Abstract
Chronic obstructive pulmonary disease (COPD) treatment guidelines do not currently include recommendations for escalation directly from monotherapy to triple therapy. This 12-week, double-blind, double-dummy study randomized 800 symptomatic moderate-to-very-severe COPD patients receiving tiotropium (TIO) for ≥3 months to once-daily fluticasone furoate/umeclidinium/vilanterol (FF/UMEC/VI) 100/62.5/25 mcg via ELLIPTA (n = 400) or TIO 18 mcg via HandiHaler (n = 400) plus matched placebo. Study endpoints included change from baseline in trough forced expiratory volume in 1 s (FEV1) at Days 85 (primary), 28 and 84 (secondary), health status (St George’s Respiratory Questionnaire [SGRQ] and COPD Assessment Test [CAT]) and safety. FF/UMEC/VI significantly improved trough FEV1 at all timepoints (Day 85 treatment difference [95% CI] 95 mL [62–128]; P < 0.001), and significantly improved SGRQ and CAT versus TIO. Treatment safety profiles were similar. Once-daily single-inhaler FF/UMEC/VI significantly improved lung function and health status versus once-daily TIO in symptomatic moderate-to-very-severe COPD patients, with a similar safety profile.
Similar content being viewed by others
Introduction
Chronic obstructive pulmonary disease (COPD) is a major cause of chronic morbidity and mortality worldwide1. It is a preventable and treatable disease, characterized by persistent respiratory symptoms and airflow limitation1. Determining the appropriate treatment requires a thorough understanding of the disease at an individual level, and assessments should cover symptomatology, exacerbation risk, and the degree of airflow limitation1. Treatment should then be tailored based on these disease characteristics and escalated, as needed, should the patient experience clinically significant symptoms and/or exacerbations1.
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2020 strategy document recommends escalating from monotherapy (long-acting muscarinic antagonist [LAMA] or long-acting β2-agonist [LABA]) to dual therapy (LAMA/LABA or inhaled corticosteroid [ICS]/LABA) or from dual therapy to triple therapy (ICS/LAMA/LABA) for patients who continue to experience clinically significant symptoms and/or exacerbations on their current maintenance therapy1. In real-life management of COPD, patients are often escalated to triple therapy by adding ICS/LABA to LAMA monotherapy, with one study showing that over a quarter of patients with newly diagnosed COPD progress to triple therapy within 24 months of diagnosis2,3. However, despite its occurrence in clinical practice, recommendations for escalation from monotherapy directly to triple therapy are currently not included in treatment guidelines. The reasons for this are varied but include heterogeneous endpoints in the clinical studies performed to date and a lack of updated recommendations based on the current body of evidence.
In a number of clinical studies, stepping up from LAMA monotherapy to ICS/LAMA/LABA triple therapy improved lung function compared with LAMA monotherapy in patients with symptomatic COPD4,5,6,7,8,9,10. Triple therapy versus LAMA monotherapy also led to statistically significant decreases (improvements) in St George’s Respiratory Questionnaire (SGRQ) score5,6,7,8,9, which measures health-related quality of life11, while a study with moderate/severe exacerbation rate as the primary endpoint showed that single-inhaler triple therapy led to a significant reduction in moderate/severe exacerbation rate versus LAMA monotherapy5. In most of these studies, triple therapy was administered using multiple inhalers, therefore evidence comparing single-inhaler triple therapy to LAMA monotherapy would be of clinical relevance. It has previously been demonstrated in Phase III trials that single-inhaler triple therapy with fluticasone furoate/umeclidinium/vilanterol (FF/UMEC/VI) significantly reduces moderate/severe exacerbations and improves lung function and health status compared with dual therapy with FF/VI or UMEC/VI (IMPACT trial) or budesonide/formoterol (FULFIL trial) in patients with symptomatic COPD who are at risk of exacerbations, while the safety profile of triple therapy reflected the known profiles of its components12,13. The current Phase IV study (study 207626) evaluated the efficacy and safety of once-daily single-inhaler FF/UMEC/VI therapy versus once-daily LAMA monotherapy with tiotropium (TIO) in patients with symptomatic COPD with moderate-to-very-severe airflow limitation.
Results
Trial population
The ITT population included 800 patients who underwent randomization (FF/UMEC/VI, N = 400; TIO, N = 400; Fig. 1). Nearly all patients (96%) completed all protocol-defined study visits, with similar discontinuation and withdrawal rates between treatment groups (Fig. 1). Baseline characteristics and demographics were similar between the two treatment groups (Table 1).
Efficacy
The mean change from baseline in trough FEV1 at Day 85 was significantly greater with FF/UMEC/VI versus TIO, with a treatment difference of 95 mL (95% confidence interval [CI]: 62, 128; P < 0.001; Fig. 2a).
The mean change from baseline in trough FEV1 was significantly greater with FF/UMEC/VI versus TIO at both Day 28 and Day 84, with treatment differences (95% CI) of 122 mL (94, 150; P < 0.001) and 87 mL (56, 118; P < 0.001), respectively (Fig. 2b).
A significantly greater mean decrease from baseline in SGRQ total score was observed with FF/UMEC/VI versus TIO at both Day 28 and Day 84. The between treatment differences (95% CI) were −3.0 (−4.7, −1.3; P < 0.001) and −3.2 (−5.0, −1.4; P < 0.001), respectively (Fig. 3a). The odds of being a SGRQ total score responder were significantly greater with FF/UMEC/VI versus TIO at Day 28 (odds ratio [OR] [95% CI]: 1.61 [1.20, 2.15]; P = 0.001) and Day 84 (OR [95% CI]: 1.62 [1.22, 2.17]; P = 0.001; Fig. 3b).
a Least squares mean (95% CI) change from baseline in SGRQ total score and b proportion of SGRQ responders (≥4-point decrease in SGRQ total score) at Day 28 and Day 84. CFB change from baseline, CI confidence interval, FF fluticasone furoate, ITT intent-to-treat, LS least squares, SGRQ St George’s Respiratory Questionnaire, TIO tiotropium, UMEC umeclidinium, VI vilanterol.
CAT score decreased significantly from baseline with FF/UMEC/VI versus TIO at Days 28 and 84. Between treatment differences (95% CI) were −0.9 (−1.5, −0.2; P = 0.006) and −1.2 (−1.9, −0.5; P = 0.001), respectively (Fig. 4a). For CAT responder analyses, ORs were in favor of FF/UMEC/VI at both Day 28 and 84. Statistical significance in favor of FF/UMEC/VI was achieved at Day 28 (OR [95% CI]: 1.49 [1.12, 1.99]; P = 0.006) but not Day 84 (OR [95% CI]: 1.15 [0.86, 1.53]; P = 0.354; Fig. 4b).
a Least squares mean (95% CI) change from baseline in CAT score and b proportion of CAT responders (≥2-point decrease in CAT score) at Day 28 and Day 84. CAT COPD Assessment Test, CFB change from baseline, CI confidence interval, FF fluticasone furoate, ITT intent-to-treat, LS least squares, TIO tiotropium, UMEC umeclidinium, VI vilanterol.
In total, 27 (7%) and 43 (11%) patients receiving FF/UMEC/VI and TIO, respectively, experienced a moderate/severe exacerbation during the 12-week study period. Severe exacerbations were seen in 5 (1%) and 3 (<1%) patients receiving FF/UMEC/VI and TIO, respectively.
The FEV1 < 50% predicted subgroup comprised 212 patients receiving FF/UMEC/VI and 203 patients receiving TIO; the FEV1 ≥ 50% predicted subgroup comprised 185 patients receiving FF/UMEC/VI and 195 patients receiving TIO (Table 2). Demographics at screening were similar across FEV1 subgroups, although lung function parameters differed substantially. Patients in the ≥50% subgroup experienced substantially more moderate COPD exacerbations in the 12 months prior to the study (Table 2), as the study inclusion criteria required a documented history of ≥2 moderate exacerbations or 1 severe exacerbation in the last 12 months for this subgroup.
Mean change from baseline in trough FEV1 was significantly greater with FF/UMEC/VI versus TIO at Days 28, 84, and 85 in both subgroups (Fig. 5). Significantly greater decreases from baseline in SGRQ total score with FF/UMEC/VI versus TIO were observed at Days 28 and 84 for the FEV1 < 50% subgroup. For the FEV1 ≥ 50% subgroup, a numerical decrease in favor of FF/UMEC/VI was observed at Day 28, and the decrease with FF/UMEC/VI versus TIO was statistically significant at Day 84 (Supplementary Fig. 1). Greater decreases from baseline in CAT scores with FF/UMEC/VI versus TIO were observed at Days 28 and 84 for both subgroups, but treatment differences were statistically significant for the FEV1 < 50% subgroup only (Supplementary Fig. 2).
Least squares mean (95% CI) change from baseline in trough FEV1 at a Day 28, b Day 84, and c Day 85. CFB change from baseline, CI confidence interval, FEV1 forced expiratory volume in 1 s, FF fluticasone furoate, ITT intent-to-treat, LS least squares, TIO tiotropium, UMEC umeclidinium, VI vilanterol.
Safety profile
The incidence of AEs, SAEs, and AESIs was similar between treatment groups, including cardiovascular effects, and there was no between-group difference in pneumonia rates (Table 3). There were no new safety findings associated with the use of an ICS, a LAMA, and a LABA in combination. Two patients died in the FF/UMEC/VI arm and one patient died in the TIO arm; these deaths were not considered to be related to study treatment.
Discussion
This study examined the effect of single-inhaler FF/UMEC/VI triple therapy versus TIO monotherapy in patients with symptomatic COPD with moderate-to-very-severe airflow limitation, as in clinical practice patients are often escalated directly from LAMA monotherapy to triple therapy with the addition of ICS/LABA. The superiority of FF/UMEC/VI versus TIO was demonstrated for the primary endpoint of change from baseline in trough FEV1 at Day 85. Furthermore, significant improvements were seen in trough FEV1 at Days 28 and 84, with the greatest improvement at Day 28 (exceeding the MCID value of 100 mL), indicating that FF/UMEC/VI leads to early and sustained benefits in lung function in this population. These findings are in line with previous studies comparing TIO monotherapy to multiple-inhaler ICS/LAMA/LABA triple therapy in patients with COPD, all of which demonstrated statistically significant improvements in pre-dose FEV1 in favor of triple therapy14. These data are also consistent with a recent study demonstrating significant improvements in pre-dose FEV1 from 4 to 52 weeks following initiation of single-inhaler triple therapy (beclometasone dipropionate, formoterol fumarate, glycopyrronium bromide) compared with TIO monotherapy in patients with symptomatic COPD with FEV1 < 50% and a history of exacerbations5. However, while sustained and significant improvements with triple therapy versus LAMA monotherapy were observed in this study, the whole clinical picture and general symptom burden must be taken into account in clinical practice. For example, while this study showed greater improvements in lung function in patients with FEV1 ≥ 50%, these results cannot necessarily be extrapolated to improving dyspnea in patients with symptomatic COPD and a history of exacerbation but preserved lung function.
Early and sustained improvements in health status, as assessed by SGRQ total score and CAT score, were also seen in the current study, with significant decreases in both scores with FF/UMEC/VI versus TIO at Days 28 and 84. Moreover, significantly more patients achieved a ≥ 4-point decrease in SGRQ total score with FF/UMEC/VI versus TIO at both Day 28 and 84. The CAT responder analysis showed a similar trend, with an OR favoring FF/UMEC/VI at both time points, although statistical significance in favor of FF/UMEC/VI was only achieved at Day 28. This was likely due to small decreases in CAT score in the TIO group at Day 84 which tipped patients over the response threshold despite a minimal change versus Day 28, resulting in the loss of statistical significance for the odds of response between the treatment groups at Day 84. These results indicate that addition of ICS and LABA therapy to LAMA monotherapy improves not only lung function but also health status in patients with symptomatic COPD with moderate-to-very-severe airflow limitation. These findings are consistent with previous studies showing that multiple-inhaler triple therapy significantly improved health status, as measured by SGRQ score, versus TIO monotherapy5,6,7,8,9. The early decreases in CAT and SGRQ total scores, seen within 28 days in the current study, are notable given that in most previous studies changes in SGRQ score were only assessed after ≥12 weeks of treatment5,6,7,9. Together, these data suggest that single-inhaler FF/UMEC/VI triple therapy leads to relatively rapid improvements in patient symptoms and quality of life in patients with symptomatic COPD with moderate-to-very-severe airflow limitation.
The post hoc subgroup analysis, conducted according to airflow limitation at screening, demonstrated significant improvements in lung function with FF/UMEC/VI versus TIO both in patients with FEV1 < 50% predicted and ≥50% predicted at baseline. These findings are consistent with a previous study, which showed that lung function benefits in patients with COPD treated with fluticasone/salmeterol plus TIO versus TIO monotherapy were more pronounced for those with severe airflow limitation (FEV1 < 50% predicted)4. Additionally, patients with FEV1 < 50% predicted at baseline experienced significant decreases in SGRQ total score and CAT score at Days 28 and 84, while those with FEV1 ≥ 50% predicted experienced numerical decreases in both scores at each time point that only reached significance at Day 84 for SGRQ total score. These data suggest that a step-up from TIO monotherapy to single-inhaler FF/UMEC/VI triple therapy improves clinical outcomes for patients with symptomatic COPD regardless of airflow limitation, with particular benefit for patients with severe airflow limitation (FEV1 < 50% predicted).
Few patients experienced a moderate/severe exacerbation in either treatment group, despite the population being at risk for exacerbation based on the inclusion criterion of FEV1 < 50% predicted or <80% predicted with a documented history of ≥2 moderate or 1 severe exacerbation in the 12 months prior to screening. The low overall number of exacerbations is likely due to the short length of the study, which along with the size of the population leaves the study underpowered to detect a between-group difference in the rate of exacerbations. Nonetheless, the proportion of patients experiencing a moderate/severe COPD exacerbation during the study was numerically higher in TIO-treated patients compared with those receiving FF/UMEC/VI. These data are consistent with a real-world observational study that showed a lower risk of COPD exacerbations in patients receiving triple therapy with fluticasone-salmeterol plus TIO compared with TIO alone15.
The safety profile of FF/UMEC/VI was similar to that of TIO, with no unexpected safety findings. Rates of SAEs and AESIs, including pneumonia and cardiovascular effects, were low and consistent with previous studies comparing multiple-inhaler triple therapy with TIO monotherapy4,5,6,7,8,9,10. The low pneumonia rates are reassuring given the association seen between pneumonia and ICS use in previous studies16.
Overall, these data show that direct escalation from TIO monotherapy to single-inhaler FF/UMEC/VI triple therapy led to rapid improvements in lung function, symptoms, and health status without an increased risk of pneumonia or other AEs in patients with symptomatic COPD with moderate-to-very-severe airflow limitation. Study limitations include the short study length, which may limit data interpretation. As such, a 1-year study focusing on other outcomes, including the rate of COPD exacerbations, is required. Nonetheless, these data provide valuable clinical information to inform treatment decisions for patients on LAMA monotherapy who continue to experience symptoms and/or exacerbations.
This study demonstrated superiority of once-daily single-inhaler FF/UMEC/VI versus TIO for lung function and patient health status, with a similar safety profile and no difference in pneumonia rates, in patients with symptomatic COPD with moderate-to-very-severe airflow limitation. These results suggest that FF/UMEC/VI is a viable treatment step-up option for optimizing outcomes in patients who continue to experience symptoms and/or exacerbations while receiving LAMA monotherapy.
Methods
Trial design
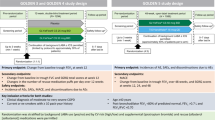
Study 207626 (NCT03474081) was a 12-week, Phase IV, parallel-group, active-controlled, double-blind, double-dummy, randomized, multicenter study comparing once-daily single-inhaler FF/UMEC/VI with TIO monotherapy in patients with symptomatic COPD and moderate-to-very-severe airflow limitation. The study was conducted in 72 centers in three countries (Poland, Russian Federation, and the USA) from March 2018 to July 2019.
Eligible patients were instructed on the proper use of the ELLIPTA and HandiHaler devices at a screening visit (Visit 1) before entering a 4-week run-in period during which they received open-label TIO 18 mcg once daily via HandiHaler and placebo once daily via ELLIPTA. Eligible patients were then randomized 1:1 (using an Interactive Web Response System) to receive either FF/UMEC/VI 100/62.5/25 mcg via ELLIPTA and placebo via HandiHaler or TIO 18 mcg via HandiHaler and placebo via ELLIPTA, all taken once daily in the morning (Visit 2). A double-dummy design was used to ensure blinding, with each patient given two inhalers (ELLIPTA and HandiHaler) to administer the active medication and placebo, and patients self-administered treatment each day. All site personnel involved in efficacy and safety assessments were also blinded to assigned treatment during the study. Rescue albuterol/salbutamol was available as needed throughout the study but withheld for ≥4 hours prior to spirometry assessments. Patients attended two on-treatment study visits (Day 28 [Visit 3] and Day 84 [Visit 4]). Final clinical assessments were conducted on Day 85 (Visit 5). A safety follow-up telephone call or on-site visit (Visit 6) was conducted ~7 days after Visit 5, at the study treatment discontinuation visit, or at the end of the study, whichever was first.
All study patients provided written informed consent. The study was approved by a national, regional, or investigational center ethics committee or institutional review board, in accordance with the International Council on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use Good Clinical Practice and applicable country-specific requirements. Further details are provided in Table 4.
Trial population
At screening, eligible patients were ≥40 years of age, current or former smokers with a history of ≥10 pack-years, had an established clinical history of COPD, had been receiving daily COPD maintenance treatment with TIO alone for ≥3 months, had a post-bronchodilator forced expiratory volume in 1 s (FEV1) of <50% predicted (or a post-bronchodilator FEV1 < 80% predicted and a documented history of ≥2 moderate exacerbations [worsening COPD symptoms requiring treatment with oral/systemic corticosteroids and/or antibiotics] or ≥1 severe exacerbation [worsening COPD symptoms requiring in-patient hospitalization] in the last 12 months), and had a COPD Assessment Test (CAT) score ≥10.
Patients with a current diagnosis of asthma, other respiratory disorders, or other clinically significant diseases were excluded, although participants with a prior history of asthma were eligible if they had a current diagnosis of COPD. Also excluded were those with α1-antitrypsin deficiency as the underlying cause of COPD, a lung resection in the last 12 months, risk factors for pneumonia or recent pneumonia and/or a moderate or severe COPD exacerbation that had not resolved ≥14 days prior to screening and ≥30 days following the last dose of oral/systemic corticosteroids, a respiratory tract infection that had not resolved ≥7 days prior to screening, or an abnormal chest x-ray at or 3 months prior to screening.
Patients were not eligible to be randomized to study treatment if they had a CAT score <10 at Visit 2, demonstrated lack of compliance to run-in treatment (<80% or >120% compliant with either ELLIPTA or HandiHaler), experienced pneumonia, had a moderate or severe COPD exacerbation, or required a change in COPD medication during the run-in period. Assessment of compliance with study treatment between visits was conducted through patient conversations and recording the number of doses left in the ELLIPTA device and the number of capsules dispensed through the HandiHaler. Full inclusion, exclusion, and randomization criteria are provided in Supplementary Note 1.
Efficacy endpoints
The primary endpoint was change from baseline in trough FEV1 at Day 85. To provide a reliable measurement of on-treatment trough FEV1 on Day 85, the final dose of study treatment was administered in clinic on Day 84, to ensure high adherence to dosing. Secondary endpoints were change from baseline in trough FEV1 at Days 28 and 84. Other endpoints included: change from baseline in SGRQ total score at Days 28 and 84; proportion of SGRQ total score responders at Days 28 and 84 (defined as ≥4-unit decrease in SGRQ total score from baseline); change from baseline in CAT score at Days 28 and 84; proportion of CAT score responders at Days 28 and 84 (defined as ≥2-unit decrease in CAT score from baseline); and moderate or severe exacerbation events. Subgroup analyses by percent predicted FEV1 at screening (FEV1 < 50% or ≥50%) were performed post hoc.
Safety assessments
On-treatment AEs were defined as those occurring from the day of starting randomized study treatment until 1 day after stopping randomized study treatment. Incidences of on-treatment adverse events (AEs), including AEs of special interest (AESIs), and serious AEs (SAEs) were recorded. AESIs included cardiovascular effects, decreased bone mineral density and associated fractures, pneumonia, and lower respiratory tract infection (excluding pneumonia). All pneumonias we confirmed clinically and by x-ray, as detailed in Supplementary Note 1.
Statistical analysis
Sample size was based on the primary endpoint of trough FEV1 at Day 85 and assumed 90% power, a two-sided 1% significance level, an estimate of residual standard deviation of 240 mL (based on mixed model repeated measures [MMRM] analyses of the Phase III IMPACT study)13 and a treatment difference of 70 mL. Under these assumptions, a total of 702 evaluable patients (351 per treatment group) were required. Assuming an 8% withdrawal rate during the run-in period and 10% withdrawal rate during the study period, it was aimed to enroll ~848 patients into the 4-week run-in period in order to randomize 780 patients.
The intent-to-treat (ITT) population included all randomized patients, excluding those randomized in error, and was used for the analyses of study population, efficacy, and safety. A participant who was recorded as a screen or run-in failure and also randomized but who did not receive any dose of study treatment was considered to be randomized in error. Any participant who received a randomization number was considered to have been randomized.
Both primary and secondary lung function endpoints were analyzed using MMRM, with covariates of baseline FEV1, visit, geographical region, and treatment; interaction terms included visit-by-baseline FEV1. A visit-by-treatment interaction term was also included to allow treatment effects to be estimated at each visit separately. The variance-covariance matrix was assumed to be unstructured. The primary treatment effect was estimated using a hypothetical strategy that only data up to the time of treatment discontinuation was used in the analysis and data following treatment discontinuation was assumed to follow the same pattern as if the patients had remained on treatment, ie. missing at random.
CAT score and SGRQ total score were analyzed using MMRM, including covariates of baseline value, visit, geographical region, and treatment; interaction terms included visit-by-baseline value and visit-by-treatment. The proportions of CAT or SGRQ responders were analyzed using a generalized linear mixed model with a logit link function and covariates of baseline score, geographical region, treatment group, visit, and visit-by-baseline and visit-by-treatment interactions. TIO was used as the reference level for treatment.
Safety endpoints were analyzed in the ITT population using descriptive statistics. AESIs were defined as AEs that have specified areas of interest for FF, UMEC, and VI, or the overall COPD population.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com by submitting an enquiry citing GSK study number 207626.
References
Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease (2020 report). https://goldcopd.org. Last Accessed Feb. 2020.
Lane, D. C., Stemkowski, S., Stanford, R. H. & Tao, Z. Initiation of triple therapy with multiple inhalers in chronic obstructive pulmonary disease: an analysis of treatment patterns from a U.S. retrospective database study. J. Manag. Care Spec. Pharm. 24, 1165–1172 (2018).
Wurst, K. E., Bushnell, G., Shukla, A., Muellerova, H. & Davis, K. J. Factors associated with time to triple therapy in newly diagnosed COPD patients in the UK general practice research database. [abstract]. Pharmacoepidemiol. Drug Saf. 23, 33 (2013).
Hanania, N. A., Crater, G. D., Morris, A. N., Emmett, A. H., O’Dell, D. M. & Niewoehner, D. E. Benefits of adding fluticasone propionate/salmeterol to tiotropium in moderate to severe COPD. Respir. Med. 106, 91–101 (2012).
Vestbo, J. et al. Single inhaler extrafine triple therapy versus long-acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (TRINITY): a double-blind, parallel group, randomised controlled trial. Lancet 389, 1919–1929 (2017).
Jung, K. S. et al. Comparison of tiotropium plus fluticasone propionate/salmeterol with tiotropium in COPD: a randomized controlled study. Respir. Med. 106, 382–389 (2012).
Welte, T. et al. Efficacy and tolerability of budesonide/formoterol added to tiotropium in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care. Med. 180, 741–750 (2009).
Aaron, S. D. et al. Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease: a randomized trial. Ann. Intern. Med. 146, 545–555 (2007).
Hoshino, M. & Ohtawa, J. Effects of adding salmeterol/fluticasone propionate to tiotropium on airway dimensions in patients with chronic obstructive pulmonary disease. Respirology 16, 95–101 (2011).
Cazzola, M. et al. A pilot study to assess the effects of combining fluticasone propionate/salmeterol and tiotropium on the airflow obstruction of patients with severe-to-very severe COPD. Pulm. Pharmacol. Ther. 20, 556–561 (2007).
Meguro, M., Barley, E. A., Spencer, S. & Jones, P. W. Development and validation of an improved, COPD-specific version of the St. George respiratory questionnaire. Chest 132, 456–463 (2007).
Lipson, D. A. et al. FULFIL trial: once-daily triple therapy for patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 196, 438–446 (2017).
Lipson, D. A. et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N. Engl. J. Med. 378, 1671–1680 (2018).
Rojas-Reyes M. X., Garcia Morales O. M., Dennis R. J. & Karner C. Combination inhaled steroid and long-acting beta(2)-agonist in addition to tiotropium versus tiotropium or combination alone for chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. CD008532 (2016).
Chatterjee, A., Shah, M., D’Souza, A. O., Bechtel, B., Crater, G. & Dalal, A. A. Observational study on the impact of initiating tiotropium alone versus tiotropium with fluticasone propionate/salmeterol combination therapy on outcomes and costs in chronic obstructive pulmonary disease. Respir. Res. 13, 15 (2012).
Kew K. M. & Seniukovich A. Inhaled steroids and risk of pneumonia for chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. CD010115 (2014).
Acknowledgements
This study was funded by GlaxoSmithKline (GSK study 207626; NCT03474081). The funders of the study had a role in the study design, data analysis, data interpretation, and writing of the report. Editorial support (in the form of writing assistance, assembling figures, collating author comments, grammatical editing, and referencing) was provided by Anne Errichelli, DPhil, at Fishawack Indicia Ltd, UK, and was funded by GSK. ELLIPTA is owned by or licensed to the GSK Group of Companies. HandiHaler is a trademark of Boehringer Ingelheim International GmbH.
Author information
Authors and Affiliations
Contributions
The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors, take responsibility for the integrity of the work as a whole, contributed to the writing and reviewing of the manuscript, and have given final approval for the version to be published. All authors had full access to the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. S.B., D.E., and M.K. were involved in acquisition of data and analysis/interpretation of data. N.B., M.C.K., D.A.L., and C.-Q.Z. were involved in the conception/design of the study and analysis/interpretation of data. C.C., T.C.C., C.H., N.M., A.P., M.A., and A.A. were involved in the analysis/interpretation of data.
Corresponding author
Ethics declarations
Competing interests
S.B. has received speaker fees from GSK, Boehringer Ingelheim, Auris Health, Veran, Veracyte, Biodesix, Pinnacle Biologics, and Circulogene. He has also previously participated in speaker’s bureau for Sunovion Pharmaceuticals and holds stocks/shares in Veracyte. M.A. has received speaker fees from AstraZeneca, Boehringer Ingelheim, GSK, MEDA, Orion Pharma, and TEVA. A.A. has received consultancy fees from Boehringer Ingelheim, Novartis, AstraZeneca, and Theravance Mylan. N.B., C.C., T.C.C., C.H., M.C.K., D.A.L., N.M., and C.-Q.Z. are employees of GSK and own stocks/shares. D.E. has received compensation for being a trial investigator for Vitalink Research. M.K. has nothing to disclose. A.P. has received consultancy fees and board membership from AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Mundipharma, and TEVA, and consultancy fees and payment for lectures from Sanofi. He has also received reimbursement of travel expenses from Avillion, reimbursement of travel expenses and payment for lectures from AstraZeneca, Boehringer Ingelheim, Chiesi, ELPEN Pharmaceutical, GSK, Menarini, MSD, Mundipharma, Novartis, TEVA, and Zambon, and research grants from AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Pfizer, Sanofi, and TEVA.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bansal, S., Anderson, M., Anzueto, A. et al. Single-inhaler fluticasone furoate/umeclidinium/vilanterol (FF/UMEC/VI) triple therapy versus tiotropium monotherapy in patients with COPD. npj Prim. Care Respir. Med. 31, 29 (2021). https://doi.org/10.1038/s41533-021-00241-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41533-021-00241-z
This article is cited by
-
Eligibility of patients with chronic obstructive pulmonary disease for inclusion in randomised control trials investigating triple therapy: a study using routinely collected data
Respiratory Research (2024)
-
Intraclass comparison of inhaled corticosteroids for the risk of pneumonia in chronic obstructive pulmonary airway disorder: a network meta-analysis and meta-regression
International Journal of Clinical Pharmacy (2024)
-
Effect of fracture risk in inhaled corticosteroids in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis
BMC Pulmonary Medicine (2023)
-
Different inhaled corticosteroid doses in triple therapy for chronic obstructive pulmonary disease: systematic review and Bayesian network meta-analysis
Scientific Reports (2022)