Abstract
This study reports the association of ICS use and the risk of type 2 diabetes mellitus (T2DM) in Swedish patients with COPD using data from real-world, primary care settings. A total of 7078 patients with COPD were included in this analysis and the 5-year cumulative incidence rate per 100,000 person years was 1506.9. The yearly incidence rate per 100,000 person years ranged from 850 to 1919. Use of ICS especially at a high dose in patients with COPD was related to an increased risk of T2DM.
Similar content being viewed by others
Introduction
The effect of inhaled corticosteroids (ICSs) on the risk of diabetes in patients with chronic obstructive pulmonary disease (COPD) remains uncertain. Some studies suggest an increased risk of onset and progression of diabetes, specifically associated with high-dose ICSs1,2,3,4, while the other studies and reviews of controlled trials report no such association5,6. The Swedish national guidelines during the study period only recommended the use of ICS in combination with long-acting beta-2 agonists (LABA) for patients with COPD with a forced expiratory volume in 1 s <50% predicted and a history of exacerbations7. However, real-world studies indicate that ICSs/LABA are often used in Swedish patients in Global Initiative for Obstructive Lung Disease group A and B8 indicating that more patients could be exposed to the potential risks of ICS treatment than necessary. This study assessed the risk of type 2 diabetes mellitus (T2DM) associated with ICSs in Swedish patients with COPD.
Results
Study patients
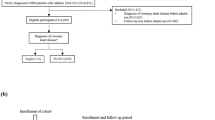
A total of 7078 patients with COPD were included in this analysis and T2DM was reported in 418 (5.9%) patients. The 5-year cumulative incidence rate per 100,000 person years was 1506.9 and the yearly incidence rate per 100,000 person years ranged from 850 to 1919. The mean age of the total study population was 68.6 years and comprised of 55.7% females. Regarding the T2DM patients, the mean age was 67.4 years and 47.1% females. The follow-up time (in person-years) in the different ICS groups ranged from 365 to 11679 and from 11.5 to 464 in patients without T2DM and patients with T2DM, respectively. Among T2DM patients, the main ICS treatment groups were “no ICS stable dose” (n = 147), “low ICS stable dose” (n = 138), and “high ICS stable dose” (n = 35). Time to T2DM diagnosis was not statistically different across the groups.
Risk of type 2 diabetes
For almost all groups, except the low-dose ICS groups with mixed ICS dosage and decrease or increase in the ICS dosage, a significant increased risk of T2DM was observed compared to the reference population. A dose-related association was observed as the risk of T2DM was 32 and 64% higher among patients using stable continuous low- and high-dose ICS over the whole study period, respectively, compared to the reference controls using no ICS. Furthermore, for the patients who were using high-dose ICS, the risk of T2DM was 100% (increased usage) and 96% (mixed usage) higher compared to the reference group (Table 1). Further, adjustment for hypertension (I10) and/or heart failure (I50), did not significantly affect the results (data not shown).
These results confirm previous studies that exposure of ICS in COPD was associated with an increased risk of onset of diabetes2,4,9,10,11.
Methods
Study design and patients
We analysed data from ARCTIC, a real-world observational study of Swedish primary care patients with COPD12,13,14,15. Ethical approval for the study was obtained from the local Ethical Regional Board in Uppsala, Sweden on 11 December 2014 (number: 2014-397) for accessing the National Health Register and for recruiting primary care centres to the study. An amendment specifying additional analysis was approved by the Ethical Regional Board in Uppsala on 6 October 2017. Data from all records were de-identified, and therefore, patient consent was not required by the ethics committee.
Following ethical approval, electronic medical record (EMR) data linked to National Health Registries were collected from COPD patients in 52 Swedish primary care centres (2000–2014). The study population consisted of patients aged ≥40 years who had received a physician’s diagnosis of COPD (International Classification of Diseases, Tenth Edition (ICD-10) code: J44), with or without asthma (ICD-10 code: J45/J46) in a primary care setting (EMR database) or in a hospital setting (according to the National Patient Register). The information on ICS usage might be incomplete in the case notes from the general practitioners. Hence, for this analysis, the study population was limited to the patients diagnosed with COPD since 01 August 2005 as the national drug prescription register with full details of all dispensed medications was operational from July 2005. In order to focus exclusively on the effects of ICS, patients with more than one dispensation of oral corticosteroids (OCSs) or patients who received more than a normal pack, i.e. 25 tablets with 5 mg prednisolone or equivalent per tablet at any time point from 1 year prior to index and during the whole study period were excluded. We excluded 1490 patients due to OCSs, ending up with 7078 patients in the study population.
Statistical analyses
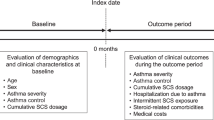
The study index date constituted the time of the first recorded physician’s diagnosis of COPD during the enrolment timeframe. In the Poisson regression model, the follow-up time from COPD index to event, i.e. either incidence of T2DM (ICD code E11 or E14, in EMR or inpatient/outpatient register, or if the patient had filled a prescription of TD2M drug, i.e. ATC code A10B) or end of study, whichever comes first was split into equally sized intervals of 365 days (the last time interval will be <365 days). Body mass index (BMI) was assessed within each of these time intervals plus allowing for 6 months of the next time interval (e.g., for the first year of follow-up, BMI was assessed from COPD diagnosis and going 18 months forward). The treatment pattern of ICS dosage over time varied among COPD patients, hence we have categorised the ICS treatment pattern during the study period into (i) stable continuous dosage during the whole study period, i.e. no ICS, low-dose ICS, and high-dose ICS; (ii) mixed ICS dosage; (iii) decrease in the ICS dosage, and (iv) increase in the ICS dosage. A patient is assigned mixed ICS dosage if the previous levels of ICS have been varying between the three main ICS categories (no ICS, low-dose ICS, and high-dose ICS). The decreased/increased ICS dosage is assigned if the patient is switching from high dose to low/no ICS dose or vice versa and maintaining the same direction of treatment pattern, i.e. decreasing or increasing. The reference population considered in the analysis are “no ICS stable dose at any time point” COPD patients. The dispensed amounts of different types of ICS (budesonide, fluticasone propionate, beclomethasone, rest of the R03BA group, and all steroid combinations in the R03AK group) were converted to budesonide equivalents. The average daily dosage of ICS was calculated based on the filled prescriptions in the given time interval and the patients were stratified by the level of ICS exposure after the index date until an event (high dose: ≥640 μg/day budesonide or equivalent; low dose: <640 μg/day budesonide or equivalent; and no ICS).
The last time interval of follow-up might vary in duration and a shorter follow-up period (<180 days) can result in misleading information on ICS dosage. To avoid this, the dosage for the last time interval was calculated based on the prescriptions of ICS received 6 months before the last follow-up interval. Patients without any follow-up or patients who have filled the first prescription within 3 months prior to the event were excluded from the analysis. Poisson regression was used to model the impact of ICS on the risk of T2DM among COPD patients (time to event). As the exposure time was split into equally sized intervals, the constant rates were assumed within the time interval. The analysis was presented as relative risk with 95% confidence interval adjusted for BMI, time since COPD, sex, and age. The statistical analysis software used was “PC SAS for Windows” Version 9.4 (SAS Institute Inc., Cary, NC).
Strengths and limitations
The present study has several important strengths. The real-world study design and the large sample size of high-quality national registry data from primary care settings adequately reflect the general population and clinical practice in Sweden. Nevertheless, this study also has certain limitations. The retrospective study design introduces the potential for bias and confounding due to variables that may not have been accounted for in our analysis. Although all patients had physician-diagnosed COPD, the accuracy of COPD diagnoses and the severity of disease could not be verified, as spirometry data were not available in many patients. Due to lack of spirometry data, it was not possible to assess the impact of disease severity on the incidence of T2DM. This study was conducted only in Swedish patients; it is therefore uncertain whether these findings can be extrapolated to a more diverse group of patients and to other healthcare systems.
Hence, we conclude that patients with COPD who initiate treatment with ICS, especially with high dose, may have an elevated risk of developing T2DM.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The data for this study were obtained from EMRs in primary care and the Swedish National Health Register. Restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are, however, available from IQVIA Solutions, Copenhagen, Denmark upon reasonable request and with permission of the Swedish National Health Register.
References
Mannino, D. M., Thorn, D., Swensen, A. & Holguin, F. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur. Respir. J. 32, 962–969 (2008).
Price, D. B. et al. Inhaled corticosteroids in COPD and onset of type 2 diabetes and osteoporosis: matched cohort study. NPJ Prim. Care Respir. Med. 29, 38 (2019).
Rana, J. S. et al. Chronic obstructive pulmonary disease, asthma, and risk of type 2 diabetes in women. Diabetes Care 27, 2478–2484 (2004).
Suissa, S., Kezouh, A. & Ernst, P. Inhaled corticosteroids and the risks of diabetes onset and progression. Am. J. Med. 123, 1001–1006 (2010).
Dendukuri, N., Blais, L. & LeLorier, J. Inhaled corticosteroids and the risk of diabetes among the elderly. Br. J. Clin. Pharmacol. 54, 59–64 (2002).
O’Byrne, P. M. et al. Risk of new onset diabetes mellitus in patients with asthma or COPD taking inhaled corticosteroids. Respir. Med. 106, 1487–1493 (2012).
Läkemedelverkets Medical Products Agency (Sweden): Läkemedelverkets expert panel. Farmakologisk behandling av kroniskt obstruktiv lungsjukdom (KOL)–behandlingsrekommendationer (In Swedish). https://lakemedelsverket.se/kol (2009).
Sundh, J. et al. Factors influencing pharmacological treatment in COPD: a comparison of 2005 and 2014. Eur. Clin. Respir. J. 4, 1409060 (2017).
Barnes, P. J. Inhaled corticosteroids in COPD: a controversy. Respiration 80, 89–95 (2010).
Price, D. B. et al. Metabolic effects associated with ICS in patients with COPD and comorbid type 2 diabetes: a historical matched cohort study. PLoS ONE 11, e0162903 (2016).
Saeed, M. I. et al. Use of inhaled corticosteroids and the risk of developing type 2 diabetes in patients with chronic obstructive pulmonary disease. Diabetes Obes. Metab. 22, 1348–1356 (2020).
Janson, C. et al. Identifying the associated risks of pneumonia in COPD patients: ARCTIC an observational study. Respir. Res. 19, 172 (2018).
Lisspers, K. et al. Gender differences among Swedish COPD patients: results from the ARCTIC, a real-world retrospective cohort study. NPJ Prim. Care Respir. Med. 29, 45 (2019).
Lisspers, K. et al. Economic burden of COPD in a Swedish cohort: the ARCTIC study. Int. J. Chron. Obstruct. Pulmon. Dis. 13, 275–285 (2018).
Stallberg, B. et al. Real-world retrospective cohort study ARCTIC shows burden of comorbidities in Swedish COPD versus non-COPD patients. NPJ Prim. Care Respir. Med. 28, 33 (2018).
Acknowledgements
This study was funded by Novartis Pharma AG (Basel, Switzerland). The authors would like to thank Harneet Kaur (Novartis) for providing writing assistance in the development of this manuscript.
Funding
Open Access funding provided by Uppsala University.
Author information
Authors and Affiliations
Contributions
All authors participated equally in the study conception, design, statistical analysis planning, and analysis and interpretation of the data and have reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
B.S. reports personal fees from AstraZeneca, Novartis, Boehringer Ingelheim, GlaxoSmithKline, Meda, Teva, and Chiesi, outside the submitted work. C.J. reports personal fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Novartis, and Teva, outside the submitted work. K. Lisspers reports personal fees from AstraZeneca, Novartis, Boehringer Ingelheim, GlaxoSmithKline, and Chiesi, outside the submitted work. G.J. has participated in the steering committee organised by Novartis for this study and served on advisory boards arranged by AstraZeneca, Novo Nordisk, and Takeda. F.S.G. and K.M. are employees of Novartis Pharma AG. B.K.B., L.J., and A.M.T.K. are employees of IQVIA and have received remuneration in relation to statistical analyses. K. Larsson has, during the past 5 years, on one or more occasion served in an advisory board, served as a speaker, and/or participated in education activities arranged by AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Chiesi, Sanofi, Novartis, Orion, and Teva.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ställberg, B., Janson, C., Lisspers, K. et al. Inhaled corticosteroids and the risk of type 2 diabetes among Swedish COPD patients. npj Prim. Care Respir. Med. 30, 47 (2020). https://doi.org/10.1038/s41533-020-00207-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41533-020-00207-7