Abstract
Despite access to diagnostic tests and effective therapies, asthma often remains misdiagnosed and/or poorly controlled or uncontrolled. In this review, we address the key issues of asthma diagnosis and management, recent evidence for levels of asthma control, the consequences of poor control and, in line with that, explore the potential reasons for poor asthma control and acute exacerbations. Based on recent evidence and current guidelines, we also aim to provide practical answers to the key questions of how to improve asthma management, with the best possible prevention of exacerbations, addressing the basics—adherence, inhaler misuse, obesity and smoking—and how to facilitate a new era of asthma care in the twenty-first century. We hope this review will be useful to busy primary care clinicians in their future interactions with their patients with both suspected and proven asthma.
Similar content being viewed by others
Introduction
Our current understanding is that asthma is a common and potentially life-threatening chronic inflammatory airway disease, with different phenotypes, characterised by variable airflow obstruction and, even in mild cases, with unpredictable, recurrent episodes of worsening symptoms1,2. Typical symptoms include wheeze, cough, shortness of breath and chest tightness that can vary in intensity over time, spontaneously or with pharmacological treatment1. Periods of symptom breakthroughs, commonly due to fluctuating inflammatory activity, can develop into exacerbations that may require urgent healthcare and, in some cases, may even be fatal1,2,3,4,5,6 (Fig. 1). Although exacerbations are more common and of greater severity in patients whose asthma is poorly controlled or more severe1,7, even patients with mild asthma are at risk of breakthrough symptoms and exacerbations1,2,8.
Inhaled corticosteroids (ICS) were first introduced as an anti-inflammatory treatment in the 1970s9,10, and despite subsequent advances in our understanding of asthma and its various phenotypes, new medications and inhaler devices, and evidence-based management guidelines, asthma-related morbidity (i.e. uncontrolled asthma and exacerbations) are still a widespread problem although mortality rates have declined1,2,7,9.
In this review, we aim to address key issues, review recent evidence and provide practical answers to the question of how we can optimise current management of asthma and bring the care of this common disease into the twenty-first century.
What are the goals of diagnosis and treatment in asthma?
Correct diagnosis is essential to ensure that every patient receives treatment appropriate to their condition and, unfortunately, misdiagnosis of asthma is still common11. Correct diagnosis of asthma is generally based on a history of symptoms, family history (e.g. atopic disease), physical examination and, as the essential part of the diagnostic process, demonstration of variable airflow limitation by spirometry or peak flow measurement, with consideration of differential diagnoses12,13 (Table 1).
Various guidelines and reports, such as the GINA Global Strategy for Asthma Management and Prevention, have been developed with the aim of providing consistency of asthma treatment around the world1,3,4,13,14,15. Most guidelines share a ‘step care’ approach to treatment, with the aim of achieving daily asthma control and preventing exacerbations (future risk) using the lowest level of medication needed to achieve these goals (Fig. 2). Controller medication should be stepped up or down in line with the observed variations in level of asthma control which can be detected by regular assessment, treatment and review1,3,5,13,14.
Available at https://www.ncbi.nlm.nih.gov/books/NBK7222/figure/A2212/ (accessed 20 January 2020). EIB exercise-induced bronchospasm, ICS inhaled corticosteroid, LABA long-acting inhaled beta2-agonist, LTRA leukotriene receptor antagonist, SABA short-acting beta2-agonist. Source: National Heart, Lung, and Blood Institute; National Institutes of Health; U.S. Department of Health and Human Services.
For many years, the lowest treatment step, recommended in most guidelines for intermittent or mild asthma, has been a short-acting β2-agonist (SABA) reliever, which relieves bronchoconstriction rapidly and effectively but does not reduce the underlying inflammation usually present even in mild asthma16,17. The recommendation for SABA alone as initial treatment for mild asthma dates back to the era when asthma was thought to be a disease only of bronchoconstriction10,18. Also, the development of SABA predated the development of ICS by many years, so SABA use became ingrained in the management of asthma10. Over-reliance on β2-agonist bronchodilators may even worsen inflammation and increase the risk of exacerbations and hospital admissions1,19,20,21,22,23. The 2019 update to the GINA guideline now recommends replacing SABA with low-dose ICS/formoterol as preferred reliever, for safety reasons, both for mild asthma and also at higher GINA steps, in patients already on ICS/formoterol maintenance therapy18 (Fig. 3).
Box 3−5A. Available at https://ginasthma.org/wp-content/uploads/2019/06/GINA-2019-main-report-June-2019-wms.pdf (accessed 20 January 2020). © 2019 Global Strategy for Asthma Management and Prevention, all rights reserved. Use is by express license from the owner.
Anti-inflammatory therapy with ICS is recommended as maintenance therapy, initially at a low dose but at higher doses for more severe asthma1,3,5. However, in patients on ICS, add-on of a long-acting β2-agonist (LABA) has been shown to be more effective than increasing the ICS dose in improving asthma control and preventing exacerbations24,25. As a result, an ICS/LABA combination inhaler is now the first choice of maintenance therapy for the majority of patients with moderate-to-severe asthma1.
Other treatment options include leukotriene receptor antagonists (LTRA), which have a less potent anti-inflammatory activity than ICS, short- or long-acting muscarinic antagonists (SAMA or LAMA) as alternative or additional bronchodilator relievers, and the recently developed injectable biologic drugs for patients with specific subtypes of severe asthma1,26.
What is meant by asthma control?
Well-controlled asthma means that patients are free from troublesome respiratory symptoms during both day and night, need little or no reliever medication (no more than two puffs SABA/week), can lead normal, productive and active lives and continue to have normal or the best possible lung function1,3,5,13. Daytime symptoms or use of reliever more than twice a week, night-time awakenings or limitation of activity all suggest only partial control and if a patient is experiencing all of these then their asthma can be considered uncontrolled1,18.
Achieving well-controlled asthma greatly reduces, but does not eliminate, the risk of breakthrough symptoms and exacerbations of asthma resulting from increases in the airway inflammation that underlies most patients’ asthma27. Even patients with mild or intermittent asthma are at risk of these exacerbations6,17. However, in many cases there is a discrepancy between what patients and healthcare professionals understand by the term ‘asthma control’8. For many patients, ‘control’ simply means being able to keep their symptoms at a manageable level through frequent use of reliever medication28,29. For healthcare professionals, the definition of asthma control is usually broadly based on guideline definitions—absence of symptoms and restrictions on daily activities, good lung function with minimal or no use of reliever and no sleep disturbances5,18.
How well are patients achieving asthma control?
Given the availability of evidence-based reports and guidelines, along with a range of effective medications and inhaler devices to deliver those medications to the target tissues, most patients nowadays should have well-controlled asthma. Regrettably, however, although hospital admissions and asthma mortality have decreased over recent decades, rates now appear to have plateaued2,7,9,30,31,32,33 (Fig. 4). Several real-world surveys have indicated that, at best, only 50% of patients with asthma meet the criteria for well-controlled asthma, indicating either that these criteria are too strict or that asthma management is inadequate12,28,29,34,35,36.
Crude asthma mortality rates during the bronchodilator and anti-inflammatory eras. Reproduced from Pavord et al.2.
A range of tools has been validated for the assessment of asthma control, including the Asthma Control Questionnaire (ACQ) and Asthma Control Test™ (ACT)37. In the INSPIRE study (n = 3415), comparing patients’ own assessment of their level of control with ACQ scores, most patients (89%) had experienced a mean of 12 periods of symptom worsening within the previous year, despite reporting that they believed their asthma was controlled or even well-controlled28. Even the 28% with asthma classed objectively as well-controlled reported an average of 6.3 asthma worsenings a year28. A study by Haughney et al.38 found that 91% (n = 468) of respondents felt that their asthma was under control, yet two-thirds (n = 339) experienced symptoms at least 2–3 times a week. Similarly, in the REALISE study (n = 8000), among patients with GINA-defined partially controlled and uncontrolled asthma, 95% and 84% respectively stated that they had controlled asthma, despite the fact that 55% reported that their daily life was affected by their asthma and 52% had been awakened at least once in the previous week29 (Fig. 5).
The mismatch between patient perceptions of their asthma control and objective assessments. Source: Price et al.29.
Few patients are aware of the treatment goals outlined in the guidelines38. A UK-wide study showed that 58% of patients were initially satisfied with the standard of their asthma management and control38. However, after being shown international asthma guidelines on the outcomes they should expect from their treatment, this declined to only 33%38.
What are the consequences of poor asthma control?
In addition to respiratory problems, poorly controlled asthma has been shown to reduce the general health-related quality of life and affect several aspects of human life such as mobility, sleeping, everyday activities, mental function, discomfort, depression, distress, vitality and sexual activity39. Poor asthma control causes symptoms affecting daily activities, well-being and quality of sleep patterns of patients, all having a negative impact on quality of life40. Poor asthma control also increases the risk of asthma deteriorating into acute exacerbations41. The most important risk factors for exacerbations are having uncontrolled asthma, a history of previous exacerbations and/or hospitalisation, over-reliance on SABA, elevated blood eosinophils and respiratory viral infections41,42,43. In a UK National Health & Wellness Survey of patients being treated with ICS/LABA, compared with well-controlled disease, poorly controlled asthma was associated with more emergency department visits (21% vs. 14%; p = 0.016) or hospitalisations (13% vs. 8%; p = 0.022) in the previous 6 months, lower mental and physical health-related quality of life (p < 0.001) and impaired work productivity (29% vs. 17%; p < 0.001) and activity scores (46% vs. 24%; p < 0.001)44. Over 70% of the patients in the INSPIRE study also reported that one of the worst things about having asthma was the panic they felt when their symptoms worsened28.
When patients have asthma exacerbations, they are likely to receive treatment with oral corticosteroids (OCS)45. Almost half of the respondents in the REALISE study reported that they had acute exacerbations requiring OCS for asthma in the previous year and almost one-quarter had visited the emergency department29. In a Swedish study, 22.5% of patients with asthma (n = 49,930) were periodic users of OCS (>0 but <5 mg/day/year) and 1.5% (n = 3299) were regular users (≥5 mg/day/year). The percentage of patients in REALISE who had an acute exacerbation resulting in OCS treatment in the previous year ranged from 26% to 29% for those with mild asthma (GINA steps 1−2) to 61% for those with more severe asthma (GINA step 4)29. Minimising exposure to OCS by improving asthma control is important as repeated or maintenance treatment with OCS increases the risk of adverse effects such as development of osteoporosis, peptic ulcer, diabetes, cataracts and fractures45,46,47.
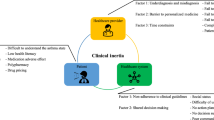
Why is asthma control poor and why do exacerbations occur?
The reasons for poor asthma control can be divided into three categories: patient-related, healthcare-related, and therapy-related48 (Table 2). The most important of the patient-related reasons for poor asthma control in the twenty-first century include obesity, tobacco smoking, over-reliance on reliever therapy and underuse of maintenance controller medication. The inability to use inhalers correctly, and poor perception of asthma symptoms also contribute to poor control1,12,43,49,50. At times of worsening symptoms, most patients increase their SABA use early and many only increase their ICS or ICS/LABA later when symptoms are at their worst28. Using only SABA during symptom breakthroughs is a paradoxical approach since SABA alone does not address the increased inflammation during occasional episodes in response to trigger factors such as exercise, cold air and aeroallergens1,8,51,52,53,54. The pathophysiological changes in response to a trigger factor result in an inflammatory flare-up and release of a wide variety of inflammatory mediators within the airways5. Regardless of the trigger, there is a rapid smooth muscle contraction, mucosal oedema and mucus hypersecretion which together lead to the development of airway obstruction and symptoms.
Many patients with poorly controlled asthma are over-reliant on their SABA for relief of symptoms28,29,31,35. They feel rapid symptom relief every time they use the SABA, whereas they feel no immediate benefit from inhaling ICS. This is often the reason for poor adherence to their ICS-based maintenance regimen8. In the seven-country AIRE study, SABA use was ~3 times greater than ICS use over a 4-week period, and in Italy and France recent ICS use was reported by less than one in nine patients who reported recent use of SABA35. Other studies show a similar imbalance in the ratio of ICS maintenance to SABA reliever treatment use (Fig. 6).
Reliance on SABA and underuse of anti-inflammatory treatment in the AIRE survey. Adapted from Rabe et al.31.
Overall, maintenance medication adherence rates in patients with asthma have consistently been shown to be around 30–40% in practice, with a systematic review finding that 24% of exacerbations and 60% of asthma-related hospitalisations could be attributed to poor adherence to ICS55,56. In the REALISE study, some 52% of patients prescribed daily anti-inflammatory maintenance treatment (n = 3481) did not take this medication daily29. The INSPIRE study gave similar results, with 50% of patients saying they adjusted how much ICS/LABA they took according to how they felt, 25% of patients stating that they did not feel they needed to take their maintenance therapy everyday when they felt well and 74% having used their SABA everyday in the previous week.
Possibly because of inadequate asthma education/knowledge and lack of advice and follow-up, many patients do not understand that the need for reliever use is a sign of deteriorating asthma and that they need instead to increase their anti-inflammatory controller medication2,29. Use of SABA alone can mask increasing inflammation until it reaches a level that requires urgent medical attention. Regular or frequent use of β2-agonists is associated with adverse effects including β-receptor downregulation57, reduced bronchoprotection against constrictor stimuli58, rebound bronchial hyperresponsiveness and reduced bronchodilator response to β-agonist during acute bronchoconstriction57. Indeed, it has been shown that SABA used alone induces production of the proinflammatory cytokine IL-6 and this production is significantly augmented during virus infection59. Concurrent treatment with ICS reduces these adverse effects59,60,61.
Using ≥3 ×200-dose canisters of SABA a year was associated with double the risk of an asthma-related exacerbation in one study20. Every additional SABA canister was associated with an 8–14% and 14–18% increase in risk of an asthma-related exacerbation in children and adults, respectively20. Multivariate analyses in adults (n = 35,864) also showed that the risk of hospitalisation was significantly associated with prescription of SABA inhalers above a baseline of 1–3 per year (4–12 SABA: OR 1.71; 95% CI 1.20–2.46)22. In a Canadian database analysis (n = 343,520), inappropriate use of SABAs in any 1-year period was associated with a 45% (OR 1.45, 95% CI 1.26-1.66) increase in the risk of asthma-related admissions in the following 3-month period23. An earlier study had demonstrated that dispensing of ≥2 SABA canisters a month was associated with increased risk of death62. The UK Royal College of Physicians in their recent National Review of Asthma Deaths found a similar association between risk of death from asthma and prescription of ≥12 SABA inhalers per year6. They also found evidence of previous under-prescribing of preventer ICS medication for those patients who died6.
This reliance on SABA treatment is reinforced by its rapid relief of symptoms, its prominence in emergency primary care and hospital management of exacerbations, and, in many countries, its low cost and availability. Repeat prescriptions for SABA may be given through online systems, email or by telephone, and in some countries patients can get their SABA ‘over the counter’. Consequently, patients may not have their use of medication reviewed or their asthma control re-assessed.
How can we improve asthma control and prevent exacerbations?
Approaches to improving asthma control include patient and HCP education, regular review and assessment of asthma status and inhaler technique along with use of a wide range of interventions and technologies to try to improve adherence to inhaled asthma medications63. These include enhancing communication skills, structured frameworks such as SIMPLES (Smoking status, Inhaler technique, Monitoring, Pharmacotherapy, Lifestyle, Education, Support) and various forms of electronic monitoring, with or without reminders for when to take controller medication63,64.
Improving adherence
A recent Cochrane review included 28 studies regarding a range of interventions to improve adherence to ICS maintenance therapy for asthma65. The authors concluded that patient education, electronic trackers or reminders and simplified regimens generally improved adherence but did not consistently translate into observable benefit for clinical outcomes65. In a study by Foster et al.66, despite personalised adherence discussions or inhaler reminders and feedback in connection with prescribed fixed combination ICS/LABA controller therapy, adherence decreased over 6 months to as low as ~38% with personalised advice whilst electronically measured adherence decreased to 60% even in patients given inhaler reminders and feedback. Even after potentially life-threatening emergency department visits, adherence to ICS maintenance decreased to 50% within the first week after discharge67.
Asthma action plans
Optimal self-management involving provision of a written asthma action plan was shown to reduce unscheduled primary care visits and hospitalisations in a Cochrane review68. However, education alone was not included in the analysis as previous work had shown that without an action plan, self-monitoring or regular review, information-only education had no significant impact on objective health outcomes68. All asthmatics should be offered a self-management action plan that advises them how to recognise and respond to a deterioration in their level of asthma control69. Self-management plans that involve patients doubling their ICS dose when symptoms worsen do not appear to be fully effective in preventing exacerbations70,71, although it has recently been shown that a temporary four-fold increase in ICS had some beneficial effects72.
Regular asthma assessment
Regular systematic asthma reviews, at least once a year, have been shown to help improve asthma control and reduce exacerbations6,18. Reviews are an ideal opportunity for the practitioner to check the patient’s inhaler technique, discuss adherence to maintenance therapy, reinforce patient education on asthma and its treatment and, for smokers, to discuss the benefits of cessation18 (Table 3). In an observational study (PACEHR) of 18,724 patients with asthma in Sweden, 96% had mild-to-moderate asthma and 4% had severe asthma requiring high-dose ICS and a second controller. Only a minority of patients had their asthma assessed in the year prior to the index date, and of the patients with severe asthma, only one in five had visited a specialist in secondary care in the year before and after an index date73. Many patients do not visit their primary care doctor or nurse for routine asthma reassessments and rely on their SABA to manage symptoms as they occur28,29.
Face-to-face interactions with a general practitioner or asthma nurse during an asthma review can motivate improvements in adherence and enable the individual’s asthma severity and inhaler technique to be assessed74. Inadequate inhalation technique has been observed in up to 90% of patients and is associated with poor asthma control and more frequent visits to emergency departments49,75,76. Even after training, 50% of people with poor technique revert to their old habits or develop new errors over time49, emphasising the need for regular checks on inhalation technique to avoid ineffective treatment and waste of medication1,3,49. During a review, asthma guidelines recommend asking about asthma symptom breakthroughs and SABA use/week, night-time awakening/coughing or exacerbations, testing lung function and using an objective assessment tool such as the ACT or ACQ.
Mental health, co-morbidities, poverty, drug abuse, financial hardship, poor literacy, pet ownership and many other personal factors can also affect a patient’s self-management of asthma and are factors that could potentially be detected and addressed during face-to-face interviews with patients77,78.
Smoking cessation and weight loss
Asthma reviews are also an opportunity for the clinician to recommend smoking cessation to patients who continue to smoke tobacco, offering treatment if the patient agrees. Patients who smoke should be told that due to smoking, their asthma control is worse, lung function decline is faster and they have a higher risk for hospitalisation79,80. The permeability of airway mucosa is increased in smokers, which could increase clearance of ICS from the airways81. Smoking also decreases histone deacetylase activity, which can reduce the ability of ICS to suppress inflammatory cytokine production (steroid resistance)81. Similarly, advice and recommendations on weight loss can be provided to patients with a high BMI, as this, like smoking, is a significant risk factor for poor asthma control and exacerbations43 and studies have shown that a 5–10% reduction in body weight improves asthma control and lung function82,83.
Exposure avoidance
Avoiding or minimising exposure to allergens and environmental irritants/pollutants can help patients with allergic or occupational asthma, although few studies have shown significant results from allergen avoidance alone84. However, recent studies using an overhead cooled laminar airflow filter device to displace aeroallergens from the breathing zone overnight in patient’s bedrooms improved quality of life and reduced airway inflammation (FeNO) and markers of systemic allergy (IgE and eosinophils) in patients with persistent atopic asthma84,85. Such devices are a new form of non-digital technology that may benefit patients with asthma in future.
How can we bring asthma care into the twenty-first century?
Digital technology
A growing number of asthma Apps are being developed for use on smartphones and other electronic devices86,87. These have the potential to aid self-management and to serve as useful tools in the patient−doctor relationship88. They can track the individual patient’s use of treatment and peak flow readings, provide dose reminders and help them to avoid exacerbation triggers such as high pollen counts or peaks in air pollution86,89. Some provide information on asthma, instructions and information on asthma medications, and what to do if symptoms worsen87.
Digitally enabled inhalers (so-called ‘smart inhalers’) are also becoming available, which can monitor medication use (time, date, number of inhalations) and, when connected wirelessly to a mobile phone, can send medication alerts/reminders for scheduled doses, which can improve both adherence and asthma-related health outcomes90,91. Data obtained from such devices can be used to deliver self-management interventions tailored to the specific needs of patients, thus increasing the efficacy of such interventions. Digitally enabled inhalers can also help to discriminate between patients with severe asthma and those who have poor inhaler technique and/or poor adherence92. These inhalers could help to identify patients with a genuine need for the newly available biologic therapies.
Portable spirometers and FeNO metres are also becoming more affordable and thus more widely available in primary care, enabling more accurate assessment of lung function and airway inflammation to be conducted by GPs or asthma nurses93.
The GINA 2019 update
GINA describes this update as the biggest change to asthma management proposed in over 30 years. Single inhaler ICS/formoterol is now recommended as the preferred reliever in place of SABA alone across the full spectrum of asthma severity (only for patients already on ICS/formoterol maintenance at GINA steps 3–5)18 (Fig. 3). Recommending use of an anti-inflammatory combination reliever for this inflammatory disease, rather than SABA alone, which can worsen inflammation8, resolves a major paradox in most previous guidelines. The new approach proposed by GINA 2019 had already been suggested by a consortium of international experts on asthma management in the Lancet Commission 2017 2. A large body of data already exists on the efficacy and safety of the budesonide/formoterol combination when used as an as-needed reliever medication in moderate-to-severe asthma94,95,96,97,98. There is also a study showing that symptom-driven use of beclometasone/salbutamol as reliever was as effective as regular use of beclometasone, with a lower cumulative ICS dose99. There are currently no data demonstrating the efficacy and safety of combining ICS/formoterol with maintenance ICS/LABA treatment that does not contain formoterol.
In mild asthma, as-needed use of budesonide/formoterol was shown in the recent SYGMA studies to be more effective and better tolerated than SABA alone94,95,100,101, and was clinically equivalent to daily maintenance therapy with budesonide with as-needed SABA as reliever, in terms of asthma control94,95,101. Use of as-needed budesonide/formoterol in SYGMA 1 reduced the rate of severe asthma exacerbations by 64% and the rate of moderate to severe exacerbations by 60% versus SABA alone while the severe exacerbation rates did not significantly differ between the as-needed budesonide/formoterol group and the budesonide maintenance group94. In the SYGMA 2 trial, as-needed budesonide/formoterol and maintenance budesonide were also equipotent in reducing the rate of severe exacerbations95. Importantly, however, the median daily doses of ICS were considerably lower with as-needed budesonide/formoterol than with daily maintenance therapy (metered dose, 57 vs. 340 μg and 66 vs. 267 μg, in SYGMA 1 and 2 respectively)94,95. The 52-week PRACTICAL and Novel START studies have confirmed these findings in a more pragmatic, real-world open label setting in which as-needed budesonide/formoterol was more effective at preventing severe asthma exacerbations than low-dose maintenance budesonide plus as-needed terbutaline, with a lower daily mean dose of budesonide (difference in PRACTICAL of 126.5 μg per day vs. maintenance; 95% CI −171.0 to −81.9; p < 0·001)100,101.
The period of worsening symptoms that usually precedes an exacerbation appears to be a ‘window of opportunity’, during which the extra doses of ICS provided by as-needed ICS/formoterol may be able to suppress the inflammatory flare-up and prevent the exacerbation from occurring or reduce its severity28,102. As-needed budesonide/formoterol has a significant advantage over as-needed SABA in that it provides the required immediate relief simultaneously with an anti-inflammatory boost of ICS during the ‘window of opportunity’2,8,94. Studies have shown that when symptoms appear or worsen, most patients instinctively reach for their SABA to relieve the symptoms and increase their use of this medication, rather than the controller needed to reduce the increased inflammation causing the worsening2,8,28,29 (Fig. 7).
Increasing short-acting β2-agonist (SABA) use but not inhaled corticosteroids (ICS) use when symptoms worsen. Adapted from Partridge et al.28.
Add-on therapies
Another recent change to asthma management is the use of LAMA as an add-on to ICS or ICS/LABA maintenance therapy for moderate-to-severe asthma. Single inhaler ICS/LABA/LAMA combinations are already in development and will soon be available103. A systematic review comparing add-on LAMA with add-on LABA found that people taking LAMA + ICS scored slightly less well for quality of life and asthma control and had adverse events more often than those taking LABA + ICS104. As an add-on to ICS/LABA in patients with poorly controlled asthma, LAMA significantly increased the time to first exacerbation and gave a modest improvement in lung function in two randomised controlled trials with 912 patients105.
LRTAs are recommended in asthma guidelines as an alternative or add-on controller, but a systematic review concluded that ICS were more effective in both adults and children, particularly in patients with moderate airway obstruction106.
Biologics
For patients with specific subtypes of severe asthma, such as eosinophilic asthma, a number of biologics have been developed for use when conventional therapy, and systematic assessment and optimising therapy of co-morbidities, does not lead to acceptable asthma control26. These include omalizumab, which targets IgE, mepolizumab, reslizumab, and benralizumab, which all target pathways to reduce eosinophil counts, and dupilumab which targets the interleukins IL-4 and IL-13. These are different approaches to reducing the underlying eosinophilic or Type 2 inflammation in asthma and have been shown to reduce exacerbation rates and improve asthma control26. All require injection, adding inconvenience to their already considerable costs, but for some patients they represent a real breakthrough in efficacy against their severe asthma, while avoiding exacerbations and hospitalisations can also make them cost-effective in the right patients32.
Which patients should be referred to asthma specialists?
Patients with difficult-to-treat asthma should be systematically assessed to find out if they have a severe asthma or other reasons explaining their poor response to treatment, such as poor adherence or inappropriate treatment. For patients who do not respond to standard step care management and have poor asthma control despite good adherence and inhalation technique, including management of environmental exposures and co-morbidities, referral to a specialist is clearly essential1,18,107,108.
Discussion
Despite improvements in understanding, availability of evidence-based management guidelines and improved medications and devices, approximately half of all patients with asthma are still not optimally controlled. A range of factors is responsible for this situation—the fluctuating nature of the disease, patients’ reluctance to take ‘steroids’ when feeling well, typical patient relief-seeking behaviour favouring SABA over ICS, the costs of medication, the different asthma phenotype responses to treatment, misperceptions of what asthma control means in practice and lack of interest in or knowledge of asthma among HCPs.
The twenty-first century now offers clinicians a range of new and different options to improve asthma control and help patients avoid exacerbations. Improved guidelines, electronic monitoring, smartphone apps, FeNO metres, portable spirometers, easy-to-use inhalers and, for patients with very severe asthma, biologic therapies, are all options that were unavailable to previous generations. Of course, regular review and assessment by knowledgeable physicians and specialist nurses, weight loss and smoking cessation will continue to play important roles, with or without these newer options.
However, the greatest impact on future care for the majority of patients, those with mild-to-moderate asthma, may come when the recommendations concerning ICS/formoterol as preferred reliever across the asthma severity spectrum are fully implemented. This would prevent over-reliance on SABA and ensure that patients receive a dose of anti-inflammatory ICS whenever they feel the need for additional relief of symptoms.
References
GINA. Global Strategy for Asthma Management and Prevention (Global Initiative for Asthma, 2018).
Pavord, I. D. et al. After asthma: redefining airways diseases. Lancet 391, 350–400 (2018).
BTS/SIGN. British Guideline on the Management of Asthma (British Thoracic Society & Scottish Intercollegiate Guidelines Network, 2019).
NICE. Asthma: Diagnosis, Monitoring and Chronic Asthma Management (National Institute for Health and Care Excellence, 2017).
National Heart Lung and Blood Institute. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma (EPR-3) (National Heart Lung and Blood Institute, 2007).
Royal College of Physicians. Why Asthma Still Kills: The National Review of Asthma Deaths (NRAD) Confidential Enquiry Report (RCP, London, 2014).
Janson, C. et al. Prevalence, characteristics and management of frequently exacerbating asthma patients: an observational study in Sweden (PACEHR). Eur. Respir. J. 52, 1701927 (2018).
O’Byrne, P. M., Jenkins, C. & Bateman, E. D. The paradoxes of asthma management: time for a new approach? Eur. Respir. J. 50, 1701103 (2017).
The Global Asthma Network. The Global Asthma Report 2018 (Auckland, New Zealand, 2018).
Tanaka, A. Past, present and future therapeutics of asthma: a review. J. Gen. Fam. Med. 16, 158–169 (2015).
Heffler, E. et al. Misdiagnosis of asthma and COPD and underuse of spirometry in primary care unselected patients. Respir. Med. 142, 48–52 (2018).
Tuomisto, L. E. et al. A 12-year prognosis of adult-onset asthma: Seinäjoki Adult Asthma Study. Respir. Med. 117, 223–229 (2016).
National Asthma Council Australia. Australian Asthma Handbook 2.0. (National Asthma Council Australia, 2019).
Dahl, R. & Bjermer, L. Nordic consensus report on asthma management. Nordic Asthma Consensus Group. Respir. Med. 94, 299–327 (2000).
Haahtela, T. et al. Update on current care guidelines: asthma. Duodecim 129, 994–995 (2013).
Muneswarao, J. et al. It is time to change the way we manage mild asthma: an update in GINA 2019. Respir. Res. 20, 183 (2019).
Dusser, D. et al. Mild asthma: an expert review on epidemiology, clinical characteristics and treatment recommendations. Allergy 62, 591–604 (2007).
Global Initiative for Asthma. 2019 GINA Main Report. www.ginasthma.org/reports (2019).
Gauvreau, G. M., Jordana, M., Watson, R. M., Cockroft, D. W. & O’Byrne, P. M. Effect of regular inhaled albuterol on allergen-induced late responses and sputum eosinophils in asthmatic subjects. Am. J. Respir. Crit. Care Med. 156, 1738–1745 (1997).
Stanford, R. H., Shah, M. B., D’Souza, A. O., Dhamane, A. D. & Schatz, M. Short-acting β-agonist use and its ability to predict future asthma-related outcomes. Ann. Allergy Asthma Immunol. 109, 403–407 (2012).
Wraight, J. M. et al. Adverse effects of short-acting beta-agonists: potential impact when anti-inflammatory therapy is inadequate. Respirology 9, 215–221 (2004).
Hull, S. A. et al. Asthma prescribing, ethnicity and risk of hospital admission: an analysis of 35,864 linked primary and secondary care records in East London. npj Prim. Care Respir. Med. 26, 16049 (2016).
FitzGerald, J. M., Tavakoli, H., Lynd, L. D., Al Efraij, K. & Sadatsafavi, M. The impact of inappropriate use of short acting beta agonists in asthma. Respir. Med. 131, 135–140 (2017).
O’Byrne, P. M., Naya, I. P., Kallen, A., Postma, D. S. & Barnes, P. J. Increasing doses of inhaled corticosteroids compared to adding long-acting inhaled β2-agonists in achieving asthma control. Chest 134, 1192–1199 (2008).
Bateman, E. D. et al. Can guideline-defined asthma control be achieved? The Gaining Optimal Asthma ControL study. Am. J. Respir. Crit. Care Med. 170, 836–844 (2004).
Godar, M., Blanchetot, C., de Haard, H., Lambrecht, B. N. & Brusselle, G. Personalized medicine with biologics for severe type 2 asthma: current status and future prospects. MAbs 10, 34–45 (2018).
Murdoch, J. R. & Lloyd, C. M. Chronic inflammation and asthma. Mutat. Res. 690, 24–39 (2010).
Partridge, M. R., van der Molen, T., Myrseth, S. E. & Busse, W. W. Attitudes and actions of asthma patients on regular maintenance therapy: the INSPIRE study. BMC Pulm. Med. 6, 13 (2006).
Price, D., Fletcher, M. & van der Molen, T. Asthma control and management in 8,000 European patients: the REcognise Asthma and LInk to Symptoms and Experience (REALISE) survey. npj Prim. Care Respir. J. 24, 14009 (2014).
Demoly, P., Annunziata, K., Gubba, E. & Adamek, L. Repeated cross-sectional survey of patient-reported asthma control in Europe in the past 5 years. Eur. Respir. Rev. 21, 66–74 (2012).
Rabe, K. F., Vermeire, P. A., Soriano, J. B. & Maier, W. C. Clinical management of asthma in 1999: the Asthma Insights and Reality in Europe (AIRE) study. Eur. Respir. J. 16, 802–807 (2000).
Ställberg, B. et al. Asthma control in primary care in Sweden: a comparison between 2001 and 2005. Prim. Care Respir. J. 18, 279–286 (2009).
Ebmeier, S. et al. Trends in international asthma mortality: analysis of data from the WHO Mortality Database from 46 countries (1993–2012). Lancet 390, 935–945 (2017).
Olaguibel, J. M. et al. Measurement of asthma control according to Global Initiative for Asthma guidelines: a comparison with the Asthma Control Questionnaire. Respir. Res. 13, 50 (2012).
Vermeire, P. A., Rabe, K. F., Soriano, J. B. & Maier, W. C. Asthma control and differences in management practices across seven European countries. Respir. Med. 96, 142–149 (2002).
Backer, V., Bornemann, M., Knudsen, D. & Ommen, H. Scheduled asthma management in general practice generally improve asthma control in those who attend. Respir. Med. 106, 635–641 (2012).
Schuler, M., Faller, H., Wittmann, M. & Schultz, K. Asthma Control Test and Asthma Control Questionnaire: factorial validity, reliability and correspondence in assessing status and change in asthma control. J. Asthma 53, 438–445 (2016).
Haughney, J., Barnes, G., Partridge, M. & Cleland, J. The Living & Breathing Study: a study of patients’ views of asthma and its treatment. Prim. Care Respir. J. 13, 28–35 (2004).
Ilmarinen, P. et al. Effect of asthma control on general health-related quality of life in patients diagnosed with adult-onset asthma. Sci. Rep. 9, 16107 (2019).
Siroux, V. et al. Quality-of-life and asthma-severity in general population asthmatics: results of the ECRHS II study. Allergy Asthma Proc. 63, 547–554 (2008).
Sears, M. R. Can we predict exacerbations of asthma? Am. J. Respir. Crit. Care Med. 199, 399–400 (2019).
Price, D. et al. Predicting frequent asthma exacerbations using blood eosinophil count and other patient data routinely available in clinical practice. J. Asthma Allergy 9, 1–12 (2016).
Bateman, E. D. et al. Development and validation of a novel risk score for asthma exacerbations: the risk score for exacerbations. J. Allergy Clin. Immunol. 135, 1457–1464 (2015).
Pavord, I. D. et al. The impact of poor asthma control among asthma patients treated with inhaled corticosteroids plus long-acting β2-agonists in the United Kingdom: a cross-sectional analysis. npj Prim. Care Respir. J. 27, 17 (2017).
Ekström, M. et al. Oral corticosteroid use, morbidity and mortality in asthma: a nationwide prospective cohort study in Sweden. Allergy. https://doi.org/10.1111/all.13874 (2019).
Price, D. B. et al. Adverse outcomes from initiation of systemic corticosteroids for asthma: long-term observational study. J. Asthma Allergy 11, 193–204 (2018).
Sullivan, P. W., Ghushchyan, V. H., Globe, G. & Schatz, M. Oral corticosteroid exposure and adverse effects in asthmatic patients. J. Allergy Clin. Immunol. 141, 110–116 (2018).
Sumino, K. & Cabana, M. D. Medication adherence in asthma patients. Curr. Opin. Pulm. Med. 19, 49–53 (2013).
McFadden, E. R. Improper patient techniques with metered dose inhalers: Clinical consequences and solutions to misuse. J. Allergy Clin. Immunol. 96, 278–283 (1995).
Barnes, P. J., Szefler, S. J., Reddel, H. K. & Chipps, B. E. Symptoms and perception of airway obstruction in asthmatic patients: clinical implications for use of reliever medications. J. Allergy Clin. Immunol. https://doi.org/10.1016/j.jaci.2019.06.040 (2019).
Papadopoulos, N. G. et al. International consensus on (ICON) pediatric asthma. Allergy 67, 976–997 (2012).
Ishmael, F. T. The inflammatory response in the pathogenesis of asthma. J. Am. Osteopath. Assoc. 111, S11–S17 (2011).
Busse, W. W. & Lemanske, R. F. Expert Panel Report 3: moving forward to improve asthma care. J. Allergy Clin. Immunol. 120, 1012–1014 (2007).
Gibson, P. G., Saltos, N. & Fakes, K. Acute anti-inflammatory effects of inhaled budesonide in asthma: a randomized controlled trial. Am. J. Respir. Crit. Care Med. 163, 32–36 (2001).
Bårnes, C. B. & Ulrik, C. S. Asthma and adherence to inhaled corticosteroids: current status and future perspectives. Respir. Care 60, 455–468 (2015).
Klok, T., Kaptein, A. A., Duiverman, E. J. & Brand, P. L. It’s the adherence, stupid (that determines asthma control in preschool children)! Eur. Respir. J. 43, 783–791 (2014).
Hancox, R. J. et al. Bronchodilator tolerance and rebound bronchoconstriction during regular inhaled β-agonist treatment. Respir. Med. 94, 767–771 (2000).
O’Connor, B., Aikman, S. L. & Barnes, P. J. Tolerance to the nonbronchodilator effects of inhaled beta 2-agonists in asthma. NEJM 327, 1204–1208 (1992).
Edwards, M. R., Haas, J., Panettieri, R. A., Johnson, M. & Johnston, S. L. Corticosteroids and beta2 agonists differentially regulate rhinovirus-induced interleukin-6 via distinct Cis-acting elements. J. Biol. Chem. 282, 15366–15375 (2007).
Barnes, P. J. Scientific rationale for using a single inhaler for asthma control. Eur. Respir. J. 29, 587–595 (2007).
Lommatzsch, M. et al. Adverse effects of salmeterol in asthma: a neuronal perspective. Thorax 64, 763–769 (2009).
Suissa, S. et al. A cohort analysis of excess mortality in asthma and the use of inhaled β-agonists. Am. J. Respir. Crit. Care Med. 149, 604–610 (1994).
van Boven, J. et al. Enhancing respiratory medication adherence: the role of health care professionals and cost-effectiveness considerations. J. Allergy Clim. Immunol. Pr. 4, 835–846 (2016).
Ryan, D., Murphy, A., Ställberg, B., Baxter, N. & Heaney, L. SIMPLES: a structured primary care approach to adults with difficult asthma. Prim. Care Respir. J. 22, 365–373 (2013).
Normansell, R., Kew, K. M. & Stovold, E. Interventions to improve adherence to inhaled steroids for asthma. Cochrane Database Syst. Rev. 4, CD012226 (2017).
Foster, J. M. et al. Inhaler reminders improve adherence with controller treatment in primary care patients with asthma. J. Allergy Clin. Immunol. 134, 1260–1268 (2014).
Krishnan, J. A. et al. Corticosteroid use after hospital discharge among high-risk adults with asthma. Am. J. Respir. Crit. Care Med. 170, 1281–1285 (2004).
Gibson, P. G. et al. Self‐management education and regular practitioner review for adults with asthma. Cochrane Database Syst. Rev. 3, CD001117 (2009).
Pinnock, H. et al. Systematic meta-review of supported self-management for asthma: a healthcare perspective. BMC Med. 15, 64 (2017).
Oborne, J., Mortimer, K., Hubbard, R. B., Tattersfield, A. E. & Harrison, T. W. Quadrupling the dose of inhaled corticosteroid to prevent asthma exacerbations: a randomized, double-blind, placebo-controlled, parallel-group clinical trial. Am. J. Respir. Crit Care Med. 180, 598–602 (2009).
Kew, K. M., Quinn, M., Quon, B. S. & Ducharme, F. M. Increased versus stable doses of inhaled corticosteroids for exacerbations of chronic asthma in adults and children. Cochrane Database Syst. Rev. 6, CD007524–CD007524 (2016).
McKeever, T. et al. Quadrupling inhaled glucocorticoid dose to abort asthma exacerbations. N. Engl. J. Med. 378, 902–910 (2018).
Larsson, K. et al. Prevalence and management of severe asthma in primary care: an observational cohort study in Sweden (PACEHR). Respir. Res. 19, 12 (2018).
Kotwani, A. & Chhabra, S. K. Effect of patient education and standard treatment guidelines on asthma control: an intervention trial. WHO SE Asia J. Public Health 1, 42–51 (2012).
Jahedi, L., Downie, S. R., Saini, B., Chan, H.-K. & Bosnic-Anticevich, S. Inhaler technique in asthma: how does it relate to patients’ preferences and attitudes toward their inhalers? J. Aerosol Med. Pulm. Drug Deliv. 30, 42–52 (2017).
Capstick, T. G. D. & Clifton, I. J. Inhaler technique and training in people with chronic obstructive pulmonary disease and asthma. Expert Rev. Respir. Med. 6, 91–103 (2012).
Gandhi, P. et al. Exploring factors influencing asthma control and asthma-specific health-related quality of life among children. Respir. Res. 14, 26 (2013).
Miles, C. et al. Barriers and facilitators of effective self-management in asthma: systematic review and thematic synthesis of patient and healthcare professional views. NPJ Prim. Care Respir. Med. 27, 57 (2017).
Tommola, M. et al. The effect of smoking on lung function: a clinical study on adult-onset asthma. Eur. Respir. J. 48, 1298–1306 (2016).
Tommola, M. et al. Cumulative effect of smoking on disease burden and multimorbidity in adult-onset asthma. Eur. Respir. J. 54, 1801580 (2019).
Stapleton, M., Howard-Thompson, A., George, C., Hoover, R. M. & Self, T. H. Smoking and asthma. J. Am. Board Fam. Med. 24, 313–322 (2011).
Stenius-Aarniala, B. et al. Immediate and long term effects of weight reduction in obese people with asthma: randomised controlled study. Bmj 320, 827–832 (2000).
Aaron, S. D. et al. Effect of weight reduction on respiratory function and airway reactivity in obese women. Chest 125, 20146–20152 (2004).
Warner, J. O. Use of temperature-controlled laminar airflow in the management of atopic asthma: clinical evidence and experience. Ther. Adv. Respir. Dis. 11, 181–188 (2017).
Boyle, R. J. et al. Nocturnal temperature controlled laminar airflow for treating atopic asthma: a randomised controlled trial. Thorax 67, 215–221 (2012).
Kagen, S. & Garland, A. Asthma and allergy mobile apps in 2018. Curr. Allergy Asthma Rep. 19, 6 (2019).
Tinschert, P., Jakob, R., Barata, F., Kramer, J. N. & Kowatsch, T. The potential of mobile apps for improving asthma self-management: a review of publicly available and well-adopted asthma apps. JMIR Mhealth Uhealth 5, e113 (2017).
Himes, B. E., Leszinsky, L., Walsh, R., Hepner, H. & Wu, A. C. Mobile health and inhaler-based monitoring devices for asthma management. J. Allergy Clin. Immunol. Pr. 7, 2535–2543 (2019).
Cook, K. A., Modena, B. D. & Simon, R. A. Improvement in asthma control using a minimally burdensome and proactive smartphone application. J. Allergy Clin. Immunol. Pr. 4, 730–737 (2016).
Chan, A. et al. The effect of an electronic monitoring device with audiovisual reminder function on adherence to inhaled corticosteroids and school attendance in children with asthma: a randomised controlled trial. Lancet Respir. Med. 3, 210–219 (2015).
Kuipers, E., Wensing, M., de Smet, P. & Teichert, M. Self-management research of asthma and good drug use (SMARAGD study): a pilot trial. Int. J. Clin. Pharm. https://doi.org/10.1007/s11096-017-0495-6 (2017).
Jochmann, A. et al. Electronic monitoring of adherence to inhaled corticosteroids: an essential tool in identifying severe asthma in children. Eur. Respir. J. 50, 1700910 (2017).
Funston, W. & Higgins, B. Improving the management of asthma in adults in primary care. Practitioner 258, 15–19 (2014).
O’Byrne, P. et al. Inhaled combined budesonide–formoterol as needed in mild asthma. N. Engl. J. Med. 378, 1865–1876 (2018).
Bateman, E. D. et al. As-needed budesonide–formoterol versus maintenance budesonide in mild asthma. N. Engl. J. Med. 378, 1877–1887 (2018).
Kuna, P. et al. Effect of budesonide/formoterol maintenance and reliever therapy on asthma exacerbations. J. Clin. Pr. 61, 725–736 (2007).
Bateman, E. D. et al. Overall asthma control achieved with budesonide/formoterol maintenance and reliever therapy for patients on different treatment steps. Respir. Res. 12, 38 (2011).
Lazarinis, N. et al. Combination of budesonide/formoterol on demand improves asthma control by reducing exercise-induced bronchoconstriction. Thorax 69, 130–136 (2014).
Papi, A. et al. Rescue use of beclomethasone and albuterol in a single inhaler for mild asthma. N. Engl. J. Med. 356, 20140–20152 (2007).
Beasley, R. et al. Controlled trial of budesonide-formoterol as needed for mild asthma. N. Engl. J. Med. 380, 2020–2030 (2019).
Hardy, J. et al. Budesonide-formoterol reliever therapy versus maintenance budesonide plus terbutaline reliever therapy in adults with mild to moderate asthma (PRACTICAL): a 52-week, open-label, multicentre, superiority, randomised controlled trial. The Lancet. https://doi.org/10.1016/S0140-6736(19)31948-8 (2019).
Balter, M. et al. Asthma worsenings: approaches to prevention and management from the Asthma Worsenings Working Group. Can. Respir. J. 15, 1B–19B (2008).
Virchow, J. C. et al. Single inhaler extrafine triple therapy in uncontrolled asthma (TRIMARAN and TRIGGER): two double-blind, parallel-group, randomised, controlled phase 3 trials. Lancet 394, 1737–1749 (2019).
Kew, K. M., Evans, D. J., Anderson, D. E. & Boyter, A. C. Long-acting muscarinic antagonists (LAMA) added to inhaled corticosteroids (ICS) versus addition of long-acting beta2-agonists (LABA) for adults with asthma. Cochrane Database Syst. Rev. 2015, CD011438 (2015).
Kerstjens, H. A. et al. Tiotropium in asthma poorly controlled with standard combination therapy. N. Engl. J. Med. 367, 1198–1207 (2012).
Chauhan, B. F. & Ducharme, F. M. Anti-leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/or chronic asthma in adults and children. Cochrane Database of Systematic Reviews, CD002314, https://doi.org/10.1002/14651858.CD002314.pub3 (2012).
Chung, K. F. et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur. Respir. J. 43, 343–373 (2014).
Porsbjerg, C. et al. Nordic consensus statement on the systematic assessment and management of possible severe asthma in adults. Eur. Clin. Respir. J. 5, 1440868 (2018).
Tommola, M., Ilmarinen, P., Tuomisto, L. E. & Kankaanranta, H. Differences between asthma-COPD overlap syndrome and adult-onset asthma. Eur. Respir. J. 49, pii 1602383 (2017).
Janson, C. et al. Bronchodilator reversibility in asthma and COPD: Findings from three large population studies. Eur. Respir. J. pii: 1900561 (2019).
Acknowledgements
This review was supported by AstraZeneca. The authors would like to thank David Candlish of inScience Communications, Springer Healthcare, Ltd, UK, for providing medical writing support, which was funded by AstraZeneca in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3). Open access funding provided by Karolinska Institute.
Author information
Authors and Affiliations
Contributions
All authors made substantial contributions to the concept, analysis and interpretation of data, and to the drafting of the manuscript or revising it critically for important intellectual content. In addition, all authors provided final approval of the manuscript.
Corresponding author
Ethics declarations
Competing interests
K.L. has, during the last 5 years, on one or more occasion served in an advisory board and/or served as speaker and/or participated in education arranged by AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Orion, Novartis, and Teva. H.K. has received institutional grants, personal fees and non-financial support from AstraZeneca, personal fees from Chiesi Pharma AB, personal fees and non-financial support from Boehringer Ingelheim, personal fees from Novartis, personal fees from Mundipharma, personal fees and non-financial support from Orion Pharma, personal fees from SanofiGenzyme, personal fees from GlaxoSmithKline, outside the submitted work. C.J. has received honoraria for educational activities and lectures from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, Meda and Teva and has served on advisory boards arranged by AstraZeneca, Boehringer Ingelheim, Novartis, Meda, Teva and GlaxoSmithKline. L.L. has received honoraria for educational activities, lectures or advisory boards from ALK, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Mundipharma, Novartis, Orion, Sanofi and Teva. B.S. has received honoraria for educational activities and lectures from AstraZeneca, Boehringer Ingelheim, Novartis, Meda, and Teva and has served on advisory boards arranged by AstraZeneca, Boehringer Ingelheim, Novartis, Meda, Teva and GlaxoSmithKline. A.L. has received fees for lectures and for educational activities from AstraZeneca, Boehringer Ingelheim, Chiesi, Novartis, GlaxoSmithKline, Mundipharma and Novartis, and has participated in advisory boards arranged by AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Pfizer and Novartis. Furthermore, he has received grants for research from Pfizer, Boehringer Ingelheim and Novartis, as well as research-related travel accommodation from AstraZeneca, Boehringer Ingelheim, Chiesi, Novartis, GlaxoSmithKline, Mundipharma, Orion and Novartis. A.L. has previously been/is currently a principal investigator in pharmaceutical company-sponsored research studies for AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline and Novartis. K.H. has received honoraria for educational activities, lectures and advisory boards from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Mundipharma, Novartis, Orion Pharma, and Teva. K.R. has, during the last 5 years, on one or more occasion served in an advisory board and/or served as speaker and/or participated in education arranged by AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, and Teva. C.S.U. has received honoraria for educational activities, lectures and advisory boards from AstraZeneca, GlaxoSmithKline, Sanofi, Mundipharma, TEVA, Boehringer Ingelheim, Novartis, Orion Pharma, Actelion, ALK-Abello, and Sandoz.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Larsson, K., Kankaanranta, H., Janson, C. et al. Bringing asthma care into the twenty-first century. npj Prim. Care Respir. Med. 30, 25 (2020). https://doi.org/10.1038/s41533-020-0182-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41533-020-0182-2
This article is cited by
-
Documentation of comorbidities, lifestyle factors, and asthma management during primary care scheduled asthma contacts
npj Primary Care Respiratory Medicine (2024)
-
Participation in scheduled asthma follow-up contacts and adherence to treatment during 12-year follow-up in patients with adult-onset asthma
BMC Pulmonary Medicine (2022)
-
The Myth of Mild: Severe Exacerbations in Mild Asthma: An Underappreciated, but Preventable Problem
Advances in Therapy (2021)
-
Indacaterol/Glycopyrronium/Mometasone: A Review in Asthma
Drugs (2021)