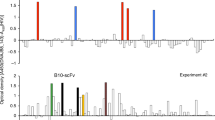
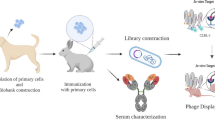
Abstract
The cell-surface glycoprotein CD98—a subunit of the LAT1/CD98 amino acid transporter—is an attractive target for cancer immunotherapies, but its widespread expression has hampered the development of CD98-targeting antibody therapeutics. Here we report that an anti-CD98 antibody, identified via the screening of phage-display libraries of CD98 single-chain variable fragments with mutated complementarity-determining regions, preserves the physiological function of CD98 and elicits broad-spectrum crystallizable-fragment (Fc)-mediated anti-tumour activity (requiring Fcγ receptors for immunoglobulins, macrophages, dendritic cells and CD8+ T cells, as well as other components of the innate and adaptive immune systems) in multiple xenograft and syngeneic tumour models established in CD98-humanized mice. We also show that a variant of the anti-CD98 antibody with pH-dependent binding, generated by solving the structure of the antibody–CD98 complex, displayed enhanced tumour-specific activity and pharmacokinetics. pH-dependent antibody variants targeting widely expressed antigens may lead to superior therapeutic outcomes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The main data supporting the results in this study are available within the paper and its supplementary information. The structure of hCD98 ECD used in this study is available from the Protein Data Bank (PDB) under the accession code 2DH2. The S1-F4/CD98 complex crystal structure generated in this study is available from the PDB under accession code 7DF1. Source data are provided with this paper.
References
Mastroberardino, L. et al. Amino-acid transport by heterodimers of 4F2hc/CD98 and members of a permease family. Nature 395, 288–291 (1998).
Nakamura, E. et al. 4F2 (CD98) heavy chain is associated covalently with an amino acid transporter and controls intracellular trafficking and membrane topology of 4F2 heterodimer. J. Biol. Chem. 274, 3009–3016 (1999).
Rosell, A. et al. Structural bases for the interaction and stabilization of the human amino acid transporter LAT2 with its ancillary protein 4F2hc. Proc. Natl Acad. Sci. USA 111, 2966–2971 (2014).
Yan, R., Zhao, X., Lei, J. & Zhou, Q. Structure of the human LAT1–4F2hc heteromeric amino acid transporter complex. Nature 568, 126–130 (2019).
Nicklin, P. et al. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell 136, 521–534 (2009).
Cormerais, Y. et al. Genetic disruption of the multifunctional CD98/LAT1 complex demonstrates the key role of essential amino acid transport in the control of mTORC1 and tumor growth. Cancer Res. 76, 4481–4492 (2016).
Timmerman, L. A. et al. Glutamine sensitivity analysis identifies the xCT antiporter as a common triple-negative breast tumor therapeutic target. Cancer Cell 24, 450–465 (2013).
Harris, I. S. et al. Glutathione and thioredoxin antioxidant pathways synergize to drive cancer initiation and progression. Cancer Cell 27, 211–222 (2015).
Bajaj, J. et al. CD98-mediated adhesive signaling enables the establishment and propagation of acute myelogenous leukemia. Cancer Cell 30, 792–805 (2016).
Canup, B. S. B., Song, H. & Laroui, H. Role of CD98 in liver disease. Ann. Hepatol. 19, 602–607 (2020).
Kaira, K. et al. CD98 expression is associated with poor prognosis in resected non-small-cell lung cancer with lymph node metastases. Ann. Surg. Oncol. 16, 3473–3481 (2009).
Furuya, M., Horiguchi, J., Nakajima, H., Kanai, Y. & Oyama, T. Correlation of L-type amino acid transporter 1 and CD98 expression with triple negative breast cancer prognosis. Cancer Sci. 103, 382–389 (2012).
Toyoda, M. et al. Prognostic significance of amino-acid transporter expression (LAT1, ASCT2, and xCT) in surgically resected tongue cancer. Br. J. Cancer 110, 2506–2513 (2014).
Toyoda, M. et al. CD98 as a novel prognostic indicator for patients with stage III/IV hypopharyngeal squamous cell carcinoma. Head Neck 37, 1569–1574 (2015).
Theodosakis, N. et al. Integrative discovery of CD98 as a melanoma biomarker. Pigm. Cell Melanoma Res. 29, 385–387 (2016).
Kaira, K. et al. Prognostic significance of L-type amino-acid transporter 1 expression in surgically resected pancreatic cancer. Br. J. Cancer 107, 632–638 (2012).
Xiao, B. et al. Silencing of intestinal glycoprotein CD98 by orally targeted nanoparticles enhances chemosensitization of colon cancer. ACS Nano 12, 5253–5265 (2018).
Arndt, C. et al. UniCAR T cell immunotherapy enables efficient elimination of radioresistant cancer cells. Oncoimmunology 9, 1743036 (2020).
Hayes, G. M. et al. Antitumor activity of an anti-CD98 antibody. Int. J. Cancer 137, 710–720 (2015).
Rossier, G. et al. LAT2, a new basolateral 4F2hc/CD98-associated amino acid transporter of kidney and intestine. J. Biol. Chem. 274, 34948–34954 (1999).
Fagerberg, L. et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell. Proteom. 13, 397–406 (2014).
Cantor, J. et al. CD98hc facilitates B cell proliferation and adaptive humoral immunity. Nat. Immunol. 10, 412–419 (2009).
Tsumura, H. et al. The targeted disruption of the CD98 gene results in embryonic lethality. Biochem. Biophys. Res. Commun. 308, 847–851 (2003).
Li, D. et al. A potent human neutralizing antibody Fc-dependently reduces established HBV infections. eLife 6, e26738 (2017).
Chao, M. P. et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell 142, 699–713 (2010).
Grillo-Lopez, A. J. et al. Rituximab: the first monoclonal antibody approved for the treatment of lymphoma. Curr. Pharm. Biotechnol. 1, 1–9 (2000).
Scott, A. M., Wolchok, J. D. & Old, L. J. Antibody therapy of cancer. Nat. Rev. Cancer 12, 278–287 (2012).
Wilson, N. S. et al. An Fcgamma receptor-dependent mechanism drives antibody-mediated target-receptor signaling in cancer cells. Cancer cell 19, 101–113 (2011).
Shields, R. L. et al. High resolution mapping of the binding site on human IgG1 for Fc gamma RI, Fc gamma RII, Fc gamma RIII, and FcRn and design of IgG1 variants with improved binding to the Fc gamma R. J. Biol. Chem. 276, 6591–6604 (2001).
Idusogie, E. E. et al. Mapping of the C1q binding site on rituxan, a chimeric antibody with a human IgG1 Fc. J. Immunol. 164, 4178–4184 (2000).
Treffers, L. W. et al. FcgammaRIIIb restricts antibody-dependent destruction of cancer cells by human neutrophils. Front. Immunol. 9, 3124 (2018).
Nimmerjahn, F., Gordan, S. & Lux, A. FcgammaR dependent mechanisms of cytotoxic, agonistic, and neutralizing antibody activities. Trends Immunol. 36, 325–336 (2015).
Seidel, U. J., Schlegel, P. & Lang, P. Natural killer cell mediated antibody-dependent cellular cytotoxicity in tumor immunotherapy with therapeutic antibodies. Front. Immunol. 4, 76 (2013).
Kamber, R. A. et al. Inter-cellular CRISPR screens reveal regulators of cancer cell phagocytosis. Nature 597, 549–554 (2021).
Yang, X. et al. Cetuximab-mediated tumor regression depends on innate and adaptive immune responses. Mol. Ther. 21, 91–100 (2013).
Liu, X. et al. CD47 blockade triggers T cell-mediated destruction of immunogenic tumors. Nat. Med. 21, 1209–1215 (2015).
Vander Heiden, M. G., Cantley, L. C. & Thompson, C. B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324, 1029–1033 (2009).
Warburg, O. On the origin of cancer cells. Science 123, 309–314 (1956).
Corbet, C. & Feron, O. Tumour acidosis: from the passenger to the driver’s seat. Nat. Rev. Cancer 17, 577–593 (2017).
Gatenby, R. A. & Gillies, R. J. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer 4, 891–899 (2004).
Robey, I. F. et al. Bicarbonate increases tumor pH and inhibits spontaneous metastases. Cancer Res. 69, 2260–2268 (2009).
Helmlinger, G., Yuan, F., Dellian, M. & Jain, R. K. Interstitial pH and pO2 gradients in solid tumors in vivo: high-resolution measurements reveal a lack of correlation. Nat. Med. 3, 177–182 (1997).
Sarkar, C. A. et al. Rational cytokine design for increased lifetime and enhanced potency using pH-activated ‘histidine switching’. Nat. Biotechnol. 20, 908–913 (2002).
Johnston, R. J. et al. VISTA is an acidic pH-selective ligand for PSGL-1. Nature 574, 565–570 (2019).
Chaparro-Riggers, J. et al. Increasing serum half-life and extending cholesterol lowering in vivo by engineering antibody with pH-sensitive binding to PCSK9. J. Biol. Chem. 287, 11090–11097 (2012).
Ramanathan, S. & Jagannathan, N. Tumor associated macrophage: a review on the phenotypes, traits and functions. Iran. J. Cancer Prev. 7, 1–8 (2014).
Feral, C. C. et al. CD98hc (SLC3A2) mediates integrin signaling. Proc. Natl Acad. Sci. USA 102, 355–360 (2005).
Lee, Y. et al. Cryo-EM structure of the human L-type amino acid transporter 1 in complex with glycoprotein CD98hc. Nat. Struct. Mol. Biol. 26, 510–517 (2019).
Igawa, T. et al. Antibody recycling by engineered pH-dependent antigen binding improves the duration of antigen neutralization. Nat. Biotechnol. 28, 1203–1207 (2010).
Kang, J. C. et al. Engineering a HER2-specific antibody–drug conjugate to increase lysosomal delivery and therapeutic efficacy. Nat. Biotechnol. 37, 523–526 (2019).
Sulea, T. et al. Structure-based engineering of pH-dependent antibody binding for selective targeting of solid-tumor microenvironment. MAbs 12, 1682866 (2020).
Zuchero, Y. J. et al. Discovery of novel blood–brain barrier targets to enhance brain uptake of therapeutic antibodies. Neuron 89, 70–82 (2016).
Ishiguro, T. et al. Anti-glypican 3 antibody as a potential antitumor agent for human liver cancer. Cancer Res. 68, 9832–9838 (2008).
Wang, Q. S. et al. The macromolecular crystallography beamline of SSRF. Nucl. Sci. Tech. 26, 12–17 (2015).
Fort, J. et al. The structure of human 4F2hc ectodomain provides a model for homodimerization and electrostatic interaction with plasma membrane. J. Biol. Chem. 282, 31444–31452 (2007).
Acknowledgements
We thank the NIBS Animal Facility for their help in the handling and care of mice, the NIBS Biological Resource Centre for DNA sequencing, and the NIBS imaging facility for assistance with the microscope experiment. This work was supported by grants from the Ministry of Science and Technology of the People’s Republic of China (973 Program #2012CB837600 to J.S.), the Beijing Municipal Science and Technology Commission, and the Beijing Key Laboratory of Pathogen Invasion and Immune Defense (Z171100002217064 to J.S.). The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
X.T., X.L. and J.S. conceptualized this study, interpreted the results and drafted the manuscript. X.T., X.L., K.W., J.L., Z.W., X.H., Y.L., X.W. and H.Z. performed experiments. X.L. and J.D. analysed the crystal structure. F.W. constructed the CD98-humanized mice. X.T. prepared figures. J.S. supervised the study. All authors commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
J.S. and X.T. are co-inventors of patent applications for the antibodies reported in this study. The other authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks Kanyi Pu, John C. Zwaagstra and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–10 and Tables 1–6.
Supplementary dataset 1
Unmodified images for the gels shown in Supplementary Fig. 3b–d.
Supplementary dataset 2
Source data for the supplementary figures.
Source data
Source Data for Fig. 1
Source data.
Source Data for Fig. 2
Source data.
Source Data for Fig. 3
Source data.
Source Data for Fig. 4
Source data.
Source Data for Fig. 5
Source data.
Source Data for Fig. 6
Source data.
Source Data for Fig. 7
Source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tian, X., Liu, X., Ding, J. et al. An anti-CD98 antibody displaying pH-dependent Fc-mediated tumour-specific activity against multiple cancers in CD98-humanized mice. Nat. Biomed. Eng 7, 8–23 (2023). https://doi.org/10.1038/s41551-022-00956-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-022-00956-5