature scenarios (25 °C, 28 °C, and 31 °C; purple, green, blue, respectively) and two residence times (1 hour and 5 hours; solid and dotted lines, respectively). The model is described in detail in the Supplementary Information Extended Methods. (b) Comparison between the box model-calculated changes in DO due to warming and the calculation approach employed for the global coral reef dataset (Extended Data Fig. 4; Equations 6–13) represented as the deviation of model DO estimates from calculation DO estimates (µmol O2 kg−1). Comparisons were made for two warming scenarios relative to the base scenario of 25 °C (+3 °C and +6 °C; blue and green, respectively) and two residence times (1 hour and 5 hours; solid and dotted, respectively) over 7 days.
Extended Data Fig. 6 Total number of hypoxic observations and events for global coral reef sites under different warming scenarios and hypoxia thresholds.
(a) Total number of observations and (b) total number of hypoxic events below different hypoxia thresholds: 153 µmol O2 kg−1, 122 µmol O2 kg−1, 92 µmol O2 kg−1, and 61 µmol O2 kg−1 (weak, mild, moderate, and severe; light blue to dark blue) for different warming scenarios including 4 Shared Socioeconomic Pathways (SSPs) and a heatwave event across global coral reef sites.
Extended Data Fig. 7 Changes in the duration, intensity, and severity of hypoxic events under warming for global coral reef sites.
Distributions of the (a) duration (hours), (b) intensity (µmol O2 kg−1), and (c) severity (µmol O2 kg−1 hr) of hypoxic events below different oxygen thresholds (≤153 µmol O2 kg−1, ≤122 µmol O2 kg−1, ≤92 µmol O2 kg−1, or ≤61 µmol O2 kg−1) under present-day conditions and 5 different warming projections (including 4 Shared Socioeconomic Pathways (SSPs); blue to red) across global coral reef sites.
Extended Data Fig. 8 Changes in the cumulative duration, intensity, and severity of hypoxic events under warming for global coral reef sites.
(a) Cumulative duration (hours), (b) cumulative intensity (µmol O2 kg−1), and (c) cumulative severity (µmol O2 kg−1 hr) of hypoxic events below different oxygen thresholds (≤153 µmol O2 kg−1, ≤122 µmol O2 kg−1, ≤92 µmol O2 kg−1, or ≤61 µmol O2 kg−1; shades of blue) for different warming scenarios including 4 Shared Socioeconomic Pathways (SSPs) and a heatwave event across global coral reef sites.
Supplementary information
Supplementary Information
Supplementary Methods, Tables 1–9 and References.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pezner, A.K., Courtney, T.A., Barkley, H.C. et al. Increasing hypoxia on global coral reefs under ocean warming. Nat. Clim. Chang. 13, 403–409 (2023). https://doi.org/10.1038/s41558-023-01619-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41558-023-01619-2
This article is cited by
-
Meta-analysis reveals less sensitivity of non-native animals than natives to extreme weather worldwide
Nature Ecology & Evolution (2023)