Abstract
Patients with lung cancer (LC) often experience delay between symptom onset and treatment. Primary healthcare professionals (HCPs) can help facilitate early diagnosis of LC through recognising early signs and symptoms and making appropriate referrals. This systematic review describes the effect of interventions aimed at helping HCPs recognise and refer individuals with symptoms suggestive of LC. Seven studies were synthesised narratively. Outcomes were categorised into: Diagnostic intervals; referral and diagnosis patterns; stage distribution at diagnosis; and time interval from diagnosis to treatment. Rapid access pathways and continuing medical education for general practitioners can help reduce LC diagnostic and treatment delay. Awareness campaigns and HCP education can help inform primary HCPs about referral pathways. However, campaigns did not significantly impact LC referral rates or reduce diagnostic intervals. Disease outcomes, such as LC stage at diagnosis, recurrence, and survival were seldom measured. Review findings highlight the need for longitudinal, powered, and controlled studies.
Similar content being viewed by others
Introduction
Lung cancer (LC) is the most common cause of cancer incidence and mortality worldwide, with 2.1 million new cases and 1.8 million deaths in 20181. It is estimated that, by 2040, the number of annual LC diagnoses and deaths will increase to 3.63 and 3.01 million respectively2. Worldwide, more than half of LCs (53%) are diagnosed in people aged between 55 and 74 years3. Data from 185 countries indicate that LC is typically diagnosed at an advanced stage, with a 5-year survival rate of 10–20%4.
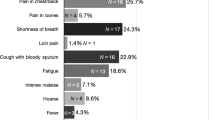
LC has a relatively broad symptom signature compared to other cancers, such as breast and testicular cancers that typically present with a single identifiable symptom (e.g., painless lump)5,6,7. Early-stage LC can be asymptomatic or can cause a range of symptoms including a persistent cough, changes to an existing cough, shortness of breath, and chest pain8,9. Systemic symptoms, such as unexplained weight loss and fatigue, are typically associated with advanced disease10. Haemoptysis is one of the strongest symptom predictors of LC8,11. The broad symptom signature of LC, and overlap with common symptoms of benign disease, may contribute to delays in presentation and diagnosis12.
Early medical help-seeking for symptoms suggestive of LC is a key enabler of early diagnosis, curative treatment, and improved survival11. However, a Swedish study found that patients diagnosed with LC experience, on average, a 6-month delay between symptom onset and initiation of treatment13. Reasons for delayed patient help-seeking include patient factors, such as symptom misappraisal, fear of a potential cancer diagnosis, and guilt associated with smoking14,15, as well as healthcare system factors, such as the high financial cost of healthcare, lack of access to healthcare, and previous bad experiences with the healthcare system15,16,17,18.
Primary healthcare professionals (HCPs) play a key role in facilitating early diagnosis through recognising people with signs and symptoms suggestive of LC and referring them appropriately19. HCP-related barriers to early diagnosis of LC may include lack of awareness of signs and symptoms of LC, inadequate access to diagnostics and rapid referral pathways, and fear of overburdening the healthcare system15,18. In this systematic review, we identify and describe the effect of interventions aimed at helping HCPs recognise and refer individuals with signs and symptoms indicative of LC to the appropriate healthcare pathway in a timely manner.
Methods
This systematic review was guided by the Cochrane Handbook for Systematic Reviews of Interventions20 and reported using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist21 (Supplementary Table 1).
Eligibility criteria
Using a modified version of the population, intervention, comparison, and outcomes (PICO) framework22, to include “S” for study design and “T” for timeframe (PICOST), the systematic review inclusion criteria were as follows: population: any HCPs. Studies were included only when patient outcomes were reported as a result of an intervention targeted towards HCPs; Intervention: any intervention, campaign, programme, trial, education, algorithm, decision tree/support, or guide aimed at improving early diagnosis of symptomatic LC; comparison: any pre-post comparison; outcomes: any outcomes (e.g., LC diagnosis among symptomatic patients, stage of LC at diagnosis, LC treatments received, and LC survival); study design: any experimental design; and timeframe: studies published between January 2011 and September 2021 in order to identify the latest evidence.
Studies were excluded if interventions were exclusively targeted at patients, did not incorporate a comparator, and/or used non-experimental designs. Studies focusing on detection of LC in asymptomatic individuals (i.e., through screening or surveillance) were also excluded. Moreover, we excluded conference proceedings, dissertations, and theses.
Search strategy
MEDLINE, CINAHL, ERIC, and Academic Search Complete were searched on September 13, 2021. Truncation “*” was used and keywords were combined using Boolean operators “OR” and “AND” and the proximity indicator “N.” The following keywords were searched based on title or abstract: (Interven* OR program* OR campaign* OR trial* OR experiment* OR educat* OR algorithm* OR “decision* tree*” OR “decision* support*” OR guid*) AND (Refer* OR consult* OR recogni* OR counsel* OR advice OR advis* OR detect* OR find* OR triag* OR direct* OR manag* OR signpost* OR know* OR aware* OR understand*) AND ((Lung* OR pulmo*) N3 (cancer* OR neoplas* OR malignan* OR tumo* OR symptom* OR sign*)) AND (“Health* profession*” OR “health care profession*” OR HCP* OR “health* work*” OR “health care work*” OR HCW* OR clinician* OR nurs* OR “public health nurs*” OR PHN* OR “community nurs*” OR “clinic nurs*” OR “practice nurs*” OR pharmac* OR chemist* OR doctor* OR physician* OR “general practitioner*” OR GP* OR consultant*).
Study extraction and synthesis
Records were screened in Covidence, an online software used to streamline the production of systematic reviews23. First, titles and abstracts were screened, and irrelevant records were excluded. Full texts of potentially eligible records were then sourced and screened. Each record was title, abstract, and full text screened twice by two independent reviewers. Screening conflicts were resolved by a third reviewer.
The following data were extracted for each study using a standardised table14,24 (Supplementary Table 2): author(s); year; country; aim; design; theoretical underpinning; sample; setting; relevant outcomes; intervention; procedures; instruments; follow-up time(s); and relevant findings. One reviewer conducted data extraction. Each extracted study was then cross-checked for accuracy by the review team. Meta-analyses were not plausible due to significant heterogeneity in study design, interventions, and outcome measures. Instead, a narrative synthesis was conducted, which involved grouping and synthesising the results according to the outcomes measured within the reviewed studies25.
Quality appraisal and level of evidence assessment
The Mixed Methods Appraisal Tool was used to appraise the methodological quality of the included randomised controlled trials (RCTs) and non-RCTs26. Quality appraisal was conducted in terms of the appropriateness of recruitment, data collection, and data analysis to the research question. Each item was voted on a “yes,” “no,” and “cannot tell” basis. The Scottish Intercollegiate Guidelines Network27 grading system was used to assess the level of evidence for each of the included studies. The eight levels of evidence range between 1++, 1+, 1−, 2++, 2+, 2−, 3, and 4. For instance, a score of 1++ corresponds to high quality meta-analyses, systematic reviews of RCTs, or RCTs with a very low risk of bias, whereas a score of 4 is assigned to expert opinions27. Quality appraisal and level of evidence assessment were conducted by one reviewer and cross-checked for correctness by the review team.
Results
Study selection
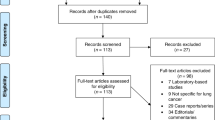
Database searching resulted in 5829 records. Following deletion of duplicates, 3556 records were screened by title and abstract and 3458 irrelevant records were excluded. The full texts of the remaining 98 records were obtained and screened. Of those, seven were included in this systematic review (Fig. 1).
Study characteristics
Most of the studies were conducted in Denmark (n = 2) and England (n = 2) and were non-RCTs (n = 5). Sample size ranged widely between 7228 and 56,02029 participants and follow-up times varied from 330 to 37 months31. Five different interventions were used across the seven studies, including: (i) Combined public and HCP LC awareness campaigns;30,32 (ii) letters and continuing medical education (CME) meetings to educate general practitioners (GPs) about referral criteria for fast-track evaluation of patients with “reasonable suspicion” of LC (maximum 72 h waiting time for evaluation, which includes low dose computed tomography [LDCT]);33,34 (iii) a cancer fast-track programme (i.e., target of 30 days between well-founded suspicion of cancer by a GP and the start of treatment). Referrals to this programme can also originate from emergency departments or other clinical departments involved in routine monitoring or screening;29 (iv) the thoracic-trained advanced practice provider-led LC strategist programme to minimise diagnostic redundancy, streamline management decisions for indeterminate nodules, and expedite curative therapy. Once patients were referred from primary care to secondary care, an individual evaluation strategy was developed and followed for them;31 and (v) multi-disciplinary meetings, screensavers, and posters to reduce delay between initial suspicion of LC and measurement of serum calcium levels28. Of note, Hypercalcaemia is a serious complication of LC and is associated with poorer prognosis28. The full characteristics of the included studies are presented in Table 1.
Quality appraisal and level of evidence assessment
All the included non-RCTs (n = 5) used appropriate data collection methods, outcome measures, and intervention administration. Outcome data were complete in all non-RCTs. Four non-RCTs had clear research questions. The study by Philips et al.31 did not have a clear aim statement, despite clearly stated hypotheses. Only one non-RCT reported that participants were representative of the target population33 and only one non-RCT reported that confounders were accounted for in the study design28. Both RCTs (n = 2) had clear research aims, performed randomisation appropriately, collected data in line with the research aims, had groups that were comparable at baseline, and reported on participant adherence to the assigned intervention30,34. However, the outcome assessor was not blinded in Gudlbrant et al.’s34 RCT.
Four studies scored 2+ on the Scottish Intercollegiate Guidelines Network27 level of evidence criteria, indicating well-conducted non-RCTs with a low risk of confounding or bias and a moderate probability that the relationship is causal29,31,32,33. Only one study scored 2++, indicating a well-conducted non-RCT with a low risk of confounding or bias and a moderate probability that the relationship is causal28. Both RCTs scored 1+ indicating well-conducted RCTs with a low risk of bias30,34. See Table 2 for quality and level of evidence assessment.
Synthesis of findings
Outcomes reported in the reviewed studies were categorised into four categories as follows: diagnostic intervals; referral and diagnosis patterns; stage distribution at diagnosis; and time interval from diagnosis to treatment.
Diagnostic interval
Four studies aimed to reduce the diagnostic interval (i.e., the time from the first presentation with symptoms of LC until diagnosis35) using the LC strategist programme31, a community- and GP-targeted cancer awareness campaign30, information on LDCT and CME sessions34, and a multimodal quality improvement project28.
A retrospective review of the LC strategist programme found that time from suspicious findings on CT chest, chest X-ray, and to a lesser extent abdominal CT, to initiation of diagnostic workup of lung nodules for treatment or surveillance was significantly shorter with the programme in comparison to routine referral (3 vs 28 days respectively, p < 0.001)31. Following referral, the median time to workup was also significantly shorter with the programme in comparison to routine referral (1 vs 7 days respectively, p < 0.001)31.
In contrast, a concurrent community- and GP-targeted breast, prostate, colorectal, and LC awareness campaign found no statistically significant difference in the total diagnostic interval at community (i.e., public intervention) level (median total diagnostic interval = 114.5 days pre-test vs 114 days post-test, mean difference = 0.06, 95% confidence interval [CI] 0.39–0.5, p = 0.79) or at GP level (median total diagnostic interval = 115 days pre-test vs 125 days post-test, mean difference = 0.02, 95%CI 0.56–0.60, p = 0.45)30. Likewise, a study measuring the effect of an intervention to inform GPs about direct access to LDCT found no statistically significant difference in primary care interval (i.e., the time from the patient’s first symptomatic presentation in primary care until referral to secondary care35) between patients of GPs who received information about indications for LDCT (intervention group) (media n = 14 days, inter quartile intervals [IQI] = 4–53) and patients of GPs who did not receive this information (control group) (media n = 18 days, IQI = 5–69, Prevalence Ratio [PR] = 0.99, 95%CI 0.65–1.54, p = 0.455)34. Moreover, no statistically significant difference was found in the diagnostic interval between patients in the intervention group (median = 44 days, IQI = 17–83) and the control group (media n = 36 days, IQI = 17-112, PR = 0.8, 95%CI 0.5–1.27, p = 0.299). However, the primary care and diagnostic intervals in the intervention group were significantly shorter if the GP also participated in a 1-h small-group-based CME session (primary care interval median = 9 days [with CME] vs 37 days [without CME], p = 0.048; diagnostic interval median=23 days [with CME] vs 66 days [without CME], p = 0.008)34.
In their quality improvement project, Apthrop et al.28 used multidisciplinary meetings, screensavers, and posters encouraging secondary care physicians to order serum calcium levels in patients with a suspected diagnosis of LC. This project aimed to help reduce delay between initial suspicion of LC and ordering serum calcium levels during initial LC diagnostic workup in England. This project led to a statistically significant reduction in overall median time to ordering serum calcium levels in patients with a suspected diagnosis of LC, from 13 days pre-test (i.e., before the quality improvement project) to 7 days post-test (p = 0.001)28.
Referral and diagnosis patterns
Three studies reported on patterns of LC referral and diagnosis following implementation of a public awareness and GP training campaign32, a cancer fast-track programme29, and GP information and CME sessions on indications for LDCT33. Athey et al.32 delivered a public and GP LC awareness campaign in six English communities with high LC incidence served by 11 GP surgeries (intervention group). This campaign ran for six weeks and used a “push-pull” approach to “push” the public to seek help for symptoms of concern and encourage GPs to “pull” symptomatic individuals into appropriate services. Five other communities served by nine GP surgeries with similar demographics served as the control group. There was a 27% increase in the number of chest X-rays ordered in the intervention group compared to a 19% increase in the control group during the campaign and six months post-test. In comparison to pre-campaign, there was a sustained increase in chest X-rays requested in the intervention group (20% relative increase) in comparison to a 2% relative reduction in the control group (Incidence Rate Ratio [IRR] = 1.22, 95%CI 1.12–1.33, p = 0.001) at 12 months post-campaign. Moreover, LC diagnoses increased by 27% (relative increase) in the intervention group and fell by 10% (relative reduction) in the control group. However, this was not statistically significant (IRR = 1.42, 95%CI 0.83–2.44, p = 0.199)32.
In a study of a cancer fast-track programme in Catalonia, Prades et al.29 noted increased use of the programme over time, with 3336 patients with suspected LC referred via the programme in 2006, compared to 3841 patients in 2009. The proportion of all new LCs that were diagnosed through this programme fell from 60.2% (95%CI 59.8–63.4%) in 2006 to 53.2% (95%CI 51.5–54.9%) in 2009. GPs were the source of 60.6% of referrals to the fast-track programme in 2006 (95%CI 59–62.3%), falling to 41.4% (95%CI 39.7–42.9%) in 2009, demonstrating increased referrals from other sources such as hospital-based clinicians and services. The LC detection rate via the programme fell from 49.9% (95%CI 48.2–51.6%) in 2006 to 39.7% (95%CI 38.1–41.2%) in 2009. Prades et al.29 reported a statistically significant increase in GP compliance with cancer fast-track referral guidelines from 70.8% in 2006 (95%CI 69.1–72.1%) to 82.3% in 2009 (95%CI 81.1–83.5%).
In a cohort study nested in an RCT, Guldbrandt33 examined the use of a fast-track referral option for GPs for patients with suspected LC and the effect of GP education and awareness training on direct referral to LDCT. This education comprised a one-hour CME session and information about LDCT, including indications and Positive Predictive Values (PPV) for LC (i.e., the ratio of patients truly diagnosed as positive to all those who had positive test results). Results showed that, out of 648 patients directly referred to LDCT, absolute numbers of referrals were significantly higher (61%, 95%CI 54–66%) among GPs working in a clinic with one or more CME-participating GPs. However, the referral rate to LDCT via fast-track was 0.13 per 1000 adults per month (95%CI 0.09–0.19) for CME-participating GPs compared to 0.14 (95%CI 0.09–0.20) for non-participating GPs. The PPV for LC diagnosis due to referral to a fast-track LC pathway was 13.3% (95%CI 8.7–19.1%) for CME-participating GPs and 6.1% (95%CI 3–11%) for non-participating GPs (2.2 higher PPV). This was found to be statistically significant (p = 0.027)33.
Stage distribution at diagnosis
Three studies reported on LC stage at diagnosis following an intervention. Athey et al.32 examined LC stage at diagnosis following a “push-pull” LC awareness campaign, Guldbrandt et al.34 examined LC stage at diagnosis following an information programme and CME sessions on LDCT for GPs, and Philips et al.31 examined LC stage at diagnosis following the LC strategist programme. Athey et al.32 found no significant stage shift three months, six months, or one year following the LC “push-pull” awareness campaign. Similarly, Guldbrandt et al.34 reported a non-statistically significant difference in stage of LC at diagnosis between the intervention group (i.e., information and CME sessions on LDCT) and control group (p = 0.586 for advanced LC and p = 0.595 for localised LC). Philips et al.31 also found non-statistically significant difference in stage at diagnosis for the seven patients in the LC strategist programme and 33 routine referral patients who underwent surgery for LC. This was the only study to report on disease free survival and overall survival. It was found that six of the seven patients (85.7%) in the LC strategist programme cohort were found to have early-stage disease with a median time of 37 days from suspicious imaging to treatment31. In these six patients, with a median duration of follow up of 33 months, disease free survival and overall survival were 100% (i.e., no LC recurrence and no LC death). As for the routine referral group, 25 of 33 patients (75.7%) were found to have early-stage LC with a median time of 68 days from suspicious imaging to treatment. In these 25 patients, there were six recurrences (76% disease free survival) and no deaths (100% overall survival) over a median time of 35 months. The differences in survival rates between the LC strategist programme group and the routine referral group were not statistically significant31.
Time interval from diagnosis to treatment
The time from LC diagnosis to treatment was measured in two studies following two specialist programmes, namely the cancer fast-track programme29 and the LC strategist programme31. The latter study found that the time from suspicious imaging to definitive management plan was 14.5 days in the LC strategist programme and 46.5 days in routine referral (p < 0.001)31. It was also found that referral to the programme moved patients into low-risk nodule surveillance approximately one month earlier relative to routine referral (12.5 vs 39 days respectively, p < 0.001). Compared to routine referral, management through the programme also significantly reduced the median number of hospital trips (4 vs 6 respectively, p < 0.001), median number of clinicians seen (1.5 vs 2 respectively, p = 0.08), median number of diagnostic studies obtained (4 vs 5 respectively, p = 0.01), median time from suspicious radiological findings to diagnosis (30.5 vs 48 days respectively, p = 0.02), and median time from suspicious radiological findings to treatment (40.5 vs 68.5 days respectively, p = 0.02)31. Moreover, time from suspicious radiological findings to surgical resection was significantly shorter in patients managed through the programme in comparison to routine referral (38 vs 69 days respectively, p = 0.05). Among patients with early-stage non-small cell LC treated with radiation therapy, the LC strategist programme led to a substantial reduction in the time from suspicious radiological findings to initiation of treatment in comparison to routine referral (62.5 vs 122.5 days respectively, p = 0.08)31. Conversely, in the cancer fast-track programme, Prades et al.29 noted a variable trend in mean time from detection of suspected LC in primary care to start of initial treatment. The 30-day target was not achieved, with mean times of 30.8 days, 38.9 days, 32.25 days, and 36.7 days in 2006, 2007, 2008, and 2009 respectively. There was also an increase in the proportion of patients waiting between 30 and 45 days (23.7% in 2006 vs 26.1% in 2009) and over 45 days (13.6% in 2006 vs 22.6% in 2009) from the time of LC detection to initiation of treatment.
Discussion
Achieving early diagnosis is an essential step in improving LC outcomes28,29,30,31,34. While more than 85% of patients subsequently diagnosed with cancer initiate their diagnostic pathway in primary care35, timely recognition and referral of people with suspected LC is complicated by various primary HCP and system-related factors. For example, a scoping review of 33 studies identified low index of suspicion, delays in obtaining access to diagnostic tests, multiple specialist consultations and lack of rapid assessment services as barriers to early diagnosis of LC36. Additionally, a qualitative study of 16 GPs from five practices in the United Kingdom found that GPs often required high levels of suspicion to refer patients to secondary care and were concerned about overloading the healthcare system by over-referring patients37. More recently, Saab et al.38 interviewed 36 primary HCPs (GPs, community pharmacists, GP practice nurses, and public health nurses) about their experience of referring individuals with suspected LC in Ireland. It was found that “typical” LC lung signs and symptoms such as cough and haemoptysis triggered referrals, whereas “atypical” signs and symptoms like back pain and pallor, were perceived as difficult to interpret. Participants suggested educating primary HCPs about early LC referral using “communications from professional organisations, webinars, interdisciplinary meetings, education by lung specialists, and patient testimonials” (p.1)38. The use of simple, clear, and visually appealing LC referral checklists and algorithms in primary care was also recommended38.
Several studies included in the present review reported on efforts to raise awareness of LC signs and symptoms among HCPs, and prompt timely referral for further diagnostic or specialist evaluation. These included: a combined public and HCP LC awareness campaign which used GP education resource cards with symptom risk assessment charts to increase symptom awareness and early specialist referral among GPs;30 a push-pull campaign that involved educating GPs and community pharmacists about chest X-ray referral criteria for symptomatic patients;32 and CME sessions for GPs addressing the indications for LDCT for signs and symptoms that raised GPs’ suspicion of LC, but fell short of satisfying the fast-track referral criteria33,34. Indeed, the effect of CME meetings on raising GPs’ awareness of cancer signs and symptoms and prompting early referral is well documented in the wider literature. Toftegaard et al.39 studied the impact of CME meetings in Denmark to support GPs in recognising and referring patients with cancer warning signs and symptoms. An evaluation of this initiative found that CME meetings significantly improved knowledge of cancer among GPs and increased the number of urgent referrals39, which is associated with better cancer survival40,41.
Interventions that were successful in reducing the diagnostic interval included a multi-modal quality improvement project in primary care28 and the LC strategist programme in secondary care31. In contrast, statistically significant reductions in diagnostic intervals were not achieved following a community- and GP-targeted awareness campaign30 as well as information for GPs on LDCT for symptomatic patients34. GP participation in a 1-h CME session on LDCT, however, was associated with shorter primary care and diagnostic intervals34, higher absolute number of referrals to LC fast-track, and higher PPV for LC diagnosis33.
Postal questionnaires offer a pro-active, if somewhat resource intensive, option for primary HCPs to prompt help-seeking among high-risk symptomatic patients. For example, Wagland et al.42 studied the impact of sending a postal symptom questionnaire, incorporating nine symptoms of LC, to patients identified as high risk for LC in eight GP practices in England. Through this intervention, a small, clinically relevant group (6.7%, n = 61/908) of primary care patients was identified who, despite reporting potential symptoms of LC, had not consulted a GP in ≥12 months. Primary care consultations significantly increased in the 3-month period following receipt of the symptom elicitation questionnaire compared to the 3-month period pre-questionnaire (p = 0.002)42. Participants who decided not to consult their GP cited concerns over wasting their own and the GP time and reported a high symptom tolerance threshold and a greater tendency to self-manage their symptoms42. These barriers are well documented in the wider literature15,16,18.
The benefits of cancer fast-track pathways/programmes are well documented in the international literature43,44,45,46. Fast-track referral criteria are typically based on the presence of combinations of, or individual, ‘alarm’ cancer signs and symptoms and/or relevant radiological findings, usually with a PPV for cancer of 3% or above47. Two of the reviewed studies evaluated the impact of specialist-led and fast-track programmes on time from suspicious radiologic findings31 and LC detection29 to the planning and initiation of treatment. In comparison to routine referral, the specialist-led LC strategist programme significantly reduced the intervals between suspicious radiologic findings and definitive management plan, diagnosis, and treatment31. In contrast, in their evaluation of a cancer fast-track programme from its inception in 2006 until 2009, Prades et al.29 reported a significant increase in waiting times from LC detection to initiation of treatment. This may be explained by factors including the complexity of LC treatment, including thoracic surgery at tertiary hospitals29.
Interventions aimed at prompting early referral and diagnostic work-up do not always lead to significant improvements in stage of LC at diagnosis and overall survival. Our systematic review demonstrated that CME sessions on the indications for LDCT34, the specialist-led LC strategist programme31, and a combined public and HCP cancer awareness campaign32, were not associated with significant differences in stage of LC at diagnosis. In addition, Philips et al.31 found non-statistically significant differences in LC recurrence and mortality in patients referred through the LC strategist programme in comparison to those referred through routine referral. Larger scale studies with more statistical power and prospective RCTs with longer follow-up are recommended31,32,34.
This review offers valuable insights into interventions aimed at improving the early diagnosis of symptomatic LC. However, a few limitations are worthy of note. While there is some evidence for the effectiveness of CME meetings and fast-track programmes, recommendations for clinical practice should be made with caution, particularly due to the small number of studies included in this review and the fact that meta-analyses were not possible due to significant heterogeneity in study design, interventions, and outcome measures. Study selection bias could have occurred, as only studies relevant to the review aims were included, the search did not include records from the grey literature or clinical trial registries, and the review was limited to studies published within a 10-year timeframe.
In conclusion, findings from this review indicate that CME meetings for primary HCPs may facilitate early LC referral, diagnosis, and survival. We also found evidence that fast-track programmes, such as the LC strategist programme31, may improve time from initial presentation with symptoms in primary care to LC diagnosis, and time from diagnosis to treatment, in addition to reducing hospital visits and the number of clinicians seen between initial presentation and initiation of treatment. However other interventions, such as awareness campaigns, were not associated with significant improvements in outcomes30,32. Outcomes such as LC stage shift and mortality rates were seldom measured in the reviewed studies. When measured, statistical significance was not reached, hence the importance of conducting future studies that are appropriately powered, controlled, and have longer follow-up.
Review findings may inform cancer control policy, including the design and implementation of interventions aimed at overcoming barriers to early LC diagnosis. These interventions may include awareness and education campaigns targeting the public and HCPs, and implementation of specialist-led fast-track referral programmes to facilitate timely diagnosis.
Data availability
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
References
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424 (2018).
World Health Organization International Agency for Research on Cancer. Cancer Tomorrow. https://gco.iarc.fr/tomorrow/en/dataviz/isotype?cancers=15&single_unit=100000&types=0 (2020).
Torre, L. A., Siegel, R. L., & Jemal, A. Lung cancer statistics. Lung Cancer and Personalized Medicine, 1–19 (Springer, Cham, 2016).
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J. Clinicians 71, 209–249 (2021).
Koo, M. M., Hamilton, W., Walter, F. M., Rubin, G. P. & Lyratzopoulos, G. Symptom signatures and diagnostic timeliness in cancer patients: a review of current evidence. Neoplasia 20, 165–174 (2018).
Saab, M. M., Landers, M. & Hegarty, J. Exploring awareness and help-seeking intentions for testicular symptoms among heterosexual, gay, and bisexual men in Ireland: a qualitative descriptive study. Int. J. Nurs. Stud. 67, 41–50 (2017).
O’Mahony, M., McCarthy, G., Corcoran, P. & Hegarty, J. Shedding light on women’s help seeking behaviour for self discovered breast symptoms. Eur. J. Oncol. Nurs. 17, 632–639 (2013).
Okoli, G. N., Kostopoulou, O. & Delaney, B. C. Is symptom-based diagnosis of lung cancer possible? A systematic review and meta-analysis of symptomatic lung cancer prior to diagnosis for comparison with real-time data from routine general practice. PLoS ONE 13, e0207686 (2018).
Chowienczyk, S., Price, S. & Hamilton, W. Changes in the presenting symptoms of lung cancer from 2000–2017: a serial cross-sectional study of observational records in UK primary care. Br. J. Gen. Pract. 70, e193–e199 (2020).
American Cancer Society. Signs and Symptoms of Lung Cancer. https://www.cancer.org/cancer/lung-cancer/detection-diagnosis-staging/signs-symptoms.html (2019).
Walter, F. M. et al. Symptoms and other factors associated with time to diagnosis and stage of lung cancer: a prospective cohort study. Br. J. Cancer 112, S6–S13 (2015).
Holmberg, L. et al. National comparisons of lung cancer survival in England, Norway and Sweden 2001–2004: differences occur early in follow-up. Thorax 65, 436–441 (2010).
Ellis, P. M. & Vandermeer, R. Delays in the diagnosis of lung cancer. J. Thorac. Dis. 3, 183 (2011).
Saab, M. M. et al. Promoting lung cancer awareness, help-seeking and early detection: a systematic review of interventions. Health Promotion Int. 36, 1656–1671 (2021).
Saab, M. M. et al. Awareness and help-seeking for early signs and symptoms of lung cancer: a qualitative study with high-risk individuals. Eur. J. Oncol. Nurs. 50, 101880 (2021).
Cassim, S. et al. Patient and carer perceived barrriers to early presentation and diagnosis of lung cancer: a systematic review. BMC Cancer 19, 25 (2019).
Cunningham, Y. et al. Lung cancer symptom appraisal among people with chronic obstructive pulmonary disease: a qualitative interview study. Psychooncology 28, 718–725 (2019).
Saab, M. M. et al. Primary healthcare professionals’ perspectives on patient help-seeking for lung cancer warning signs and symptoms: a qualitative study. BMC Prim. Care 23, 119 (2022).
Bradley, S. H., Kennedy, M. & Neal, R. D. Recognising lung cancer in primary care. Adv. Ther. 36, 19–30 (2019).
Higgins, J. P. T. et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane. www.training.cochrane.org/handbook (2022).
Page, M. J. et al. Updating guidance for reporting systematic reviews: development of the PRISMA 2020 statement. J. Clin. Epidemiol. 134, 103–112 (2021).
Schardt, C., Adams, M. B., Owens, T., Keitz, S. & Fontelo, P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med. Inform. Decis. Mak. 7, 1–6 (2007).
The Cochrane Collaboration. Covidence. https://community.cochrane.org/help/tools-and-software/covidence (2022).
Saab, M. M. et al. Referring high-risk individuals for lung cancer screening: a systematic review of interventions with healthcare professionals. Eur. J. Cancer Prev. 31, 540–550 (2022).
Popay, J. et al. Guidance on the conduct of narrative synthesis in systematic reviews. A product from the ESRC Methods Programme. Version 1, b92. https://www.lancaster.ac.uk/media/lancaster-university/contentassets/documents/fhm/dhr/chir/NSsynthesisguidanceVersion1-April2006.pdf (2006).
Hong, Q. N. et al. The Mixed Methods Appraisal Tool (MMAT) version 2018 for information professionals and researchers. Educ. Inf. 34, 285–291 (2018).
Scottish Intercollegiate Guidelines Network. Healthcare Improvement Scotland: A Guideline Developer’s Handbook. https://www.sign.ac.uk/assets/sign50_2011.pdf (2011).
Apthorp, C. et al. Assessment of serum calcium in patients referred for suspected lung cancer: a quality improvement project to enhance patient safety in clinical practice. Future Healthc. J. 8, e109 (2021).
Prades, J., Espinas, J. A., Font, R., Argimon, J. M. & Borras, J. M. Implementing a Cancer Fast-track Programme between primary and specialised care in Catalonia (Spain): a mixed methods study. Br. J. Cancer 105, 753–759 (2011).
Emery, J. D. et al. The Improving Rural Cancer Outcomes Trial: a cluster-randomised controlled trial of a complex intervention to reduce time to diagnosis in rural cancer patients in Western Australia. Br. J. Cancer 117, 1459–1469 (2017).
Phillips, W. W. et al. Lung Cancer Strategist Program: a novel care delivery model to improve timeliness of diagnosis and treatment in high-risk patients. Healthcare 9, 100563 (2021).
Athey, V. L., Suckling, R. J., Tod, A. M., Walters, S. J. & Rogers, T. K. Early diagnosis of lung cancer: evaluation of a community-based social marketing intervention. Thorax 67, 412–417 (2012).
Guldbrandt, L. M., Rasmussen, T. R., Rasmussen, F. & Vedsted, P. Implementing direct access to low-dose computed tomography in general practice—method, adaption and outcome. PLoS ONE 9, e112162 (2014).
Guldbrandt, L. M. et al. The effect of direct access to CT scan in early lung cancer detection: an unblinded, cluster-randomised trial. BMC Cancer 15, 1–11 (2015).
Hansen, R. P., Vedsted, P., Sokolowski, I., Søndergaard, J. & Olesen, F. Time intervals from first symptom to treatment of cancer: a cohort study of 2,212 newly diagnosed cancer patients. BMC Health Serv. Res. 11, 1–8 (2011).
Malalasekera, A. et al. How long is too long? A scoping review of health system delays in lung cancer. Eur. Respir. Rev. 27, 180045 (2018).
Wagland, R. et al. Facilitating early diagnosis of lung cancer amongst primary care patients: the views of GPs. Eur. J. Cancer Care 26, e12704 (2017).
Saab, M. M. et al. Referring patients with suspected lung cancer: a qualitative study with primary healthcare professionals in Ireland. Health Promotion Int. 37, 1–12 (2022).
Toftegaard, B. S., Bro, F., Falborg, A. Z. & Vedsted, P. Impact of continuing medical education in cancer diagnosis on GP knowledge, attitude and readiness to investigate–a before-after study. BMC Fam. Pract. 17, 1–10 (2016).
Toftegaard, B. S., Bro, F., Falborg, A. Z. & Vedsted, P. Impact of a continuing medical education meeting on the use and timing of urgent cancer referrals among general practitioners-a before-after study. BMC Fam. Pract. 18, 1–13 (2017).
Møller, H. et al. Use of the English urgent referral pathway for suspected cancer and mortality in patients with cancer: cohort study. BMJ 351, h5102 (2015).
Wagland, R. et al. Promoting help-seeking in response to symptoms amongst primary care patients at high risk of lung cancer: a mixed method study. PLoS ONE 11, e0165677 (2016).
Stapley, S. et al. The risk of pancreatic cancer in symptomatic patients in primary care: a large case–control study using electronic records. Br. J. Cancer 106, 1940–1944 (2012).
Howell, D. A. et al. Time-to-diagnosis and symptoms of myeloma, lymphomas and leukaemias: a report from the Haematological Malignancy Research Network. BMC Blood Disord. 13, 1–9 (2013).
Din, N. U. et al. Age and gender variations in cancer diagnostic intervals in 15 cancers: analysis of data from the UK Clinical Practice Research Datalink. PLoS ONE 10, e0127717 (2015).
Zhou, Y. et al. Variation in ‘fast-track’ referrals for suspected cancer by patient characteristic and cancer diagnosis: evidence from 670 000 patients with cancers of 35 different sites. Br. J. Cancer 118, 24–31 (2018).
National Collaborating Centre for Cancer. Suspected Cancer: Recognition And Referral. https://www.nice.org.uk/guidance/ng12/evidence/full-guideline-pdf-2676000277 (2015).
Acknowledgements
The authors would like to thank the National Cancer Control Programme, Health Services Executive, Ireland for funding this research.
Author information
Authors and Affiliations
Contributions
M.M.S., H.E.B., U.K., Á.L. and J.H. contributed to study conception. M.M.S., M.Mc.C., M.O’.D., L.J.S., P.L.-W., B.N., S.F., M.O’.M. and N.L. performed screening, data extraction, and quality appraisal. M.M.S. and M.Mc.C. drafted the manuscript and all authors provided critical revisions and editing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Saab, M.M., McCarthy, M., O’Driscoll, M. et al. A systematic review of interventions to recognise, refer and diagnose patients with lung cancer symptoms. npj Prim. Care Respir. Med. 32, 42 (2022). https://doi.org/10.1038/s41533-022-00312-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41533-022-00312-9
This article is cited by
-
Our contribution to systematic review and meta-analysis in primary care respiratory medicine
npj Primary Care Respiratory Medicine (2023)
-
Applications and advancements of nanoparticle-based drug delivery in alleviating lung cancer and chronic obstructive pulmonary disease
Naunyn-Schmiedeberg's Archives of Pharmacology (2023)