Abstract
A significant subset of patients with metastatic breast cancer develops brain metastasis. As efficacy of systemic therapies has improved and patients live longer with metastatic breast cancer, the incidence of breast cancer brain metastases has increased. Brain metastases pose a clinical challenge in diagnosis, treatment, and monitoring across all breast cancer subtypes, and better tools are needed. Liquid biopsy, which enables minimally invasive sampling of a patient’s cancer, has the potential to shed light on intra-cranial tumor biology and to improve patient care by enabling therapy tailoring. Here we review current evidence for the clinical validity of liquid biopsy in patients with breast cancer brain metastases, with a focus on circulating tumor cells and circulating tumor DNA.
Similar content being viewed by others
Introduction
Central nervous system (CNS) metastases (including brain metastasis [BM] and leptomeningeal disease [LMD]) are the most common intracranial malignancy1. About one in five patients with metastatic breast cancer (MBC) will develop BM during their course of disease1. In autopsy studies, the prevalence is higher, reaching 40% of patients with lethal MBC1. Traditionally, BM were associated with very poor prognosis, however, novel therapies and treatment modalities are changing this paradigm in specific patient subsets. Patients with HER2-positive and triple-negative breast cancer subtypes have higher incidence of BM of up to 50% in some series with recent data suggesting an increase in the overall incidence of MBC-related BM (BCBM)2. This increase may be secondary to the efficacy of novel systemic therapies, specifically that of HER2-directed agents. LMD is a specific form of CNS metastasis, in which tumor cells infiltrate the leptomeninges, and associated with a dismal prognosis3. The clinical incidence of LMD is estimated to occur in up to 7% of breast cancer patients, enriched for patients with aggressive phenotypes (e.g., high grade, triple negative) and lobular histology4.
The pathophysiology of BM and LMD development is an area of active research. The mechanisms involved in tumor cells’ seeding are complex and involve multiple additional players, including the blood-brain barrier (BBB) and the microenvironment1. The BBB describes an insulating physiologic structure of microvasculature which closely regulates the passage of molecules and cells into and out of the brain microenvironment. The BBB is composed of endothelial cells, pericytes, astrocytes, and dual basement membrane. Metastasizing tumor cells are thought to reach the brain hematogenously, penetrate the BBB, and then colonize and proliferate in the brain tissue. Metastatic deposits then arise at the junction of white-gray matter and vascular watershed areas, where most BM are diagnosed in clinical practice1,5. In experimental models, brain penetration necessitates significantly longer periods when compared to other tissues, potentially explaining the preference towards slower-flowing vessels to enable more time for the metastasizing cells to complete their passage through the BBB5. Following metastasis formation, the BBB architecture is distorted and the insulating features are altered in a formation known as the blood-tumor barrier (BTB).
Recent studies have shown the presence of functional lymphatic vessels into the CNS6, but is unlikely that it has a role in BM development. Although the meningeal lymphatic system is involved in both brain tumor cells spreading into the external lymphatic system and in antitumor immune response7, this occurs through the lymphatic drainage from the CNS to the periphery. Given the absence of an inflow lymphatic system, this does not appear to be a possible route for metastatic tumor cells to penetrate the brain.
Studies have shown that an immune-suppressive environment develops in BM, further supporting tumor growth1,5. BCBM have a distinct set of features when compared to BM from other tumor types; more commonly there are multiple metastases, the interval between primary diagnosis and BM diagnosis is longer, and patients with BCBM are more often subsequently diagnosed with LMD as compared to other primary tumor types8.
The mutational landscape of BCBM is different from primary breast tumors or extracranial metastases. BCBM are enriched for alterations in specific pathways such PTEN loss and HER2 amplification9,10. Intriguingly, the genomic alterations in BCBM can be divergent from other metastases or from the primary tumors, highlighting the need to analyze brain lesions (or brain-derived circulating factors) in order to gain insights into their biology, and to identify targets for therapeutic interventions11.
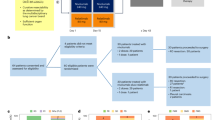
Historically, only a limited number of systemically-administered cytotoxic agents were considered as having intracranial BBB-penetrant activity12. During the past two decades, with the rise of personalized medicine tools enabling tumor genotyping combined with the availability of targeted therapies (e.g., receptor tyrosine kinase inhibitors, PARP inhibitors), the clinical need to analyze BM for biomarkers has further evolved. Historically, analysis of CNS metastasis clinical samples began with material obtained from surgical specimens (e.g., craniotomy), and later on shifted to analysis of less-invasive sources, such as plasma or cerebrospinal fluid (CSF) from lumbar puncture. In the current manuscript, we will mainly review the contemporary data utilizing minimally-invasive analyses of BCBM-derived factors, mainly cell-free DNA (cfDNA) and circulating tumor cells (CTCs) (Fig. 1 and Table 1).
Circulating tumor cells
CTCs are cells shed from the primary tumor or metastatic sites into the peripheral blood circulation. Overall, in patients with MBC not enriched for patients with BCBM, CTCs can be identified in up to 70% of cases using a 1 CTC/7.5 mL cut-off, and in up to 50% of cases using a 5 CTC/7.5 mL cut-off13. Various assays have been developed to detect CTCs in the bloodstream. First, CTCs are enriched based on biological or physical properties; second, immunological, molecular, or functional assays are used to identify CTCs among the surrounding white blood cells14. Among others, nuclear staining and expression of EpCAM and cytokeratins have been most frequently used to discriminate between CTCs from non-tumoral mesenchymal blood cells14. The CellSearch® assay, which is the only U.S. Food and Drug Administration (FDA)-approved method to detect CTCs in MBC, identifies CTCs based on the following criteria: EpCAM+, cytokeratin+, CD45−, DAPI+15.
Although the exact mechanism leading to development of BCBM is unknown, the extravasation of CTCs through the BBB into the CNS is likely to occur. However, how to identify and characterize these BM-initiating CTCs (BMICs) is under investigation. Interestingly, preclinical studies showed that mesenchymal-like, EpCAM-negative CTCs can generate BM16,17,18. This is of particular interest considering that most CTC detection methods target EpCAM-positive cells, and thus may miss BMICs. Therefore, alternate approaches may be more suitable for CTCs detection in patients with BCBM. To shed light on this question, Klotz and colleagues generated patient-derived CTC lines via ex-vivo cultures of CTCs isolated from patients with BCBM19. They observed that these cell lines can generate metastases when injected into immune-deficient mice, with a distribution pattern that reflects organ involvement in corresponding patients. Moreover, the authors identified Semaphorin 4D (SEMA4D) and MYC as key markers of BMICs, the former as mediator of BBB transmigration, and the latter as regulator for adaptation of CTCs in the brain microenvironment19.
In patients with MBC, enumeration of CTCs correlates with treatment response and survival13, whereas genomic and transcriptomic profiling of CTCs may provide information about tumor biology20,21. In patients with primary brain tumors or BM from solid tumors, prevalence of peripheral CTCs is relatively low, especially in case of brain-only disease due to the presence of the BBB22,23,24,25. Moreover, whether peripheral CTCs do reflect the biology of brain metastasis in patients with both intra- and extra-CNS disease is debated. Hence, ‘neurosystemic dissociation’ of response is not rare in patients with BCBM26. In addition, recent data indicate that CTCs can be detected in other non-blood sources, including CSF25,27. To date, CSF-CTCs proved to be a potentially helpful biomarker for diagnosis of LMD, an assessment treatment response and prognosis, and as a source of genomic information for tumor subtyping and identification of actionable alterations.
Diagnosis of LMD via CTCs
Traditionally, a diagnosis of LMD relies on a combination of clinical signs and symptoms, radiographic findings, and conventional cytology of CSF samples28. Albeit the gold standard, CSF cytology has a relatively low sensitivity, estimated as 44–67% with a single assessment, and up to 84–91% with repeated sampling29. In addition to low abundance of tumor cells in the CSF, this technique may be limited by technical and analytical challenges. Sampling via lumbar puncture requires expertise, CSF sample volume has to be at least 3 mL, and analysis must be performed rapidly given fast clearance of tumor cells after sampling27. In case of negative cytology, LMD may be prompted by symptoms and typical findings at brain and/or spine MRI, such as leptomeningeal contrast enhancement, cranial nerve enlargement, ventriculitis, and/or non-obstructive hydrocephalus28. Thus patients may be diagnosed with LMD even in the absence of a positive CSF cytology.
In order to increase sensitivity of LMD diagnosis, several studies investigated the prevalence of CTCs in CSF and its correlation with cytology, repurposing assays initially validated for peripheral CTCs27,29,30,31. To date, the use of CSF-CTCs for LMD diagnosis reached a significantly higher sensitivity than cytology at first lumbar puncture (78–100% vs 44–67%), and a specificity of 84–100%29. In contrast to conventional cytology, which relies on standard staining and immunohistochemistry for cytokeratin (CK) and/or epithelial membrane antigen (EMA), CTC assays enrich for epithelial tumor cells by more sensitive methods. The CellSearch system includes anti-EpCAM antibodies conjugated with ferromagnetic particles in addition to fluorescently conjugated antibodies15, whereas others are based on multiple fluorescently conjugated antibodies targeting a variety of cell surface markers that are subsequently enumerated by flow cytometry29.
However, none of these studies proved the clinical utility of CTCs for patients with MBC. The small sample size, inclusion of many tumor subtypes and non-randomized design of these studies are major limitations. Moreover, the interpretation of false-positive results in these studies is challenging. As the ground truth was represented by cytology in most of these studies, false-positives may represent true-positives missed by false-negative cytology. Or false-positives may represent excessive sensitivity of CTC-based assays for what clinically may or may not behave as classic LMD and require LMD-focused therapies. Hence, the optimal cut-off for diagnosis has not been defined. Considering the evidence generated so far, the Response Assessment in Neuro-Oncology (RANO) Leptomeningeal Metastasis and the RANO Brain Metastasis Working Groups recommend considering CTCs as tools for high-sensitivity detection, but presence/absence of malignancy should be confirmed by formal cytology29.
Prognostic value of CTCs
In 2004, the prognostic significance of plasma CTCs in MBC was first reported32. In this seminal study, CTCs were assessed in 177 patients both before starting a new line of therapy and at the first follow-up visit. At both time points, patients with a CTC count ≥5/7.5 mL had a significantly shorter progression-free and overall survival. Of note, CTC level was the most significant independent predictors of both endpoints, and these results led to the FDA approval of CellSearch as a prognostic assay in MBC.
Although several studies further confirmed the prognostic role of CTCs in this setting13, CTC count failed to show a meaningful predictive value. In two prospective, randomized, phase III studies, serial CTC monitoring and early treatment switching based on CTC count failed to improve long-term outcomes33,34. Hence, major international guidelines do not recommend routine use of CTCs to monitor response to therapy among patients with MBC35,36.
In patients with BCBM, peripheral CTC count similarly correlates with worse prognosis. The phase III LANDSCAPE trial assessed the efficacy of capecitabine plus lapatinib in patients with HER2-positive MBC and newly diagnosed, untreated brain metastasis. As a preplanned secondary objective, the prognostic role of CTCs at baseline and day 21 was investigated. Using a cut-off of 1 CTC/7.5 mL, prevalence of CTC-positive was 49% (20/41 patients) at baseline and 18% (7/38) at day 21, with CTC clearance observed in 11 patients. Despite the small sample size, significantly better outcomes were observed in patients with early CTC clearance than in CTC-positive patients at day 21, in terms of both CNS response (80% vs 29%, p = 0.01) and 1-year overall survival (83.9% versus 42.9%, p = 0.02)24. Similarly, Riebensahm and colleagues observed a significant association between poor survival after BCBM diagnosis and the presence of peripheral CTCs17. Interestingly, dynamics of peripheral CTCs appears to also correlate with response to brain radiation, with evidence of persistent vital CTCs in poor responders versus an increase in apoptotic CTCs in patients with local response to therapy37.
Although only a few small studies assessed CTCs in CSF and their correlation with outcomes in patients with BCBM and/or LMD, a trend towards high CTC count and short survival, and a correlation between decline in CTC count and response to intrathecal therapy was observed31,38. In patients with LMD, the prognostic value of CSF-CTCs was recently confirmed by a large retrospective analysis. In this study, in which 35 out of 101 patients included had MBC, risk of death was more than double in patients with a CSF-CTC count at or above the optimal cutoff of 61 CSF-CTCs/3 mL (hazard ratio 2.84, p = 0.002)39. More prospective studies are needed to understand whether CTCs in CSF can be routinely used to assess prognosis and guide therapy.
Molecular profiling and tumor subtyping via CTCs
Breast cancer is highly heterogeneous, with diverse subclones that evolve under selective pressure. Although breast tumors are usually classified as either hormone receptor (HR)-positive/HER2-negative, HER2-positive, or triple-negative based on expression of receptors at diagnosis, the emergence of clones with phenotypically distinct characteristics may occur. Hence, a subtype switch, defined as either receptor gain or loss, is not rarely observed40. This phenomenon appears to be particularly frequent in patients with BCBM41,42 and has been ascribed to selective tropism of BM-initiating clones. As mentioned, HER2-positive BCBM clones have a high tropism for the brain microenvironment43.
As all other “cytology” specimens, CTCs can be characterized at the protein, RNA, and genome levels.
DNA sequencing of CSF-CTCs and matched primary tissue in patients with LM showed that although some alterations are shared, others can be identified in the CTCs only44,45. This finding reflects what was previously shown in large studies comparing mutational profiles of BM and primary tissues, and suggests the subclonal origin and branch evolution of BM11. Similarly, profiling of peripheral CTCs and matched tumor tissue in 3 patients with BCBM showed chromosomal aberrations with a high genomic clonality and mutations in pathways previously associated with BM including the Notch and PI3K pathways, cell cycle regulations, epithelial-mesenchymal transition, and chromatin remodeling17. Furthermore, transcriptomic analysis of CTCs allows for investigation of gene expression profiles. For example, via whole-genome mRNA microarray of peripheral CTCs, Boral and colleagues described a unique “CTCs gene signature” that was distinct from primary breast cancer tissues16. By comparing staining and transcriptional profiling of CTCs between patients with and without BM, the authors also identified that patients with BCBM have more CTCs with high ki67 staining, and a 126-gene signature that was significantly altered between the two groups, with higher Notch signaling, hyperactivation of pro-inflammatory and immunomodulatory networks among patients with BCBM16.
Finally, CTC analysis allows the assessment of protein expression. Given its therapeutic role, most studies have focused on HER2 expression by CTCs. In line with the subtype switch observed in tissue analyses, many cases of HER2 gain have been observed across different series and reported in up to 40.6% of cases27,46,47. Hence, HER2-positive CTCs may reflect clonal HER2 heterogeneity eventually missed by tissue analyses due to sampling bias, and thus be used as a predictive biomarker of benefit from anti-HER2 therapy. Two clinical trials that investigated the efficacy of anti-HER2 therapy in patients with HER2-negative MBC and HER2-positive peripheral CTCs failed to demonstrate a convincing benefit (response rate of 5% with trastuzumab-navelbine; 9.1% with trastuzumab emtansine)48,49. However, whether HER2 assessment on CSF-CTCs would be effective to detect and target HER2 gain in patients with BM is unknown. For instance, HER2 staining on CTCs may be helpful to identify a subgroup of tumors with HER2 score 0 at immunohistochemistry who may derive benefit from trastuzumab deruxtecan, an anti-HER2 antibody-drug conjugate with high brain penetration which is currently approved only for HER2-positive and HER2-low breast cancer50,51.
Taken together, CSF-CTCs might shed light on intracranial disease dynamics and enable studying its features, but further work is needed to decipher the clinical role of these cells in patient care. In summary, CTCs are promising biomarkers in patients with BCBM and/or LMD and may help to profile the unique molecular landscape of brain lesions, to confirm LMD diagnosis, and to monitor for treatment response. However, evidence proving their clinical utility is still lacking and prospective trials are warranted.
Circulating tumor DNA
The term cfDNA refers to double-stranded DNA fragments bound to histones in circulation that can be detected in blood and other body fluids. Although most plasma cfDNA is derived from white blood cells, in patients with cancer a proportion of cfDNA consists of circulating tumor DNA (ctDNA) released by tumor cells via secretion or following apoptosis or necrosis52. The fraction of cfDNA composed by ctDNA is highly variable according to tumor burden and histology, host factors, and type of fluid sampled52. Moreover, sensitivity of ctDNA assays is highly variable and depends on the analyzed volume, ranging from 1 × 10−6 of cutting-edge minimal residual disease assays to 0.1% of commercially available next-generation sequencing panels53,54,55. With modern assays, ctDNA can be identified in almost all patients with MBC, given the relatively high amount of circulating DNA and genomic aberrations56.
The amount of plasma ctDNA correlates with tumor burden in patients with MBC. Hence, monitoring ctDNA dynamics has been shown to correlate with treatment response57,58,59,60. However, discordancy between CT scan results and ctDNA dynamics has also been observed61, and its clinical utility still has to be proven14. Moreover, ctDNA analysis can identify tumor alterations that may help in treatment choice, both as predictor of response mutations (e.g., PIK3CA mutations for PIK3CA-inhibitors) and resistance (e.g., ESR1 mutations).
However, similar to CTCs, sensitivity of ctDNA assays is much lower in patients with brain-only metastasis62,63,64,65. Hence, CSF may represent an alternative source for isolation of ctDNA. Although potential applications of ctDNA and CTCs are similar for patients with BCBM, the two techniques are different and not completely overlapping, and might be considered complementary.
Diagnosis of LMD via ctDNA
The presence of BTB and the direct contact between intracranial tumors and CSF make it an excellent source of ctDNA. Moreover, the CSF contains fewer white blood cells, thus ctDNA assays have increased sensitivity for low variant allele fraction (VAF) variants due to the reduced noise caused by non-tumor cfDNA66.
Isolation of ctDNA from CSF has been investigated as an alternative approach of LMD diagnosis. Although most of the evidence available relies on retrospective studies including few patients across different tumor types, in all cases ctDNA outperformed cytology in LMD diagnosis63,67. Hypothetically, ctDNA could even anticipate LMD diagnosis, as it has been detected in one patient without any signs or symptom of LMD, but with LMD diagnosed at autopsy63.
In addition to traditional ctDNA targeted DNA sequencing assays, a few studies have investigated ultra-low pass whole-genome sequencing (ULP-WGS) on CSF-ctDNA for LMD diagnosis. ULP-WGS is a relatively low-cost and rapid tool that relies on low coverage (0.1x) WGS and, differently to targeted approaches, allows to estimate tumor content in ctDNA without prior knowledge of the mutational profile. Moreover, it is optimized to achieve high sensitivity even in low volume samples68. In a series of 30 cases (24 LMD-positive and 6 LMD-negative cases), ctDNA fraction in CSF was found to be significantly higher in patients with LMD, compared to patients without intracranial involvement (median 0.57 vs 0.03; p < 0.0001) using ULP-WGS69. Considering a ctDNA fraction cut-off of 0.10, all patients with LMD were ctDNA-positive whereas all patients without LMD were ctDNA-negative. In line with prior reports, ctDNA fraction was significantly lower in plasma than CSF in LMD cases, and 12/22 samples were below the detection level69. A similar analysis on 30 patients across different tumor types reported an accuracy of 94% (sensitivity 93%, specificity 100%) to detect LMD. Of note, 5 patients with BM abutting the CSF were ctDNA-positive despite the absence of radiographic, symptomatic, or cytologic evidence of LMD, suggesting the limitations of ctDNA to diagnose LMD in the setting of BCBM, or the enhanced sensitivity to detect sub-clinical disease70.
As an alternative to ULP-WGS, an equally fast and affordable technique to assess ctDNA fraction is the modified fast aneuploidy screening test-sequencing system (mFAST-SeqS) method, which employs selective amplification of long interspaced nuclear elements (LINE-1 sequences) that are sparce throughout the genome. In a series of 121 patients, the mFAST-SeqS had a false-negative rate of 23.1% and a false-positive rate of 5.4%. Of note, 4/14 cytology-negative, aneuploidy-positive patients further developed LMD. Despite the low sample size, which does not allow for determination of the best cut-off in this setting, these data demonstrated the feasibility of mFAST-SeqS for LMD diagnosis and higher sensitivity when compared to cytology alone71.
Molecular profiling of ctDNA
ctDNA profiling via whole exome or targeted sequencing allows not only for quantification of tumor fraction, but also for identification of molecular alterations with prognostic and/or predictive value.
In a pivotal study, De Mattos-Arruda and colleagues compared the mutational profile of CSF and plasma ctDNA in patients with primary brain tumors or BM63. They found CSF ctDNA to be representative of brain tumors, as identified alterations were confirmed in brain tumor tissue samples. Furthermore, CSF-ctDNA was more informative than plasma ctDNA; although VAF of gene alterations in the CSF and plasma were comparable in patients with abundant visceral burden, in patients with brain-only disease the allele fractions in CSF ctDNA were significantly higher than in plasma (ctDNA found in 58% of cases vs. 0%, respectively). The authors also observed how CSF genotyping allows detection of several gene mutations absent from plasma ctDNA. Of note, some of the identified alterations in CSF ctDNA were specific of the brain metastasis and not detected in extracranial lesions63. Hence, sequencing of CSF ctDNA may reliably provide the mutational profile of brain lesions in most patients with BM or primary brain tumors. This is of particular interest given the potential clinically relevant value of liquid biopsy to genotype BCBM, in light of the low sensitivity of plasma ctDNA in patients with brain-only disease (or ‘neurosystemic dissociation’). CSF profiling has been shown to identify both candidate mechanisms of resistance and potentially druggable alterations in patients with BCBM, such as PIK3CA mutations or PTEN loss26,67,72,73.
Furthermore, novel computational analyses of cfDNA are now exploring fragmentation patterns to reveal the occupancy of nucleosomes in cells-of-origin74. This structural study, called nucleosome profiling, has been primarily used for cancer detection and prediction of tumor tissue-of-origin75. However, profiling of nucleosome accessibility and transcriptional regulation may be also used to infer transcriptional profiles. In patients with breast cancer, the Griffin framework proved to properly perform estrogen receptor (ER) subtyping from ULP-WGS of ctDNA and discriminate between ER-positive and ER-negative tumors76. Given the frequency of ER loss and HER2 gain in BCBM, the availability of a CSF-based assay to assess receptor status of BCBM\LMD would allow to bypass the current need for invasive procedures41,77. Although the clinical utility of these technologies still must be proven, this would be of great interest to better target the dynamic evolution of CNS metastasis over time.
ctDNA as surrogate biomarker of response
ctDNA dynamics in plasma correlates with treatment response in patients with MBC78,79,80. In a proof-of-concept study including 30 women with MBC, Dawson and colleagues proved that ctDNA dynamics is more sensitive than traditional tumor markers (CA 15-3) and CTCs in predicting response and progression59. Of note, most patients in this study were tested using an “old”, tumor-informed ctDNA assay tracking only PIK3CA and TP53 alterations via digital PCR or tagged-amplicon deep sequencing, as the whole-genome sequencing approach was used only for 9 patients. Hence, only 30 out of the 52 patients initially screened had tumor alterations identified on the primary tissue allowing for informed ctDNA tracking. Modern techniques based on tissue whole-exome or -genome sequencing allow to track multiple mutations, thus increasing sensitivity significantly even in tumor with low mutational burden.
More recent analyses from different patient cohorts confirmed the role of ctDNA in predicting response and disease progression57,80, although its clinical utility still has to be proven14. In patients with BCBM, CSF ctDNA levels similarly showed to correlate with disease burden, treatment response, and prognosis63,69,70,71.
Interestingly, tumor-informed approaches showed to be more sensitive in CSF than plasma. Hence, only ctDNA VAF in CSF, and not in plasma, was shown to decrease with surgical resection and/or responses to systemic therapy and increase with tumor progression in a small cohort of patients with minimal or no extracranial disease63.
Tumor-agnostic methods such as ULP-WGS and mFAST-SeqS have been also applied for sequential monitoring of CSF ctDNA, and both showed to properly correlate with treatment response and survival69,70,71. In particular, ctDNA suppression during intrathecal treatment was shown to correlate with both response to therapy and survival69. Of note, ctDNA levels were found to significantly increase after surgery and radiation therapy, stressing the need to account for confounding factors to properly interpret ctDNA dynamics70.
Altogether, ctDNA (especially from CSF) is a promising approach to study and follow-up for intracranial metastatic lesions. However, its clinical application is still limited by the invasiveness of CSF sampling, which requires either repeated lumbar punctures or placement of an Ommaya catheter, and by the lack of robust clinical data proving its clinical utility.
Other approaches
In addition to ctDNA and CTCs, patients with BCBM were found to have distinctive profiles of various molecules, both in blood and CSF, compared to patients without BCBM. For example, patients with BCBM have higher plasma levels of specific long non-coding RNA81, specific microRNAs82, and proteins such as lactate dehydrogenase-A83, Tau84, and Angiopoietin-like 485. Intriguingly, lower quantity of plasma exosomes with an increased protein content were found to be associated with the presence of BCBM86. In an elegant mouse model, the complement protein C3 was found to be secreted by intracranial breast cancer cells to increase the permeability of the BBB and facilitate the entry of factors to support additional tumor growth87,88. Furthermore, based on differential metabolic adaptations between BCBM and extracranial metastases89, analysis of CSF metabolites also demonstrated distinct features in patients with BCBM, e.g., lower alanine and lactic acid levels90.
Unfortunately, despite a plethora of preclinical evidence involving non-cfDNA biomarkers, almost all the reports remain anecdotal without translation into clinical studies. We believe that one of the major challenges to promote this field is the relative unavailability of CSF and brain metastases tissues. Therefore, more investment in the establishment of biobanks is desirable to facilitate better and more robust biomarkers research.
Future perspectives
Liquid biopsy for studying CNS lesions is largely challenged by anatomic constraints. Analysis of CSF is technically simpler than plasma, but requires invasive procedures (e.g., lumbar puncture) to obtain it. The most convenient liquid biopsy approach for BCBM and/or LMD would involve blood, but the limited available data suggest that CSF ctDNA analysis may best reflect CNS disease, whether as a diagnostic test or for tumor genotyping. Of course, the utility of CSF ctDNA analysis would ideally be demonstrated in a prospective cohort with a robust and standardized assay. Importantly, the relatively low prevalence of BCBM\LMD and the challenges in access to CSF means that practically speaking, multi-center collaborations using robust and cutting-edge assays will be required to generate answers to the numerous questions in this field. In addition, we recommend additional research with chromatin and methylation-focused liquid biopsy assays in the setting of CNS metastasis. Chromatin and methylation analyses might shed light on tumor cells’ gene expression or even identify damage to neighboring healthy tissues as a biomarker for a growing metastasis75,76,91.
Overall, we believe that past and future studies in this field are limited due to several key barriers (Fig. 2), which must be addressed in order to make a significant progress. First, to fulfill the vision of blood-based liquid biopsy, assay performance should be considerably optimized to allow detection of brain-derived ctDNA in the plasma of patients with BCBM, e.g., the development of ultra-sensitive tumor-uninformed assays can be a potential solution to this challenge. Second, for technical reasons, it may be that CSF-based assays will always outperform blood-based assays, and there is dramatic room for improvement in our current standard panel of conventional cytology, cell counts, glucose, and total protein. To do this will require prospective studies to demonstrate the impact and value of ctDNA-based, CTC-based, or other novel assays in the care of patients with BCBM and/or LMD. Third, multi-institutional collaboration and resource investment in the creation of BM and LMD biobanks, with clinical data, linked to tumor, CSF, and plasma samples will significantly facilitate translational research efforts in this field. Fourth, BBB\BTB permeability is one of the main obstacles for detecting plasma ctDNA which represents intra-cranial lesions. Understanding the currently unknown mechanisms of ctDNA transport through the BBB is an important step for improving systemic detection of brain-derived ctDNA. Lastly, liquid biopsy use should be incorporated into clinical trials involving patients with BCBM and LMD. Such trials will also facilitate the collection of precious bio-specimens for future analysis and set the stage for testing various assays with a true potential to enter clinical routine. Education and outreach to both health care professionals and patients regarding the importance and potential future impact of CSF collection for research purposes, despite the obvious barriers, are critical to the success of such biobanking efforts.
In conclusion, liquid biopsy in patients with BCBM has a great potential to impact clinical care. Further research focused on the current barriers is clearly required to overcome the biological and technical challenges ahead.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
Achrol, A. S. et al. Brain metastases. Nat. Rev. Dis. Prim. 5, 5 (2019).
Frisk, G. et al. Incidence and time trends of brain metastases admissions among breast cancer patients in Sweden. Br. J. Cancer 106, 1850–1853 (2012).
Franzoi, M. A. & Hortobagyi, G. N. Leptomeningeal carcinomatosis in patients with breast cancer. Crit. Rev. Oncol. Hematol. 135, 85–94 (2019).
Pawlowska, E., Romanowska, A. & Jassem, J. Radiotherapy for leptomeningeal carcinomatosis in breast cancer patients: a narrative review. Cancers 14, 3899 (2022).
Cacho-Diaz, B. et al. Tumor microenvironment differences between primary tumor and brain metastases. J. Transl. Med. 18, 1 (2020).
Louveau, A. et al. Structural and functional features of central nervous system lymphatic vessels. Nature 523, 337–341 (2015).
Hu, X. et al. Meningeal lymphatic vessels regulate brain tumor drainage and immunity. Cell Res. 30, 229–243 (2020).
Berghoff, A. S. et al. Descriptive statistical analysis of a real life cohort of 2419 patients with brain metastases of solid cancers. ESMO Open 1, e000024 (2016).
Zhang, L. et al. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature 527, 100–104 (2015).
Huang, R. S. P. et al. Clinicopathologic and genomic landscape of non-small cell lung cancer brain metastases. Oncologist 27, 839–848 (2022).
Brastianos, P. K. et al. Genomic characterization of brain metastases reveals branched evolution and potential therapeutic targets. Cancer Disco. 5, 1164–1177 (2015).
Lin, N. U., Bellon, J. R. & Winer, E. P. CNS metastases in breast cancer. J. Clin. Oncol. 22, 3608–3617 (2004).
Bidard, F.-C. et al. Clinical validity of circulating tumour cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. Lancet Oncol. 15, 406–414 (2014).
Alix-Panabières, C. & Pantel, K. Clinical applications of circulating tumor cells and circulating tumor DNA as liquid biopsy. Cancer Disco. 6, 479–491 (2016).
Riethdorf, S. et al. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the CellSearch system. Clin. Cancer Res. 13, 920–928 (2007).
Boral, D. et al. Molecular characterization of breast cancer CTCs associated with brain metastasis. Nat. Commun. 8, 196 (2017).
Riebensahm, C. et al. Clonality of circulating tumor cells in breast cancer brain metastasis patients. Breast Cancer Res. 21, 101 (2019).
Zhang, L. et al. The identification and characterization of breast cancer CTCs competent for brain metastasis. Sci. Transl. Med. 5, 180ra48 (2013).
Klotz, R. et al. Circulating tumor cells exhibit metastatic tropism and reveal brain metastasis drivers. Cancer Disco. 10, 86–103 (2020).
Paoletti, C. et al. Comprehensive mutation and copy number profiling in archived circulating breast cancer tumor cells documents heterogeneous resistance mechanisms. Cancer Res. 78, 1110–1122 (2018).
Rothé, F. et al. Interrogating breast cancer heterogeneity using single and pooled circulating tumor cell analysis. npj Breast Cancer 8, 79 (2022).
Hanssen, A. et al. Frequency of circulating tumor cells (CTC) in patients with brain metastases: implications as a risk assessment marker in oligo-metastatic disease. Cancers 10, 527 (2018).
Mego, M. et al. Characterization of metastatic breast cancer patients with nondetectable circulating tumor cells. Int. J. Cancer 129, 417–423 (2011).
Pierga, J.-Y. et al. Circulating tumor cells and brain metastasis outcome in patients with HER2-positive breast cancer: the LANDSCAPE trial. Ann. Oncol. 24, 2999–3004 (2013).
Westphal, M. et al. Circulating tumor cells and extracellular vesicles as liquid biopsy markers in neuro-oncology: prospects and limitations. Neurooncol Adv. 4, ii45–ii52 (2022).
Siravegna, G. et al. Genotyping tumour DNA in cerebrospinal fluid and plasma of a HER2-positive breast cancer patient with brain metastases. ESMO Open 2, e000253 (2017).
Darlix, A. et al. Detection of circulating tumor cells in cerebrospinal fluid of patients with suspected breast cancer leptomeningeal metastases: a prospective study. Clin. Chem. 68, 1311–1322 (2022).
Le Rhun, E. et al. EANO-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up of patients with leptomeningeal metastasis from solid tumours. Ann. Oncol. 28, iv84–iv99 (2017).
Boire, A. et al. Liquid biopsy in central nervous system metastases: a RANO review and proposals for clinical applications. Neuro. Oncol. 21, 571–584 (2019).
Lin, X. et al. Cerebrospinal fluid circulating tumor cells: a novel tool to diagnose leptomeningeal metastases from epithelial tumors. Neuro. Oncol. 19, 1248–1254 (2017).
Patel, A. S. et al. Identification and enumeration of circulating tumor cells in the cerebrospinal fluid of breast cancer patients with central nervous system metastases. Oncotarget 2, 752–760 (2011).
Cristofanilli, M. et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N. Engl. J. Med. 351, 781–791 (2004).
Cabel, L. et al. Clinical utility of circulating tumour cell-based monitoring of late-line chemotherapy for metastatic breast cancer: the randomised CirCe01 trial. Br. J. Cancer 124, 1207–1213 (2021).
Smerage, J. B. et al. Circulating tumor cells and response to chemotherapy in metastatic breast cancer: SWOG S0500. J. Clin. Oncol. 32, 3483–3489 (2014).
Henry, N. L. et al. Biomarkers for systemic therapy in metastatic breast cancer: ASCO guideline update. J. Clin. Oncol. 40, 3205–3221 (2022).
Pascual, J. et al. ESMO recommendations on the use of circulating tumour DNA assays for patients with cancer: a report from the ESMO Precision Medicine Working Group. Ann. Oncol. 33, 750–768 (2022).
Petersen, C. et al. CTCs in patients with brain metastases under radiotherapy: do they indicate treatment response? [abstract]. Radiother. Oncol. 133, S252–S253 (2019).
Malani, R. et al. Cerebrospinal fluid circulating tumor cells as a quantifiable measurement of leptomeningeal metastases in patients with HER2 positive cancer. J. Neuro-Oncol. 148, 599–606 (2020).
Diaz, M. et al. Quantitative assessment of circulating tumor cells in cerebrospinal fluid as a clinical tool to predict survival in leptomeningeal metastases. J. Neuro-Oncol. 157, 81–90 (2022).
Schrijver, W. A. M. E. et al. Receptor conversion in distant breast cancer metastases: a systematic review and meta-analysis. J. Natl Cancer Inst. 110, 568–580 (2018).
Hulsbergen, A. F. C. et al. Subtype switching in breast cancer brain metastases: a multicenter analysis. Neuro. Oncol. 22, 1173–1181 (2020).
Jung, J. et al. Discordances in ER, PR, and HER2 between primary breast cancer and brain metastasis. J. Neuro-Oncol. 137, 295–302 (2018).
Palmieri, D. et al. Her-2 overexpression increases the metastatic outgrowth of breast cancer cells in the brain. Cancer Res 67, 4190–4198 (2007).
Li, X. et al. Clinical significance of detecting CSF-derived tumor cells in breast cancer patients with leptomeningeal metastasis. Oncotarget 9, 2705–2714 (2018).
Magbanua, M. J. M. et al. Molecular profiling of tumor cells in cerebrospinal fluid and matched primary tumors from metastatic breast cancer patients with leptomeningeal carcinomatosis. Cancer Res. 73, 7134–7143 (2013).
Kumthekar, P. et al. BIOM-05—The HER2 flip: HER2 amplification of tumor cells in the cerebrospinal fluid (CSF-TCs) of patients with solid tumor leptomeningeal metastasis [abstract]. Neuro Oncol. 23, vii4–vii5 (2022).
Priedigkeit, N. et al. Intrinsic subtype switching and acquired ERBB2/HER2 amplifications and mutations in breast cancer brain metastases. JAMA Oncol. 3, 666–671 (2017).
Jacot, W. et al. Actionability of HER2-amplified circulating tumor cells in HER2-negative metastatic breast cancer: the CirCe T-DM1 trial. Breast Cancer Res. 21, 121 (2019).
Parsons, H. A. et al. Phase II single-arm study to assess trastuzumab and vinorelbine in advanced breast cancer patients with HER2-negative tumors and HER2-positive circulating tumor cells. JCO Precis Oncol. 5, 896–903 (2021).
Cortes, J. et al. Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. N. Engl. J. Med. 386, 1143–1154 (2022).
Modi, S. et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N. Engl. J. Med. 387, 9–20 (2022).
Kustanovich, A., Schwartz, R., Peretz, T. & Grinshpun, A. Life and death of circulating cell-free DNA. Cancer Biol. Ther. 20, 1057–1067 (2019).
Lanman, R. B. et al. Analytical and clinical validation of a digital sequencing panel for quantitative, highly accurate evaluation of cell-free circulating tumor DNA. PLoS ONE 10, e0140712 (2015).
Parsons, H. A. et al. Sensitive detection of minimal residual disease in patients treated for early-stage breast cancer. Clin. Cancer Res 26, 2556–2564 (2020).
Gydush, G. et al. Massively parallel enrichment of low-frequency alleles enables duplex sequencing at low depth. Nat. Biomed. Eng. 6, 257–266 (2022).
Kingston, B. et al. Genomic profile of advanced breast cancer in circulating tumour DNA. Nat. Commun. 12, 2423 (2021).
O’Leary, B. et al. Early circulating tumor DNA dynamics and clonal selection with palbociclib and fulvestrant for breast cancer. Nat. Commun. 9, 896 (2018).
Liang, D. H. et al. Cell-free DNA as a molecular tool for monitoring disease progression and response to therapy in breast cancer patients. Breast Cancer Res. Treat. 155, 139–149 (2016).
Dawson, S.-J. et al. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N. Engl. J. Med. 368, 1199–1209 (2013).
Kodahl, A. R. et al. Correlation between circulating cell-free PIK3CA tumor DNA levels and treatment response in patients with PIK3CA-mutated metastatic breast cancer. Mol. Oncol. 12, 925–935 (2018).
Frenel, J. S. et al. Serial next-generation sequencing of circulating cell-free DNA evaluating tumor clone response to molecularly targeted drug administration. Clin. Cancer Res. 21, 4586–4596 (2015).
Bettegowda, C. et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci. Transl. Med. 6, 224ra24 (2014).
De Mattos-Arruda, L. et al. Cerebrospinal fluid-derived circulating tumour DNA better represents the genomic alterations of brain tumours than plasma. Nat. Commun. 6, 8839 (2015).
Garcia-Murillas, I. et al. Assessment of molecular relapse detection in early-stage breast cancer. JAMA Oncol. 5, 1473–1478 (2019).
Magbanua, M. J. M. et al. Circulating tumor DNA in neoadjuvant-treated breast cancer reflects response and survival. Ann. Oncol. 32, 229–239 (2021).
Tivey, A., Church, M., Rothwell, D., Dive, C. & Cook, N. Circulating tumour DNA - looking beyond the blood. Nat. Rev. Clin. Oncol. 19, 600–612 (2022).
Pentsova, E. I. et al. Evaluating cancer of the central nervous system through next-generation sequencing of cerebrospinal fluid. J. Clin. Oncol. 34, 2404–2415 (2016).
Adalsteinsson, V. A. et al. Scalable whole-exome sequencing of cell-free DNA reveals high concordance with metastatic tumors. Nat. Commun. 8, 1324 (2017).
Fitzpatrick, A. et al. Assessing CSF ctDNA to improve diagnostic accuracy and therapeutic monitoring in breast cancer leptomeningeal metastasis. Clin. Cancer Res. 28, 1180–1191 (2022).
White, M. D. et al. Detection of leptomeningeal disease using cell-free DNA from cerebrospinal fluid. JAMA Netw. Open 4, e2120040 (2021).
Angus, L. et al. Detection of aneuploidy in cerebrospinal fluid from patients with breast cancer can improve diagnosis of leptomeningeal metastases. Clin. Cancer Res. 27, 2798–2806 (2021).
Dugan, M., Fisher, D., Sweed, N., Blouw, B. & Mayer, J. Abstract PD4-03: characterization of HER2 amplification in the cerebrospinal fluid of patients with Leptomeningeal Disease in stage IV patients with breast cancer [abstract]. Cancer Res. 82, PD4-03 (2022).
Zhao, Y. et al. Evaluating the cerebrospinal fluid ctDNA detection by next-generation sequencing in the diagnosis of meningeal Carcinomatosis. BMC Neurol. 19, 331 (2019).
Liu, Y. At the dawn: cell-free DNA fragmentomics and gene regulation. Br. J. Cancer 126, 379–390 (2022).
Sadeh, R. et al. ChIP-seq of plasma cell-free nucleosomes identifies gene expression programs of the cells of origin. Nat. Biotechnol. 39, 586–598 (2021).
Doebley, A. L. et al. A framework for clinical cancer subtyping from nucleosome profiling of cell-free DNA. Nat. Commun. 13, 7475 (2022).
Sperduto, P. W. et al. Estrogen/progesterone receptor and HER2 discordance between primary tumor and brain metastases in breast cancer and its effect on treatment and survival. Neuro. Oncol. 22, 1359–1367 (2020).
Liu, B. et al. The circulating tumor DNA (ctDNA) alteration level predicts therapeutic response in metastatic breast cancer: novel prognostic indexes based on ctDNA. Breast 65, 116–123 (2022).
Cullinane, C. et al. Association of circulating tumor DNA with disease-free survival in breast cancer: a systematic review and meta-analysis. JAMA Netw. Open 3, e2026921 (2020).
Darrigues, L. et al. Circulating tumor DNA as a dynamic biomarker of response to palbociclib and fulvestrant in metastatic breast cancer patients. Breast Cancer Res. 23, 31 (2021).
Wang, S. et al. JAK2-binding long noncoding RNA promotes breast cancer brain metastasis. J. Clin. Invest. 127, 4498–4515 (2017).
Sato, J. et al. Brain metastasis-related microRNAs in patients with advanced breast cancer. PLoS ONE 14, e0221538 (2019).
Dong, T. et al. Tumor LDH-A expression and serum LDH status are two metabolic predictors for triple negative breast cancer brain metastasis. Sci. Rep. 7, 6069 (2017).
Darlix, A. et al. The prognostic value of the Tau protein serum level in metastatic breast cancer patients and its correlation with brain metastases. BMC Cancer 19, 110 (2019).
Dao, T. et al. Expression of angiopoietin-like 4 fibrinogen-like domain (cANGPTL4) increases risk of brain metastases in women with breast cancer. Oncotarget 11, 1590–1602 (2020).
Carretero-Gonzalez, A. et al. Characterization of plasma circulating small extracellular vesicles in patients with metastatic solid tumors and newly diagnosed brain metastasis. Oncoimmunology 11, 2067944 (2022).
Boire, A. et al. Complement component 3 adapts the cerebrospinal fluid for leptomeningeal metastasis. Cell 168, 1101–1113 e13 (2017).
Drusco, A. et al. A differentially expressed set of microRNAs in cerebro-spinal fluid (CSF) can diagnose CNS malignancies. Oncotarget 6, 20829–20839 (2015).
Ferraro, G. B. et al. Fatty acid synthesis is required for breast cancer brain metastasis. Nat. Cancer 2, 414–428 (2021).
Ballester, L. Y. et al. Analysis of cerebrospinal fluid metabolites in patients with primary or metastatic central nervous system tumors. Acta Neuropathol. Commun. 6, 85 (2018).
Lubotzky, A. et al. Liquid biopsy reveals collateral tissue damage in cancer. JCI Insight 7, e153559 (2022).
Acknowledgements
A.G. is funded by the Conquer Cancer - Israel Cancer Research Fund Career Development Award. S.M. is supported by the Italian Association for Cancer Research (AIRC) and Fondazione Bonadonna. The authors express sincerest gratitude to the Kiki Fund for Breast Cancer Leptomeningeal Metastases Research.
Author information
Authors and Affiliations
Contributions
Drafted initial version of the manuscript: S.M. and A.G. Critical revision of the manuscript for intellectual content: S.M., H.A.P., N.U.L., and A.G. Accountability for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: S.M., H.A.P., N.U.L., and A.G. Final approval: S.M., H.A.P., N.U.L., and A.G.
Corresponding author
Ethics declarations
Competing interests
H.A.P. reports research funding from Puma Biotechnology (paid to institution) and advisory roles at Illumina, Caris and SAGA Diagnostics. N.U.L. reports institutional research support from Genentech, Pfizer, Merck, Seattle Genetics, Zion Pharmaceuticals, Olema Pharmaceuticals, AstraZeneca; consulting honoraria from Puma, Seattle Genetics, Daichii-Sankyo, AstraZeneca, Denali Therapeutics, Prelude Therapeutics, Olema Pharmaceuticals, Aleta BioPharma, Affinia Therapeutics, Voyager Therapeutics, Janssen, Blueprint Medicines; stock and other ownership interests in Artera Inc. (<$50k and <5% as it relates to consulting activities – options are not currently valued or in-hand); and royalties from Up to Date (book). The remaining authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Morganti, S., Parsons, H.A., Lin, N.U. et al. Liquid biopsy for brain metastases and leptomeningeal disease in patients with breast cancer. npj Breast Cancer 9, 43 (2023). https://doi.org/10.1038/s41523-023-00550-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41523-023-00550-1