Abstract
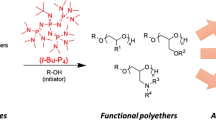
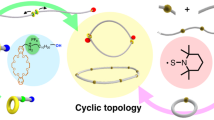
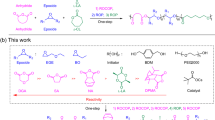
The selective synthesis of ultrahigh-molar-mass (UHMM, >2 million Da) cyclic polymers is challenging as an exceptional degree of spatiotemporal control is required to overcome the possible undesired reactions that can compete with the desired intramolecular cyclization. Here we present a counterintuitive synthetic methodology for cyclic polymers, represented here by polythioesters, which proceeds via superbase-mediated ring-opening polymerization of gem-dimethylated thiopropiolactone, followed by macromolecular cyclization triggered by protic quenching. This proton-triggered linear-to-cyclic topological transformation enables selective, linear polymer-like access to desired cyclic polythioesters, including those with UHMM surpassing 2 MDa. In addition, this method eliminates the need for stringent conditions such as high dilution to prevent or suppress linear polymer contaminants and presents the opposite scenario in which protic-free conditions are required to prevent cyclic polymer formation, which is capitalized to produce cyclic polymers on demand. Furthermore, such UHMM cyclic polythioester exhibits not only much enhanced thermostability and mechanical toughness, but it can also be quantitatively recycled back to monomer under mild conditions due to its gem-disubstitution.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Full experimental details and the data supporting the findings of this study are available within the article and its Supplementary Information. Source data are provided with this paper.
References
Tezuka, Y. Topological Polymer Chemistry: Progress of Cyclic Polymers in Syntheses, Properties, and Functions (Word Scientific, 2012).
Miao, Z. et al. Cyclic polyacetylene. Nat. Chem. 13, 792–799 (2021).
Bielawski, C. W., Benitez, D. & Grubbs, R. H. An ‘endless’ route to cyclic polymers. Science 297, 2041–2044 (2002).
Haque, F. M. & Grayson, S. M. The synthesis, properties and potential applications of cyclic polymers. Nat. Chem. 12, 433–444 (2020).
Laurent, B. A. & Grayson, S. M. An efficient route to well-defined macrocyclic polymers via ‘click’ cyclization. J. Am. Chem. Soc. 128, 4238–4239 (2006).
Sun, P., Chen, J., Liu, J. A., & Zhang, K. Self-accelerating click reaction for cyclic polymer. Macromolecules 50, 1463–1472 (2017).
Kapnistos, M. et al. Unexpected power-law stress relaxation of entangled ring polymers. Nat. Mater. 7, 997–1002 (2008).
Pasquino, R. et al. Viscosity of ring polymer melts. ACS Macro Lett. 2, 874–878 (2013).
Doi, Y. et al. Melt rheology of ring polystyrenes with ultrahigh purity. Macromolecules 48, 3140–3147 (2015).
Gambino, T., Martínez de Ilarduya, A., Alegría, A. & Barroso-Bujans, F. Dielectric relaxations in poly(glycidyl phenyl ether): effects of microstructure and cyclic topology. Macromolecules 49, 1060–1069 (2016).
Ziebarth, J. D. et al. Comparison of critical adsorption points of ring polymers with linear polymers. Macromolecules 49, 8780–8788 (2016).
Kammiyada, H., Ouchi, M. & Sawamoto, M. A study on physical properties of cyclic poly(vinyl ether)s synthesized via ring-expansion cationic polymerization. Macromolecules 50, 841–848 (2017).
Chen, B., Jerger, K., Frechet, J. M. & Szoka, F. C. Jr. The influence of polymer topology on pharmacokinetics: differences between cyclic and linear PEGylated poly(acrylic acid) comb polymers. J. Control. Release 140, 203–209 (2009).
Nasongkla, N. et al. Dependence of pharmacokinetics and biodistribution on polymer architecture: effect of cyclic versus linear polymers. J. Am. Chem. Soc. 131, 3842–3843 (2009).
Yamamoto, T. & Tezuka, Y. Cyclic polymers revealing topology effects upon self-assemblies, dynamics and responses. Soft Matter 11, 7458–7468 (2015).
Arno, M. C. et al. Exploiting topology-directed nanoparticle disassembly for triggered drug delivery. Biomaterials 180, 184–192 (2018).
Natansohn, A. & Rochon, P. Photoinduced motions in azo-containing polymers. Chem. Rev. 102, 4139–4175 (2002).
Xu, X. et al. The first example of main-chain cyclic azobenzene polymers. Macromol. Rapid Commun. 31, 1791–1797 (2010).
Hoskins, J. N. & Grayson, S. M. Synthesis and degradation behavior of cyclic poly(ε-caprolactone). Macromolecules 42, 6406–6413 (2009).
Williams, R. J., Dove, A. P. & O’Reilly, R. K. Self-assembly of cyclic polymers. Polym. Chem. 6, 2998–3008 (2015).
Josse, T., De Winter, J., Gerbaux, P. & Coulembier, O. Cyclic polymers by ring-closure strategies. Angew. Chem. Int. Ed. 55, 13944–13958 (2016).
Chang, Y. A. & Waymouth, R. M. Recent progress on the synthesis of cyclic polymers via ring-expansion strategies. J. Polym. Sci., Part A: Polym. Chem. 55, 2892–2902 (2017).
Wang, T.-W. & Golder, M. R. Advancing macromolecular hoop construction: recent developments in synthetic cyclic polymer chemistry. Polym. Chem. 12, 958–969 (2021).
Yoon, K. Y. et al. Scalable and continuous access to pure cyclic polymers enabled by ‘quarantined’ heterogeneous catalysts. Nat. Chem. 14, 1242–1248 (2022).
Boydston, A. J., Xia, Y., Kornfield, J. A., Gorodetskaya, I. A. & Grubbs, R. H. Cyclic ruthenium-alkylidene catalysts for ring-expansion metathesis polymerization. J. Am. Chem. Soc. 130, 12775–12782 (2008).
Sarkar, S. et al. An OCO3− trianionic pincer tungsten(VI) alkylidyne: rational design of a highly active alkyne polymerization catalyst. J. Am. Chem. Soc. 134, 4509–4512 (2012).
Gonsales, S. A. et al. Highly tactic cyclic polynorbornene: stereoselective ring expansion metathesis polymerization of norbornene catalyzed by a new tethered tungsten-alkylidene catalyst. J. Am. Chem. Soc. 138, 4996–4999 (2016).
Roland, C. D., Li, H., Abboud, K. A., Wagener, K. B. & Veige, A. S. Cyclic polymers from alkynes. Nat. Chem. 8, 791–796 (2016).
Wang, T. W., Huang, P. R., Chow, J. L., Kaminsky, W. & Golder, M. R. A cyclic ruthenium benzylidene initiator platform enhances reactivity for ring-expansion metathesis polymerization. J. Am. Chem. Soc. 143, 7314–7319 (2021).
McGraw, M. L., Clarke, R. W. & Chen, E. Y.-X. Synchronous control of chain length/sequence/topology for precision synthesis of cyclic block copolymers from monomer mixtures. J. Am. Chem. Soc. 143, 3318–3322 (2021).
McGraw, M. L. et al. Mechanism of spatial and temporal control in precision cyclic vinyl polymer synthesis by Lewis pair polymerization. Angew. Chem. Int. Ed. 61, e202116303 (2022).
Song, Y., He, J., Zhang, Y., Gilsdorf, R. A. & Chen, E. Y.-X. Recyclable cyclic bio-based acrylic polymer via pairwise monomer enchainment by a trifunctional Lewis pair. Nat. Chem. 15, 366–376 (2023).
Culkin, D. A. et al. Zwitterionic polymerization of lactide to cyclic poly(lactide) by using N-heterocyclic carbene organocatalysts. Angew. Chem. Int. Ed. 46, 2627–2630 (2007).
Guo, L., Lahasky, S. H., Ghale, K. & Zhang, D. N-heterocyclic carbene-mediated zwitterionic polymerization of N-substituted N-carboxyanhydrides toward poly(alpha-peptoid)s: kinetic, mechanism, and architectural control. J. Am. Chem. Soc. 134, 9163–9171 (2012).
Brown, H. A. & Waymouth, R. M. Zwitterionic ring-opening polymerization for the synthesis of high molecular weight cyclic polymers. Acc. Chem. Res. 46, 2585–2596 (2013).
Piedra-Arroni, E., Ladaviere, C., Amgoune, A. & Bourissou, D. Ring-opening polymerization with Zn(C6F5)2-based Lewis pairs: original and efficient approach to cyclic polyesters. J. Am. Chem. Soc. 135, 13306–13309 (2013).
Reisberg, S. H., Hurley, H. J., Mathers, R. T., Tanski, J. M. & Getzler, Y. D. Y. L. Lactide cyclopolymerization kinetics, X-ray structure, and solution dynamics of (tBu-SalAmEE)Al and a cautionary tale of polymetalate formation. Macromolecules 46, 3273–3279 (2013).
Asenjo-Sanz, I., Veloso, A., Miranda, J. I., Pomposo, J. A. & Barroso-Bujans, F. Zwitterionic polymerization of glycidyl monomers to cyclic polyethers with B(C6F5)3. Polym. Chem. 5, 6905–6908 (2014).
Hong, M. & Chen, E. Y.-X. Completely recyclable biopolymers with linear and cyclic topologies via ring-opening polymerization of gamma-butyrolactone. Nat. Chem. 8, 42–49 (2016).
Zhu, J. B., Watson, E. M., Tang, J. & Chen, E. Y.-X. A synthetic polymer system with repeatable chemical recyclability. Science 360, 398–403 (2018).
Naruse, K., Takasu, A. & Higuchi, M. Direct observation of a cyclic vinyl polymer prepared by anionic polymerization using N-heterocyclic carbene and subsequent ring-closure without highly diluted conditions. Macromol. Chem. Phys. 221, 2000004 (2020).
Li, H., Ollivier, J., Guilaume, S. M. & Carpentier, J.-F. Tacticity control of cyclic poly(3-thiobutyrate) prepared by ring-opening polymerization of racemic β-thiobutyrolactone. Angew. Chem. Int. Ed. 58, 618–623 (2022).
Hong, M. & Chen, E. Y.-X. Chemically recyclable polymers: a circular economy approach to sustainability. Green Chem. 19, 3692–3706 (2017).
Coates, G. W. & Getzler, Y. D. Y. L. Chemical recycling to monomer for an ideal, circular polymer economy. Nat. Rev. Mater. 5, 501–516 (2020).
Jehanno, C. et al. Critical advances and future opportunities in upcycling commodity polymers. Nature 603, 803–814 (2022).
Abel, B. A., Snyder, R. L. & Coates, G. W. Chemically recyclable thermoplastics from reversible-deactivation polymerization of cyclic acetals. Science 373, 783–789 (2021).
Haussler, M., Eck, M., Rothauer, D. & Mecking, S. Closed-loop recycling of polyethylene-like materials. Nature 590, 423–427 (2021).
Jung, M. E. & Piizzi, G. gem-Disubstituent effect: theoretical basis and synthetic applications. Chem. Rev. 105, 1735–1766 (2005).
Zhou, L. et al. Chemically circular, mechanically tough and melt-processable polyhydroxyalkanoates. Science 380, 64–69 (2023).
Xiong, W. et al. Geminal dimethyl substitution enables controlled polymerization of penicillamine-derived β-thiolactones and reversed depolymerization. Chem. 6, 1831–1843 (2020).
Stellmach, K. A. et al. Modulating polymerization thermodynamics of thiolactones through substituent and heteroatom incorporation. ACS Macro Lett. 11, 895–901 (2022).
Li, X. L., Clarke, R. W., Jiang, J. Y., Xu, T. Q. & Chen, E. Y.-X. A circular polyester platform based on simple gem-disubstituted valerolactones. Nat. Chem. 15, 278–285 (2023).
Vazdar, K. et al. Design of novel uncharged organic superbases: merging basicity and functionality. Acc. Chem. Res. 54, 3108–3123 (2021).
Schmitt, M., Faliveve, L., Caporaso, L., Cavallo, L. & Chen, E. Y.-X. High-speed organocatalytic polymerization of a renewable methylene butyrolactone by a phosphazene superbase. Polym. Chem. 5, 3261–3270 (2014).
Sweeny, W. & Casey, D. J. Propiothiolactone polymers. US patent 3,367,921A (1965).
Kumaki, J., Nishikawa, Y. & Hashimoto, T. Visualization of single-chain conformations of a synthetic polymer with atomic force microscopy. J. Am. Chem. Soc. 118, 3321–3322 (1996).
Acknowledgements
Funding was provided by the US Department of Energy, Office of Energy Efficiency and Renewable Energy, Advanced Materials and Manufacturing Technologies Office (AMMTO) and Bioenergy Technologies Office (BETO). This work was performed as part of the Bio-Optimized Technologies to keep Thermoplastics out of Landfills and the Environment (BOTTLE) Consortium and was supported by AMMTO and BETO under Contract DE-AC36-08GO28308 with the National Renewable Energy Laboratory, operated by Alliance for Sustainable Energy, LLC. The BOTTLE Consortium includes members from Colorado State University. The work by L.T.R. and E.Y.-X.C. was supported by the US National Science Foundation (grant no. NSF-2305058). We thank R. R. Gowda for dn/dc measurements.
Author information
Authors and Affiliations
Contributions
E.Y.-X.C. conceived the project and directed the research. L.Z., L.T.R., C.S. and E.C.Q. designed and conducted experiments and analysed the results. L.Z. wrote the initial paper, L.T.R. and E.C.Q. contributed to subsequent versions. E.Y.-X.C. edited the initial and subsequent versions and reviewed the entire paper.
Corresponding author
Ethics declarations
Competing interests
E.Y.-X.C. and L.Z. are named inventors on a pending PCT/US patent application (2023/021512) submitted by Colorado State University, which covers chemically circular polymers. The other authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Matthew Golder, Sophie Guillaume, Julia Pribyl Ham and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Tables 1–4 and Figs. 1–44.
Source data
Source Data Fig. 2a
Statistical source data.
Source Data Fig. 3
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, L., Reilly, L.T., Shi, C. et al. Proton-triggered topological transformation in superbase-mediated selective polymerization enables access to ultrahigh-molar-mass cyclic polymers. Nat. Chem. (2024). https://doi.org/10.1038/s41557-024-01511-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41557-024-01511-2