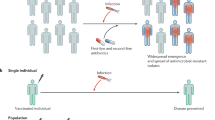
Abstract
Vaccine R&D is typically a lengthy process taking >10 years. However, vaccines still fail in clinical development because of unreliable animal models or absent immunological correlates of protection. Without a correlate of protection, phase-1 and -2 studies of safety and immunogenicity can fail to predict phase-3 efficacy. Indeed, the history of vaccine development is replete with promising phase-1 and -2 results and failed phase-3 efficacy trials. To avoid this misfortune, we present Reverse Vaccine Development for vaccines against antimicrobial-resistant (AMR) pathogens. In this approach, instead of evaluating efficacy in phase 3, proof-of-principle efficacy is evaluated as early as possible in a population with a high incidence of disease, which may differ from the population intended for registration, and can be a controlled human infection population. To identify a correlate of protection in these populations, the vaccine-elicited immune response is compared between protected and unprotected subjects. If a correlate is identified, it can help to refine the vaccine dosage, schedule, and formulation, and facilitate the assessment of vaccine efficacy in other populations with different attack rates, subject characteristics, and disease manifestations. This may be the only way to provide life-saving vaccines to populations affected by AMR-pathogen diseases at incidences that are typically low and unsuited to phase-3 efficacy trials. The availability of a correlate of protection early in clinical development can potentially prevent failures of large phase-3 trials and unnecessary exposures of populations to inefficacious vaccines that have resulted in disinvestment in the development of vaccines against AMR pathogens.
Similar content being viewed by others
Introduction
A recent estimation of the impact of antimicrobial resistance (AMR) on global human health has indicated a medical need comparable and likely larger than HIV and malaria. Unfortunately, developing vaccines against AMR pathogens is being hindered by a lack of understanding of their correlates of protection. Reverse Vaccine Development, which we are pioneering along with the development of a vaccine against Staphylococcus aureus, is proposed as an approach for easing and accelerating the clinical development of vaccines against AMR pathogens.
Vaccine development can be long, difficult, and costly. However, it becomes easier when a correlate of protection is known. For example, the speed of SARS-CoV-2 vaccine development was not only due to the extraordinary effort of companies and health authorities, but also to knowledge of the likely mechanism of protection: antibody to the spike protein preventing interaction with the host cell. Hence, neutralizing antibody titers became the common thread linking preclinical and clinical development that guided the selection of vaccine candidates eliciting high neutralizing antibody titers in mice; and subsequently, high neutralizing titers were shown to correlate with clinical efficacy. Recently, SARS-CoV-2 vaccines have been found to induce memory CD8+ T-cell responses in the first week of breakthrough infection that correlate with control of infection1.
Unfortunately, for many human pathogens, including those on the World Health Organization’s (WHO) list of global priority pathogens of antibiotic-resistant bacteria, the mechanism of protection remains unknown, and candidate vaccines against these pathogens have failed (e.g., S. aureus and Pseudomonas aeruginosa). Nonetheless, vaccines against AMR pathogens continue to be developed, supported by genomics, proteomics, and immunomics to identify antigens. However, without a correlate of protection, late-stage clinical development is risky: many thousands of research participants may be needed for efficacy evaluations, and after many years of R&D and large expense, the trials may fail.
During the development of a vaccine candidate against S. aureus, we realized the need for a new vaccine development paradigm wherein data generation on efficacy and the immune response, for identifying a correlate of protection, should occur early, instead of in phase 3, reversing the typical order and why we named the approach Reverse Vaccine Development. This approach relies on the availability of a population with a high rate of disease, which may differ from the population intended for registration.
The demonstration of efficacy (proof of concept) in the high attack-rate population enables a comparison of the protected and unprotected subjects for their immune response to the vaccine—to identify a correlate of protection. This approach is especially important when animal models are unreliable for determining correlates of protection. Knowledge of the correlate of protection in early clinical trial phases may prevent failures of large phase-3 trials, unnecessary exposures of populations to inefficacious vaccines, and disinvestment in the development of vaccines against AMR pathogens. Reverse Vaccine Development has the potential to transform the R&D of vaccine candidates against AMR pathogens that lack an animal-model-derived correlate of protection.
Standard clinical trial design
Standard clinical study designs for vaccines start with a phase-1 assessment of safety and immunogenicity in ~50-300 healthy volunteers. In phase 2, these same endpoints are evaluated in a few hundred subjects in the target population intended for registration, and early evaluation of efficacy endpoints may also be included. Finally, phase-3 trials assess efficacy and further assess safety and the immune response in the targeted population, typically in thousands to tens of thousands of subjects.
Phase 3 trials can be very large, and without a correlate of protection they are at higher risk for failure. For example, when the Streptococcus pneumoniae heptavalent vaccine was being developed, the surrogate of protection, opsonophagocytosis, had not yet been established. Additionally, the incidence of streptococcal pneumonia and invasive pneumococcal disease was relatively low in the target population. Therefore, a very large phase-3 efficacy study of >84,000 subjects was needed. Large phase-3 trials for preventive vaccines are unfortunately a common problem (Table 1).
Reverse Vaccine Development
Reverse Vaccine Development starts with a phase 1/2 study that assesses safety, immunogenicity, and efficacy. If the vaccine is First-Time-in-Human (FTiH), a phase-1 safety lead-in study may be needed that escalates dose, without adjuvant and then with adjuvant. If no safety issues emerge, the phase-2 study can proceed to evaluate immunogenicity and efficacy (Fig. 1).
Importantly, the population used for the efficacy assessment needs to have a high attack rate. But the attack rate may not be high in the population intended for registration. Therefore, Reverse Vaccine Development requires a population with a high attack rate that is available for study.
For our S. aureus vaccine candidate, such a population is available: patients with a community acquired skin and soft tissue infection (CA-SSTI) whose risk for recurrence is high. Such populations are also available for other infectious diseases.
An efficacy evaluation of a candidate vaccine early in clinical development is of key importance. If the vaccine is found not efficacious, unnecessary exposure of subjects to the vaccine is avoided. If the vaccine is efficacious, correlates of protection can be explored.
How correlates of protection are studied depends on the study outcome. If the vaccine was partially efficacious, the immune response to the vaccine can be analyzed for differences between vaccinees who became infected and those who were protected to identify an immunological signature of the protective vaccine response.
If an immune correlate of protection is identified, it can help to refine the dosage, schedule, and formulation of the vaccine. A correlate of protection can also provide a benchmark for the potential of other populations to be protected by the vaccine, including the phase-3 study population. This can be of critical importance and the only way to provide lifesaving vaccines to populations where the rates of devastating infectious diseases are usually low and not suited to phase-3 efficacy trials.
Standard clinical trial designs for S. aureus vaccines failed to demonstrate efficacy
S. aureus is a leading cause of community acquired skin infections, surgical site infections, bacteremia, and pneumonia. These infections are associated with a massive burden of morbidity, mortality, hospital length of stay, and patient cost. Additionally, S. aureus rapidly acquires antibiotic resistance, which seriously threatens human health. Current antibiotics and care bundles leave a tremendous unmet medical need worldwide. Further, there are no licensed vaccines on the market, despite the significant efforts of public and private initiatives.
Four vaccine candidates against S. aureus have been evaluated in clinical efficacy trials: (1) StaphVAX, a conjugate vaccine developed by Nabi Biopharmaceuticals, targeting capsular polysaccharides type 5 (CP5) and 8 (CP8)2; (2) V710, a vaccine targeting the iron-scavenging protein IsdB, developed by Merck3; (3) the Pfizer-developed, four-component vaccine candidate, SA4ag (containing CP5, CP8, ClfA, and MntC)4; and (4) NDV-3A, a vaccine targeting the Als-3 adhesin/invasion protein from Candida albicans that has structural similarity to the S. aureus protein clumping factor A (developed by Novadigm)5. These candidates were advanced to clinical development based on in-vitro assays and mouse models, despite their unknown translatability to humans. Their clinical development used a standard design, assessing safety and immunogenicity in healthy subjects in phase 1 and 2, followed by the assessment of efficacy in the target population in phase-3 trials. The phase-1 and -2 trials raised no safety concerns, and the elicited antibody was significantly greater than control and included functional antibodies. Despite this promise, the phase-3 efficacy trials failed. The high economic losses and the exposure of many subjects to the inefficacious vaccines resulted in disappointment and disinvestment.
Reverse Vaccine Development for evaluating our S. aureus vaccine candidate
To avoid an extensive clinical trial that evaluates immunogenicity that may not predict efficacy, we are engaged in the early evaluation of the efficacy of our vaccine candidate in individuals who were recently cured of S. aureus community acquired skin and soft tissue infection (CA-SSTI) and are at high risk for recurrence — using prevention of recurrence as the efficacy endpoint (Fig. 2). Because this population’s attack rate is high, the efficacy evaluation requires relatively few subjects. Additionally, CA-SSTI rarely has a severe outcome. Further, CA-SSTI is the most common disease associated with S. aureus in communities and hospitals. These characteristics make CA-SSTI patients a good population for a proof-of-principle efficacy evaluation.
First, to characterize the CA-SSTI population, we confirmed the high CA-SSTI recurrence rate by a retrospective chart review of cases in patients aged ≥18 years at three US medical centers between 2006 and 20166. We identified index cases (i.e., first occurrence cases) in one calendar year that we assessed for any recurrent infection in the following 12 months. Most cases were without key comorbidities. Across the centers, 16.4%–19.0% of the index cases recurred one or more times.
Based on these data, we designed a clinical trial defined as phase 1/2, observer-blinded, randomized, and placebo-controlled—to assess the safety, immunogenicity, and efficacy of GSK’s S. aureus vaccine candidate (NCT04420221, clinicaltrials.gov). This paradigm study in Reverse Vaccine Development comprises two epochs, (1) a lead-in safety study in which the vaccine is administered to healthy adults, and (2) an immunogenicity and efficacy study in adults with a recent history of CA-SSTI. The safety lead-in study uses staggered enrollment of four groups, eight healthy subjects per group, consecutively enrolled if no safety issues are identified in the prior group. In the second epoch, the index cases of CA-SSTI are enrolled. The first 40 CA-SSTI subjects are evaluated for safety. The absence of safety issues in this group triggers enrollment to accrue a total of ~300 subjects who receive the vaccine and ~300 who receive placebo, for assessing efficacy of the vaccine in reducing CA-SSTI recurrences vs. placebo (with 0% as the Lower Limit of the 85% Confidence Interval). In addition, subjects are assessed for total IgG titers, functional titers, and T-cell responses—for exploring a correlate of protection.
Exploring correlates of protection: systems serology, data science/machine learning
The success of Reverse Vaccine Development depends on the number and validity of the immunological readouts. Antibody titers alone are likely insufficient. Although antibody functionality integrates several immune properties, it may poorly represent comprehensive vaccine immunity. Multiple immunological parameters are needed.
Systems serology offers unbiased and comprehensive data for identifying previously unappreciated processes and mechanisms. For example, immunogenicity and efficacy data from a phase-3 trial of the HIV vaccine RV144 suggest that non-neutralizing Fc functional antibodies can play a protective role against the virus7. For conducting such an analysis, multiple serological parameters are required (i.e., antibody titers, affinity, isotype, and functionality).
Cellular responses should be evaluated to understand the flavor of the induced T-cell response or if the vaccine increases the frequency of T cells (CD4 and CD8) specific for the vaccine antigens. For example, a Th-1 skewed response is assumed to contribute to the activation of phagocytes (e.g., through Inf-γ) and promote pathogen clearance, and Th-17 responses can be important for facilitating phagocytosis8.
Transcriptional profiling provides a complementary and broad view of the immune response to a vaccine. In addition to profiling the T-cell response, transcriptomics can identify signatures of innate immunity, such as those emerging in response to some adjuvants9,10.
The assessment of multiple immunological signals that correlate with each other in mediating protection can increase the chance of identifying a signature of protection. Such patterns and associations between different readouts can be identified using machine learning, decreasing the possibility that the signature of protection is due to random chance, which is particularly important when the number of readouts is large relative to the number of protected and unprotected subjects (Fig. 3).
Memory responses should be assessed. While high neutralizing antibody titers or other measures of humoral immunity may correlate with protection at early times post vaccination, these signatures may wane and not inform longer term protection against disease and severe disease. Therefore, it is important to assess memory responses to breakthrough infections. For example, in the case of COVID-19 mRNA vaccination, the predominant systemic adaptive immune effectors available to limit viral replication during the first week of SARS-CoV-2 breakthrough infection were pre-existing antibodies and rapidly activated memory T cells11. Furthermore, in a separate study, activation of spike-specific T cells during the first week of breakthrough infection correlated with lower peak viral load and faster viral clearance1.
Assessment of background immunity is crucial as the efficacy of a vaccine and its correlate of protection may differ between an endemic population with high background immunity vs. a non-endemic population.
Reverse Vaccine Development for pathogens lacking a correlate of protection
Reverse Vaccine Development is most suited to the development of vaccines against pathogens for which a correlate of protection is not known, such as those on the World Health Organization’s (WHO) list of global priority pathogens of antibiotic-resistant bacteria. An absence of a correlate of protection has hindered preclinical and/or clinical development of most of the CDC and WHO bacterial priority pathogens (e.g., S. aureus, P. aeruginosa, Clostridium difficile, Escherichia coli, Neisseria gonorrhoeae, Streptococcus pyogenes, Klebsiella pneumoniae, Acinetobacter baumannii, Enterococcus spp., Helicobacter pylori, Candida spp., and Campylobacter jejuni). For all these pathogens, the absence of a correlate of protection limits the meaningfulness of preclinical studies and risks clinical failure. Examples of vaccines that lacked a correlate of protection and have failed to show efficacy in clinical trials are shown in Table 2.
Requirements for using Reverse Vaccine Development
The defining concept of Reverse Vaccine Development is the evaluation of efficacy early in clinical development with the goal of identifying a proof-of-concept correlate of protection. The exploration of the correlate must be based on efficacy and immunogenicity endpoints selected to have robust readouts. Further, when the strain variability of the pathogen is significant and the vaccine targets only some strains (e.g., targeting variable polysaccharide capsule or non-conserved protein antigens), microbiology endpoints are needed. Finally, for the study to be feasible, a relatively small, high attack-rate population must be available for study, which need not be the population intended for licensure. Importantly, a correlate of protection identified in this population (training set) needs to be confirmed in a holdout set or second matched population (test set).
While the study population can be a natural high attack-rate population, e.g., the patients suffering from S. aureus SSTI discussed above, a controlled human infection model (CHIM; aka, human challenge studies)12 can also be used as the study population. As compared to a naturally infected population, a CHIM has advantages and disadvantages. The advantages include the ability to control the population being infected regarding their naturally acquired immunity, co-infections, genetics, microbiome, nutrition, and environment, as well as the ability to control the timing, route, and/or dose of the infection and the infecting microorganism so that no disease is caused or the disease is self-limiting or can be controlled with cures or treatments13. The knowledge of the time of the infection allows a detailed characterization of the post-infection time course of the host immune response. The ability to control the pathogen dosage enables the study of pathogen dosage effects on the clinical and immune responses14. Importantly, the ability to collect pre-exposure samples provides a background to understand the correlate of protection, and whether it may differ between symptomatic primary infection and symptomatic re-infection. Because of these benefits, there has been an increase in calls for CHIMs in areas where study volunteers may have had prior exposure to the pathogen being studied and other pathogens15,16,17. Additionally, as compared to trials of vaccines in high attack-rate populations, CHIMs can be smaller, shorter, less expensive, and expose fewer participants to experimental vaccines13. Furthermore, CHIMs can be valuable for identifying lead vaccine candidates to test in larger studies, and thereby accelerate vaccine development to realize public health benefit sooner, strengthening the ethical rationale against the risk of harming participants18.
However, CHIMs have disadvantages, as previously described by Abo et al. 12 and summarized here: (1) The challenge strain may poorly represent naturally circulating pathogen strains, so the efficacy and correlate of protection of the vaccine may not extrapolate to field settings; (2) For safety reasons, challenge models use disease of low severity that might not apply to disease of greater severity in natural populations; (3) A high pathogen inoculum to achieve a high attack rate can overwhelm a vaccine’s protection, and low attack rates in placebo arms have affected efficacy rate determinations; and, (4) As compared to the target population, CHIM participants can differ in comorbidities, immunity, age, immunogenetics, and microbiome, which can influence a vaccine’s efficacy and correlate of protection. For a correlate of protection identified in a CHIM to be used to guide vaccine development, it needs to be validated in a field trial, typically a phase 2b study19. CHIMs that overcome these potential difficulties with participants, pathogens, and diseases, as with studies of natural high attack-rate subpopulations, offer the potential to de-risk and accelerate late-stage vaccine development.
Bridging from the high attack-rate population to other populations
A vaccine that prevents a disease in one population generally requires a phase-3 efficacy trial to demonstrate efficacy in another population. Such a requirement for multiple phase-3 trials burdens clinical development programs to the point of infeasibility in some cases, especially when a low incidence of infection demands trials of thousands of subjects. However, a Reverse Vaccine Development study design can potentially identify a correlate of protection that can be used as an immunological bridge to assess a vaccine’s potential efficacy in other populations. When populations differ mainly in the characteristics of patients (e.g., age, health status, ethnicity, gender) or the cause of the infection (e.g., community acquired pneumonia vs. ventilator-associated pneumonia), but the disease manifestation is largely the same, the validation of the correlate of protection in subsequent populations may eliminate the need for large efficacy trials (Fig. 4).
However, some pathogens can cause quite different diseases. For example, S. aureus can cause CA-SSTI, Surgical Site Infection (SSI), Bloodstream Infection (BSI), and pneumonia — and a vaccine’s efficacy against these diseases may vary. Similarly, P. aeruginosa, E. coli, K. pneumoniae, Enterococcus spp., S. pyogenes, A. baumannii, and Candida spp. can each cause different diseases.
In such instances, a Reverse Vaccine Development study design that identifies a correlate of protection can still provide value. For example, the correlate of protection could enable a reduced sample size. The correlate can be assessed in a small number of patients with the different disease manifestation. If the immune correlate is seen in the infected patients, the vaccine may not protect against the different manifestation, and the efficacy assessment may require a larger enrollment. Alternatively, if the infected participants show no correlate, but most vaccinees show the correlate and remain uninfected, then the infected participants likely had a suboptimal immune response, and dose escalation may be needed.
The development of a vaccine without a correlate of protection leaves only arbitrary immunological endpoints on which to base the selection of vaccine dosage, schedule, and adjuvant. Conversely, a known immune correlate can be used early in clinical development to optimize these elements that may be crucial for improving vaccine efficacy.
Limitations
Reverse Vaccine Development requires the availability of a suitable surrogate population that may be a naturally occurring, high attack-rate population or a human challenge study, which is possible for some pathogens.
An early efficacy indication in a surrogate population reduces risk for phase-3 study failure. However, the identification of a correlate of protection remains challenging despite the increasing numbers of immune parameters and machine learning algorithms to assess signatures of protection. Should an immune signature be identified by cross-validation in a relatively small, high attack-rate population with a defined immune background, and it is validated in a holdout or matched test population, its relevance to the population intended for marketing approval remains a risk.
A naturally infected population used for Reverse Vaccine Development comprises hundreds of study participants with each participant providing several longitudinal samples for assessing multiple immune parameters, which adds cost exceeding that of a conventional phase 1/2 study. However, we estimate that the risks and cost are worth the potential benefits of having a proof-of-concept correlate of protection to guide the selection of vaccine dosage, schedule, and adjuvant, and to provide an immunological bridge to assess vaccine efficacy in other populations, such as those where a large phase 3 clinical trial may not be feasible.
Conclusion
Reverse Vaccine Development assesses vaccine efficacy in the early phases of clinical development in a high attack-rate population and has the potential to identify a correlate of protection. Alternatively, depending on the pathogen, a CHIM may serve as the high attack-rate population. Early identification of proof-of-concept efficacy lowers the risk of failure in large phase-3 trials. Additionally, a proof-of-concept efficacy demonstration offers the opportunity to explore immune correlates of protection in a relatively small-sized trial, using machine learning to integrate multiple immune readouts. If an immune correlate is confirmed, it can guide the vaccine dosage, schedule, and adjuvant to improve the immune response and efficacy. Additionally, an immune correlate identified early in clinical development in a high attack-rate population can be used to corroborate efficacy in other populations that differ in characteristics such as disease incidence, age, health status, ethnicity, or disease manifestation (e.g., CA-SSTI or SSI). Reverse Vaccine Development has the potential to facilitate the development of vaccines against AMR pathogens.
References
Koutsakos, M. et al. SARS-CoV-2 breakthrough infection induces rapid memory and de novo T cell responses. Immunity 56, 879–892.e874 (2023).
Fattom, A. et al. Efficacy profile of a bivalent staphylococcus aureus glycoconjugated vaccine in adults on hemodialysis: phase III randomized study. Hum. Vacc. Immunother. 11, 632–641 (2015).
Fowler, V. G. et al. Effect of an investigational vaccine for preventing staphylococcus aureus infections after cardiothoracic surgery: a randomized trial. J. Am. Med. Assoc. 309, 1368–1378 (2013).
Hassanzadeh, H. et al. Efficacy of a 4-antigen staphylococcus aureus vaccine in spinal surgery: the STaphylococcus aureus suRgical Inpatient Vaccine Efficacy (STRIVE) randomized clinical trial. Clin. Infect. Dis. 77, 312–320 (2023).
Millar, E. V. et al. Safety, immunogenicity, and efficacy of NDV-3A against staphylococcus aureus colonization: a phase 2 vaccine trial among US army infantry trainees. Vaccine 39, 3179–3188 (2021).
Vella, V. et al. Staphylococcus aureus skin and soft tissue infection recurrence rtes in outpatients: a retrospective database study at 3 US medical centers. Clin. Infect. Dis. 73, e1045–e1053 (2021).
Chung, A. W. et al. Dissecting polyclonal vaccine-induced humoral immunity against HIV using systems serology. Cell 163, 988–998 (2015).
Bagnoli, F., Bertholet, S. & Grandi, G. Inferring reasons for the failure of Staphylococcus aureus vaccines in clinical trials. Front. Cell Infect. Microbiol. 2, 16 (2012).
Coccia, M. et al. Subsequent AS01-adjuvanted vaccinations induce similar transcriptional responses in populations with different disease statuses. PLoS One 17, e0276505 (2022).
Didierlaurent, A. M. et al. Enhancement of adaptive immunity by the human vaccine adjuvant AS01 depends on activated dendritic cells. J. Immunol. 193, 1920–1930 (2014).
Painter, M. M. et al. Prior vaccination promotes early activation of memory T cells and enhances immune responses during SARS-CoV-2 breakthrough infection. Nat. Immunol. 24, 1711–1724 (2023).
Abo, Y. N. et al. Strategic and scientific contributions of human challenge trials for vaccine development: facts versus fantasy. Lancet Infect. Dis. 23, e533–e546 (2023).
Jamrozik, E. & Selgelid, M. J. Human Challenge Studies in Endemic Settings : Ethical and Regulatory Issues 1–7 (Springer International Publishing, 2021).
Morrison, H., Jackson, S. & McShane, H. Controlled human infection models in COVID-19 and tuberculosis: current progress and future challenges. Front. Immunol. 14, 1211388 (2023).
Baay, M. F. et al. Human challenge trials in vaccine development, Rockville, MD, USA, September 28–30, 2017. Biologicals 61, 85–94 (2019).
Elliott, A. M. et al. Ethical and scientific considerations on the establishment of a controlled human infection model for schistosomiasis in Uganda: report of a stakeholders’ meeting held in Entebbe, Uganda. AAS open Res. 1, 2 (2018).
Gordon, S. B. et al. A framework for controlled human infection model (CHIM) studies in Malawi: report of a wellcome trust workshop on CHIM in low income countries held in Blantyre, Malawi. Wellcome open Res. 2, 70 (2017).
Sekhar, A. & Kang, G. Human challenge trials in vaccine development. Semin Immunol. 50, 101429 (2020).
Giersing, B. K. et al. How can controlled human infection models accelerate clinical development and policy pathways for vaccines against Shigella? Vaccine 37, 4778–4783 (2019).
Bonten, M. J. et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N. Engl. J. Med. 372, 1114–1125 (2015).
Gurtman, A. et al. The development of a staphylococcus aureus four antigen vaccine for use prior to elective orthopedic surgery. Hum. Vacc. Immunother. 15, 358–370 (2019).
Akapirat, S. et al. Characterization of HIV-1 gp120 antibody specificities induced in anogenital secretions of RV144 vaccine recipients after late boost immunizations. PLoS One 13, e0196397 (2018).
Adlbrecht, C. et al. Efficacy, immunogenicity, and safety of IC43 recombinant pseudomonas aeruginosa vaccine in mechanically ventilated intensive care patients—a randomized clinical trial. Crit. Care 24, 74 (2020).
Failure of type specific streptococcus pyogenes vaccine to prevent respiratory infections. U. S. Nav. Med. Bull. 46, 709–718 (1946).
Acknowledgements
GlaxoSmithKline Biologicals funded this work. Peter D’Arpa contributed scientific writing.
Author information
Authors and Affiliations
Contributions
F.B. conceptualized the Reverse Development approach, and contributed to writing and reviewing. I.G., V.K.V. and S.P.: contributed to writing and reviewing. All authors approved the completed version of the manuscript and are accountable for the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare the following competing interests: all authors are employees of the GSK group of companies and hold shares in the GSK group of companies. F.B. is inventor of S. aureus vaccines patents.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bagnoli, F., Galgani, I., Vadivelu, V.K. et al. Reverse development of vaccines against antimicrobial-resistant pathogens. npj Vaccines 9, 71 (2024). https://doi.org/10.1038/s41541-024-00858-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41541-024-00858-4