Abstract
Human epidermal growth factor receptor 2 (HER2) positive breast cancer accounts for 20–25% of all breast cancers. Multiple HER2-targeted therapies have been developed over the last few years, including the tyrosine kinase inhibitors (TKI) lapatinib, neratinib, tucatinib, and pyrotinib. These drugs target HER2 and other receptors of the epidermal growth factor receptor family, therefore each has unique efficacy and adverse event profile. HER2-directed TKIs have been studied in the early stage and advanced settings and have shown promising responses. There is increasing interest in utilizing these drugs in combination with chemotherapy and /or other HER2-directed agents in patients with central nervous system involvement, TKIs have shown to be effective in this setting for which treatment options have been previously limited and the prognosis remains poor. The aim of this review is to summarize currently approved TKIs for HER2+ breast, key clinical trials, and their use in current clinical practice.
Similar content being viewed by others
Introduction
Breast cancer (BC) remains the most common type of cancer in women and the second leading cause of cancer related deaths in the United States (US)1. Over two million new cases of BC were diagnosed in 2018 worldwide according to the World Health Organization2. It is estimated that 276,480 women will be diagnosed with BC in the US in 20201,3. Human epidermal growth factor receptor 2 (HER2+; ErbB2/neu) BC accounts for 20–25% of all BCs; around 360,000 new cases in the world and 48,000 in the US yearly1,3.
BC that overexpress HER2 are aggressive and associated with poor prognosis4,5. The development of HER2 directed therapies has revolutionized the treatment paradigm and the outcomes of patients with HER2 + BC. Multiple HER2 targeted agents have been approved over the past 5 years for BC, including several tyrosine kinase inhibitors (TKI). In this review we summarize the currently approved TKI for HER2 + BC, key clinical trials, and their use in current clinical practice.
HER2-positive breast cancer
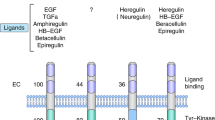
HER2 is an oncoprotein located in the long arm of chromosome 176,7. HER2 is part of the epidermal growth factor receptor family, which is composed by epidermal growth factor receptor (EGFR)/HER1, HER2, HER3, and HER4 and controls cell growth, survival differentiation, and migration6,7,8.
The HER2 extracellular domain has no known ligand and is activated by the formation of homo or heterodimers6,7. These dimers lead to phosphorylation of tyrosine kinase residues in the cytoplasmic domain which function as docking sites for proteins that activate the phosphatidylinositol triphosphate kinase (PI3K) and mitogen-activated protein kinase (MAPK) signaling pathways (Fig. 1), leading to cell cycle progression and proliferation6. Overexpression and amplification are the most common HER2 abnormalities in BC, detected by immunohistochemistry (IHC) or fluorescence in-situ hybridization (FISH), respectively9,10.
There are four members of the HER2 receptor family, these are the targets of lapatinib, neratinib, pyrotinib and tucatinib. The HER2 extracellular domain has no known ligand and is activated by the formation of homo or heterodimers (exemplified by a HER2-HER3 heterodimer in the figure). These dimers lead to phosphorylation of tyrosine kinase residues in the cytoplasmic domain which function as docking sites for proteins that activate the PI3K and MAPK signaling pathways downstream leading to cell cycle progression and proliferation.
HER2-directed therapies
Trastuzumab was approved in the late 1990’s and it was the first HER2 directed therapy approved for the treatment of BC11. Since then, several HER2 targeted therapies have been approved including pertuzumab which is another HER2 directed monoclonal antibody and the antibody-drug conjugates ado-trastuzumab emtansine and fam-trastuzumab deruxtecan. Margetuximab, a HER2-directed antibody designed to alter Fc-receptor affinity to CD16 and induce CD16-mediated cytotoxicity was also recently approved12.
Small molecule TKIs represent another option for patients with early stage or advanced HER2 + BC. Afatinib is an irreversible inhibitor of EGFR, HER2, and HER4 approved for the treatment of lung cancer with EGFR mutations13. Afatinib was one of the first TKIs studied in BC and showed promising results in phase 1–2 studies but failed to demonstrate efficacy in phase 3 trials13,14,15,16,17. Lapatinib, neratinib, pyrotinib, and tucatinib are small molecule TKIs that have shown good outcomes in phase 3 trials for patients with HER2 + BC18,19,20. These TKIs have shown to be effective as monotherapy or in combination with chemotherapy, and other HER2 directed agents in the early stage and metastatic settings18,19,20,21,22,23. Studies have shown that patients who progress on trastuzumab may benefit from a HER2 directed TKI with or without trastuzumab19,20. This efficacy may be due to different mechanisms of action and target within the HER2 receptor which allows the TKI to overcome mechanisms of resistance to trastuzumab24,25.
Early stage breast cancer
Lapatinib
Lapatinib ditosylate (GW572016, Kykerb® GlaxoSmithKline) is an orally bioavailable reversible small molecule inhibitor of EGFR and HER2. Lapatinib blocks phosphorylation of the tyrosine kinase residues inhibiting cell proliferation by blocking the MAPK and PIK3 pathways26. Preclinical studies demonstrated increased apoptosis in HER2 and EGFR dependent cells in vitro and in vivo26. The main pharmacologic characteristics of lapatinib are described in Table 1.
NeoALTTO, GeparQuinto GBG 44, NSABP B-41, and CALBG 40601 were four large phase 3, randomized trials that studied the role of lapatinib in the neoadjuvant setting (summarized in Table 2)27,28,29,30. In the CALBG 40601 study, patients were randomized to receive paclitaxel plus lapatinib and trastuzumab (THL) or paclitaxel plus lapatinib (TL) or paclitaxel plus trastuzumab (TH) in the neoadjuvant setting. After 7 years of follow up, there was an improvement in recurrence-free survival (THL 93%, TH 79% and TL 69%; hazard ratio 0.32, 95% CI 0.14–0.71, p = 0.005) and overall survival (OS) (THL 96%, TH 88%, TL 84%; hazard ratio 0.34, 95% CI 0.12–0.94, p = 0.37) in patients that received paclitaxel in combination with lapatinib and trastuzumab when compared with patients that received paclitaxel with one of the HER2 targeted agents (the TL arm was closed early). This was the first time the HER2-enriched BC intrinsic subtype was found to be predictive of response to lapatinib31,32,33,34. Lapatinib is not currently approved in the neoadjuvant setting and is unlikely to be approved based on published studies since newer TKIs appear to be more effective. However, the biomarker data being generated from the many neoadjuvant trials will be very informative for future research.
Lapatinib has also been studied in the adjuvant setting (selected studies summarized in Table 2). The TEACH study randomized patients who received neoadjuvant chemotherapy without trastuzumab to receive lapatinib or placebo. There was no difference in disease free survival (DFS) between the arms35. In the ALTTO study, patients were randomized to four treatment groups: trastuzumab (T) with or without chemotherapy, lapatinib (L) with chemotherapy, sequential treatment with T for 12 weeks followed by L or concurrent therapy T + L. Higher rates of DFS were noted in the combination arms; however, none were statistically significant and patients treated with lapatinib had higher rates of diarrhea, rash, and hepatic toxicity36. Lapatinib was associated with increased toxicity with a limited improvement in outcomes, therefore this drug is not currently used in the neoadjuvant or adjuvant setting.
Neratinib
Neratinib (HKI-272, Nerlynx®, Puma Biotechnology) is an irreversible inhibitor of EGFR, HER2, and HER437. Preclinical studies demonstrated that neratinib leads to cell cycle arrest and decreased proliferation of HER2 + cells37. The key pharmacologic characteristics of neratinib are described in Table 1.
The I-SPY 2 (NCT01042379) trial is an adaptive phase 2 trial that is evaluating targeted agents in combination with standard chemotherapy in the neoadjuvant setting. In this study, patients received paclitaxel in combination with neratinib followed by doxorubicin and cyclophosphamide. Patients in the control group received trastuzumab in combination with paclitaxel, again followed by doxorubicin and cyclophosphamide. This study included 52% hormone receptor (HR) positive tumors and 57% HER2+. A pathologic complete response (pCR) was achieved in 56% of patients in the neratinib group vs 33% in the control group in patients with HER2+/HR– BC. For HER2+/HR + disease the pCR rates were 30 and 17%, respectively38.
The exteNET study included patients with stage 2–3 HER2 + BC that had completed neoadjuvant or adjuvant trastuzumab in combination with chemotherapy. A total of 2840 women were randomized to receive adjuvant neratinib or matching placebo for 12 months39. Neratinib provided greater benefit in invasive-free survival to patients with HR + disease (hazard ratio 0.51, 95% CI 0.33–0.77, p = 0.0013) than to those with HR- BC (hazard ratio 0.93, CI 0.60–1.43, p = 0.74)39. At 8 years of follow up, in all patients neratinib was associated with an improvement in OS with an absolute benefit of 2.1% and an absolute benefit in invasive DFS of 9.1%40. The patients that received the TKI had lower rates of central nervous system (CNS) events40. Patients in this study did not receive pertuzumab or TDM1, both currently used as standard of care in early stage BC, therefore the degree of benefit of neratinib in tumors pretreated with these medications remains unknown.
Current approach
Neratinib is the only TKI currently approved for early-stage BC in the US. This drug was approved in 2017 as extended adjuvant anti-HER2 therapy after completion of trastuzumab, based on the exteNET trial39. Neratinib is often used in patients with HR + and HER2 + high-risk tumors however, its use is often limited due to high incidence of diarrhea. Antidiarrheal prophylaxis and dose escalation have been shown to decrease the incidence and severity of this toxicity41.
There has been a trend in BC to utilize more agents in the early stage setting to decrease the incidence of recurrences and progression to metastatic disease. There are several ongoing trials to assess other combinations that include TKIs in the neoadjuvant and adjuvant settings (Table 3). Many of these studies are incorporating TKI in the initial adjuvant or neoadjuvant therapy rather than in the extended adjuvant setting. Based on the impressive findings of the HER2CLIMB study, there is particular interest in the use of tucatinib. Another arm of the described ISPY2 trial (NCT01042379) is ongoing to assess the combination of tucatinib with standard chemotherapy in the neoadjuvant setting and the compassHER-RD study (NCT04457596) is evaluating the use of ado-trastuzumab-emtansine with or without tucatinib in patients with residual disease after neoadjuvant therapy42,43.
Advanced breast cancer
Lapatinib
Lapatinib was initially studied in the metastatic setting in phase 1 and 2 trials44,45. In a phase 2 study the use of lapatinib in patients with advanced HER2 + BC resulted in objective response rate (ORR) of 24%, the median time to response (TTR) was 7.9 months, and the median duration of response (DOR) was 28.4 weeks. Four patients developed asymptomatic reductions in ejection fraction (EF)45. These findings lead to development of several phase 3 clinical trials.
A phase 3 trial compared capecitabine plus or minus lapatinib in patients with locally advanced or metastatic and previously treated HER2 + BC. In the combination arm, the median time to progression was 8.8 months vs 4.4 months with capecitabine alone (hazard ratio 0.49, 95% CI 0.34–0.71, p < 0.001). Similarly, the PFS was 8.8 months vs 4.1 months, respectively (hazard ratio 0.47, 95% CI 0.33–0.67, p < 0.001). Eleven patients in the capecitabine arm developed brain metastases vs four in the combination arm (p = 0.10)46. Johnston et al. compared lapatinib plus letrozole vs letrozole alone in postmenopausal women with locally advanced or metastatic HR + BC. In women with endocrine receptor and HER2 + disease, the addition of lapatinib improved PFS when compared with letrozole alone, 8.2 months vs 3 months, respectively (hazard ratio 0.71, p = 0.019)47. The COMPLETE (MA.31) study compared HER2 directed therapy with lapatinib vs trastuzumab plus paclitaxel for 24 weeks followed by the same HER2 directed agent until disease progression in women with HER2 + metastatic BC. The PFS was 9.1 months in the lapatinib arm and 13.6 months with trastuzumab (hazard ratio 1.48, p > 0.001)48. Lapatinib is currently approved by the United States Food and Drug Administration (US FDA) for advanced or metastatic HER2 + BC in combination with capecitabine or with letrozole for patients that received prior therapy including an anthracycline, a taxane, and trastuzumab49.
Neratinib
NEfERT-T was initially a phase 3 study but accrual was reduced from 1200 to 480 therefore it was no longer powered as a phase 3 study and results were considered preliminary according to the conclusions of the authors50. In this trial, women with previously untreated metastatic HER2 + BC were randomized to receive neratinib plus paclitaxel vs trastuzumab and paclitaxel. The median PFS was 12.9 months in both groups. The ORR, clinical benefit rate and DOR were similar for both groups50. The phase 3 NALA trial compared lapatinib plus capecitabine vs neratinib plus capecitabine in patients with previously treated metastatic HER2 + BC with at least two prior lines of therapy. PFS was improved in the neratinib arm (hazard ratio 0.76, 95% CI = −0.63–0.93, p = 0.0059). There was no statistically significant difference in OS. The neratinib arm also had higher ORR and DOR. Patients with HR- disease had the greatest benefit from this combination (HR- hazard ratio 0.76, 95% CI 0.57–1.01 and for HR + hazard ratio 0.94, 95% CI 0.72–1.22). In contrast with the exteNET trial in which patients with HR + disease had a greater benefit from this TKI in the extended adjuvant setting (DFS HR + hazard ratio 0.51 vs 0.93 for HR-)20,39. Based on these studies, neratinib is approved by the US FDA in combination with capecitabine for patients who received at least two lines of HER2 directed therapy.
Tucatinib
Tucatinib (ONT-380, Tukysa®, Seattle Genetics) is a highly selective reversible HER2 inhibitor51. Tucatinib showed efficacy in preclinical models as monotherapy and in combination with chemotherapy and trastuzumab51. Additionally, xenograft models showed that BC were more sensitive to tucatinib than to lapatinib, the response to tucatinib was improved with the addition of trastuzumab52. The key pharmacologic characteristics of tucatinib are described in Table 1.
Tucatinib was approved by the US FDA in April of 2020 for the treatment of HER2 + metastatic BC, based on the phase 3 HER2CLIMB study. This was the first drug approved under Project Orbis, a collaboration between the US FDA and other international regulators to allow uniform access to therapies and clinical trials globally. In HER2CLIMB, patients with metastatic HER2 + BC who were previously treated with trastuzumab, pertuzumab, and ado-trastuzumab emtansine were randomized in a 2:1 ratio to receive trastuzumab, capecitabine, and tucatinib vs trastuzumab, capecitabine plus placebo. The PFS at 1 year was 33.1% for the tucatinib arm and 12.3% for the placebo arm (hazard ratio for disease progression or death was 0.54, 95% CI 0.42–0.71, p = <0.001). The OS at 2 years was 44.9% in the tucatinib arm and 26.6% in the placebo arm. The median PFS was 7.8 months and 5.6 months, respectively19. A recent update of the HER2CLIMB trial revealed that the benefit of tucatinib in PFS occurred irrespective of HR status53. HER2CLIMB included patients that received trastuzumab, pertuzumab, and ado-trastuzumab emtansine. However, the trial did not specify the setting. If a patient developed metastatic disease while receiving adjuvant trastuzumab and pertuzumab and was given ado-trastuzumab emtansine as first line treatment, at the time of progression the patient would have been eligible for HER2CLIMB. The HER2CLIMB regimen can be considered for patients with metastatic BC who received at least one HER2-targeted based regimen.
There is growing interest in the combination of tucatinib with antibody drug conjugates. A phase 1b trial by Borger et al.54 studied the combination of ado-trastuzumab emtansine and tucatinib in 57 patients with heavily pretreated metastatic BC; the ORR was 48% and the median PFS was 8.2 months. There are ongoing trials phase 3 trials assessing safety and efficacy of antibody drug conjugates and tucatinib in the metastatic setting (summarized in Table 3).
Pyrotinib
Pyrotinib is an irreversible inhibitor of EGFR, HER2, and HER4. This TKI was approved in China in 2018 in combination with capecitabine for patients with advanced or metastatic HER2 + BC. A phase 2 study by Ma et al.18 compared lapatinib plus capecitabine to pyrotinib plus capecitabine. The median PFS was 18 months in the pyrotinib arm and 7 months in the lapatinib arm and the response rate was 78.7% and 57.1% respectively. The most common side effects were hand foot syndrome, diarrhea, and neutropenia18. In the phase 3 study by Jiang et al.55, pyrotinib plus capecitabine was compared to placebo plus capecitabine in women with metastatic BC. The median PFS was 11 months in the pyrotinib arm and 4.1 months in the placebo arm. Interestingly, patients that progressed on the placebo arm were then treated with pyrotinib monotherapy and had a single agent response rate of 38% with a median PFS of 5.5 months55. The PHOEBE trial compared pyrotinib plus capecitabine vs lapatinib vs capecitabine in 267 patients with metastatic HER2 + BC. The median PFS was significantly longer in the pyrotinib arm (12.5 months) than in the lapatinib arm (6.8 months, p < 0.0001). Diarrhea and hand foot syndrome were more common in the pyrotinib arm56. There are multiple ongoing trials assessing the use of pyrotinib in different settings, it remains unclear if its use will expand to other countries.
Brain metastases
Brain metastases occur in up to 50% of patients with HER2 + BC and the development of central nervous system (CNS) metastases is associated with dismal outcomes57,58. The use of systemic therapy for brain metastases has been historically limited due to low CNS penetrance and limited efficacy of these medications59,60,61. Recently, a subgroup analysis of the KAMILLA study, which assessed the efficacy of ado-trastuzumab emtansine in metastatic HER2 + BC, revealed that 42.9% of the patients with baseline measurable brain metastases (n = 126) had a reduction in the size of the CNS lesions by at least 30%61. Of the patients with baseline brain metastases that did not undergo radiation therapy, 49.3% of the 67 had an at least 30% reduction of the size of their brain metastases61. Although systemic treatments for HER2 + BC brain metastases are improving, there is still an unmet need for better and durable therapies for this group of patients and TKIs represent a promising option for these patients57,58.
Lapatinib has shown to have CNS penetrance. In a phase 2 study of 242 patients with treated HER + BC brain metastases, CNS ORR was observed in 20% of patients treated with capecitabine and lapatinib62. Similarly, in the single arm phase 2 LANDSCAPE trial the combination of lapatinib and capecitabine in 45 patients with untreated BC brain metastases, reported 65.9% of the patients had an intracranial response, all partial responses63. A retrospective subgroup analysis of the EMILIA trial (which compared capecitabine plus lapatinib vs ado-trastuzumab emtansine) showed similar PFS for both arms in patients with baseline BC brain metastases. However, the PFS was 9.6 months in the ado-trastuzumab emtansine arm and 6.4 months in the capecitabine plus lapatinib arm (hazard ratio 0.65; P < 0.001). In patients with CNS disease there was a significant improvement in OS in the ado-trastuzumab emtansine arm (26.8 vs 12.9 months, hazard ratio 0.38 p = 0.008)64. In the phase 3 CEREBEL trial 540 patients with metastatic HER2 + BC were randomized to receive capecitabine plus trastuzumab vs capecitabine plus lapatinib. The incidence of brain metastases as first site of relapse was 3% in the lapatinib group and 5% in the trastuzumab group (p = 0.360). PFS and OS were longer in the trastuzumab arm and serious adverse events were more commonly reported in the lapatinib arm65.
Neratinib has also been studied in patients with brain metastases. The phase 2 study TBCRC 022 assessed the combination of neratinib and capecitabine in 49 participants with BC brain metastases with or without prior lapatinib. Patients were eligible if they had at least one lesion measuring 10 mm or more and with CNS progression after prior local therapies. The CNS ORR was 49% in participants not previously treated with lapatinib and 33% in patients who previously received this medication. The median PFS was 5.5 and 3.1 months, respectively66. In the NEfERT-T study (neratinib plus paclitaxel vs trastuzumab and paclitaxel) symptomatic or progressive CNS disease occurred in 8.3% patients in the neratinib arm and 17.3% of the trastuzumab arm (p = 0.002), the cumulative incidence of brain metastases was 20.2% in the neratinib arm and 10.1% in the trastuzumab arm (p = 0.002). Of note, twice as many patients had baseline CNS involvement in the trastuzumab arm compared to the neratinib arm, these patients were diagnosed with symptoms prior to the study as NrfERT-T did not include baseline screening for brain metastases50. The phase 3 NALA trial included patients with stable or asymptomatic brain metastases. This study showed improved PFS and time to CNS intervention with neratinib-capecitabine when compared with lapatinib-capecitabine and included participants with stable and asymptomatic brain metastases. In NALA, 22.8% of patients on the neratinib arm and 29.2% on the lapatinib arm required CNS-directed interventions (p = 0.043)20. These studies have shown CNS penetration and clinical efficacy of neratinib in pretreated patients with HER2 + BC brain metastases which may be improved compared to lapatinib.
Tucatinib in combination with trastuzumab and capecitabine showed impressive results in the HER2CLIMB trial. This study was unique in that it included 291 patients with brain metastases (n = 198 in the tucatinib arm and n = 93 in the placebo arm), which were classified as active or stable based on a baseline magnetic resonance imaging. Forty percent of the patients had treated or stable CNS disease, 37% treated and progressing, and 22% untreated brain metastases. The risk of intracranial progression or death was reduced by 68% in the tucatinib arm. The intracranial ORR was 47.3% in the tucatinib arm vs 20% in the control arm (p = 0.03). The median central nervous system (CNS) PFS was 9.9 months vs 4.2 months and the median PFS was 18 months vs 12 months, respectively. In the subset of patients whom did not receive radiation therapy, the CNS PFS was 8.1 months in the tucatinib arm vs 3.1 months in the control arm. The 1-year OS in the tucatinib arm was 70.1 vs 46.7% in the control arm. CNS progression occurred in 21 patients in the tucatinib arm and nine in the control arm; these patients underwent local therapy and then continued their study treatment. In those patients the time from random assignment to second progression was 15.9 months in the tucatinib arm and 9.7 months in the control arm (p = 0.009) and the median time from progression in the brain to second progression or death was 7.6 and 3.1 months, respectively67. Tucatinib was the first FDA approval to specifically include patients with brain metastases in the indication statement. Based on these results, there has been growing interest to use tucatinib in the first line metastatic setting for patients with BC brain metastases68. The ongoing TOPAZ trial (NCT04512261) is assessing the combination of tucatinib with the immune checkpoint inhibitor pembrolizumab and trastuzumab in patients with HER2 + BC brain metastases.
HER2 mutations
Although amplification and overexpression are the most commonly present alterations in HER2, activating mutations have been described as an alternative driver mechanism and a poor prognostic factor in BC9,10. HER2 mutations are found in 2.8% of BCs. Though not routinely performed, next generation sequencing use is increasing in the metastatic setting, and its use will likely continue to grow as more targeted agents are developed and approved9,10.
Neratinib has been studied in HER2 mutant tumors in the phase 2 SUMMIT trial69. Forty-six patients with HR+ and HER2 mutant metastatic BC received the combination of neratinib, trastuzumab, and fulvestrant. In this cohort, 12 allelic variations of HER2 were identified with L755S accounting for 33%. Clinical activity was evaluated in 30 out of the 46 patients and 12 (40%) achieved a partial response and none achieved a complete response. The median DOR was 8.4 months. And the median PFS was 8.3 months70. Other TKIs are being studied in this setting9.
Mechanisms of resistance to HER2-directed therapies
Intrinsic and acquired mechanisms of resistance to HER2-targeted agents have been described. HER2 reactivation or signaling downstream from the HER2 receptor are some of the commonly described mechanisms of resistance. HER2 mutations are a well-known intrinsic and acquired mechanisms of resistance69,71. Different types of mutations have been described, including activating mutations and mutations that modify the receptor preventing binding of monoclonal antibodies25,71,72. L755S is the most frequently identified acquired activating mutation of HER2 which is more commonly seen in metastatic tumors. L755S has been described as an acquired mechanism of resistance to lapatinib and cross resistance to tucatinib has been reported in vitro. However, the resistant cells appear to be sensitive to neratinib, which is a pan-HER2 TKI71,73. More studies are needed to determine the clinical significance of this mutations to allow us to tailor treatment based on the specific mutations.
Another known mechanism of resistance is the incomplete blockade of the HER2 family receptor which can lead to upregulation of several HER family receptors74. This mechanism can be overcome by adding pertuzumab to trastuzumab or combining it with a potent TKI with the goal of targeting different HER2 family receptors25. For example, an increase in HER3 transcription has been described after treatment with lapatinib. This mechanism is mediated by AXL activation. A multi-kinase inhibitor, such as foretinib a MET inhibitor, which has activity against AXL can overcome this mechanism of resistance in vitro75,76.
Alterations in downstream signaling pathways have also been described as a resistance mechanism. For example, activating mutations in PIK3CA or low levels of expression of tumor suppressor genes (such as PTEN) can lead to tumor proliferation25,77,78. The cyclin pathway also plays a role in resistance to HER2-targeted agents. Preclinical data has shown that the sensitivity to HER2-directed therapies can be restored by targeting cyclin dependent kinases 4 and 679. Bidirectional cross talk between HER2 and estrogen receptors is another known resistance mechanism for tumors that express both receptors. As estrogen receptor can serve as an escape pathway to HER2 inhibition, concurrent blockade can prevent this phenomenon in vitro80.
The tumor immune infiltrate also plays a key role in the response to trastuzumab. Tumors with higher quantities of tumor infiltrating lymphocytes (TIL) have been associated with a better response to trastuzumab81,82,83. In the MA.31 study, patients with lower levels of cytotoxic stromal TIL had a greater benefit with trastuzumab when compared to lapatinib. Future studies are needed to determine the predictive role of TIL in the response to different HER2 TKI84. PDL1 expression has been proposed as a mechanism of resistance to trastuzumab. The combination of HER-targeted agents with immune checkpoint inhibitors has shown to be effective in patients with PDL1 positive metastatic disease who were previously treated with trastuzumab85,86. There are multiple studies ongoing to continue to identify mechanisms of resistance and strategies to overcome resistance, as well as the role of TKIs in this setting25,70.
Safety profile
Each TKI targets different HER-receptors therefore these drugs are associated with unique toxicity profiles. Table 4 summarizes the most commonly reported adverse events (AE) based on landmark studies for HER2 + metastatic BC.
Gastrointestinal toxicities
Although all the HER2 TKIs are associated to diarrhea, neratinib, and pyrotinib use leads to higher rates in all grades (83 and 97% respectively), likely due to effects on EGFR. The use of neratinib requires intensive loperamide prophylaxis and the FDA recommends to start loperamide with the first dose of neratinib and continue for the first two cycles to maintain 1–2 bowel movements a day. This is particularly important to ensure adherence in the extended adjuvant setting66,87. The CONTROL trial assessed the use of different regimens of antidiarrheal prophylaxis in patients receiving adjuvant neratinib. Dose escalation was started at 120 mg daily for a week, followed by 160 mg daily for a week then increased to 240 mg daily in one arm and in the other arm it was started at 160 mg daily for 14 days, followed by 200 mg daily for 14 days and then increased to 240 mg daily. There were four regimens for diarrhea prophylaxis that included loperamide, budesonide, and/or colestipol. These interventions, particularly budesonide and loperamide as well as dose escalation, lead to lower incidence, duration, and severity of diarrhea. In the CONTROL trial 3–12% of patients required dose reductions when compared to 26% of the patients in the exteNET trial. All patients receiving neratinib should receive antidiarrheal prophylaxis and dose escalation should be considered41. Similarly, tucatinib has been associated with diarrhea in 81% of cases, with only 12% being grade 3 and 0.5% grade 4. Antidiarrheal prophylaxis was not specified in the HER2CLIMB trial, however loperamide and/or budesonide can be utilized as prophylaxis and to treat this common side effect19,88. Other common gastrointestinal AE are listed in Table 4.
Cardiotoxicity
Cardiac toxicity has been reported in patients taking TKI which is particularly relevant as these medications are often given in combination or after other cardiotoxic treatments, such as anthracyclines, trastuzumab, and/or radiation therapy. Many of the pivotal clinical trials excluded patients with baseline cardiac abnormalities therefore the rates of cardiac toxicity may have been underestimated. Lapatinib was initially associated with a decrease in the left ventricular ejection fraction (LVEF) in 2–5% of patients49. Neratinib has not been associated with this toxicity87. Tucatinib was associated with a decrease in LVEF in 1% of patients88. Given that the incidence of symptomatic decrease in LVEF is low there and there are no clear recommendations for cardiac monitoring, however cardiac imaging (echocardiogram) can be considered in a case by case basis for patients with known heart failure or with multiple cardiovascular risk factors who have received cardiotoxic cancer-directed therapies.
Conclusions and future directions
HER2-targeted TKIs have been approved for specific populations of BC patients (Fig. 2). With the increasing number of therapies approved for HER2 + BC, there are a lot of unanswered questions; particularly about efficacy and safety of drug combinations and toxicities. Another unanswered question is if there is cross resistance of TKI and how to select TKI based on prior HER2 directed treatments.
Figure 3 shows the current proposed treatment algorithm based on the US FDA approved HER2-targeted agents. Note that there are multiple options for metastatic disease beyond second line. Tucatinib can be considered in the third line setting based on the results of the phase 3 HER2CLIMB study, particularly in those with CNS involvement19,67. Other agents can be considered as fourth line or beyond, based on prior therapies, toxicity profile, and comorbidities. Fam-trastuzumab deruxtecan showed durable responses in heavily pretreated patients with metastatic HER2 + BC in a phase 2 single arm study89. We are awaiting for the results of the phase 3 trials to better assess effectiveness and toxicity profile and to determine the optimal line of therapy for this promising antibody-drug conjugate89. Margetuximab was recently approved in the metastatic setting for patients that received at least two prior lines of therapy based on the phase 3 SOPHIA study90. Other options include the previously mentioned lapatinib and neratinib in combination with capecitabine20,46,47. The details about these trials and drugs are outside of the scope of this review.
This is a suggested treatment algorithm for early stage and advanced human epidermal growth factor 2 positive BC, based on the current United States Food and Drug Administration approvals. Radiation therapy and endocrine therapy should be incorporated when appropriate. There are multiple options for metastatic disease beyond second line, tucatinib can be considered in the third line setting based on the results of the phase 3 HER2CLIMB study. Other agents can be considered as fourth line or beyond, based on prior therapies, toxicity profile and comorbidities. *Subgroup analysis of APHINITY showed that patients with node positive disease have a greater benefit from adjuvant dual HER2 blockade. **Benefit seen in patients with hormone receptor positive and HER2 positive disease. There are no data about the use of neratinib after treatment with pertuzumab and/or ado-trastuzumab emtansine. ***Tucatinib is FDA approved in the second line setting after treatment with trastuzumab, pertuzumab and ado-trastuzumab emtansine. Can be considered for patients with CNS involvement.
Does BC phenotype particularly HR status, plays a role in the TKI selection? Lapatinib is the only TKI approved in combination with endocrine therapy, however, 48–60% of patients enrolled in the mentioned landmark phase 3 studies for the other TKIs had HR+ disease and all groups benefited from TKI therapy. A recent update of the HER2CLIMB trial revealed that the benefit of tucatinib was irrespective of HR status53. Based on the current information, HR status should not play a significant role while selecting HER2-targeted TKI19,20,46,47,53. There is an ongoing study to determine the safety and efficacy of the combination of a HER2 TKI with a cyclin dependent kinase 4 and 6 inhibitor (NCT03054363). Preclinical models suggested that the combination of tucatinib, abemaciclib and fulvestrant lead to significant tumor regressions52. However, a clinical trial that attempted to combine abemaciclib with tucatinib and trastuzumab (NCT03846583) was withdrawn. Both tucatinib and abemaciclib are metabolized by CYP3A and tucatinib has shown to inhibit CYP2C8, CYP2C9, and CYP3A which could increase the plasma concentration of both drugs and therefore increase their toxicity making the combination difficult to tolerate91,92.
It is possible that tucatinib or other highly selective HER2 TKI will be used more broadly in the near future, as the results from the initial trials were impressive, including CNS efficacy, and it appears to be better tolerated. The COMPASS-RD trial will examine tucatinib added to ado-trastuzumab emtansine in the adjuvant setting. The HER2CLIMB2 study is also assessing the use of ado-trastuzumab emtansine with or without tucatinib in patients with HER2 + metastatic BC. The single arm phase 2 HER2CLIMB4 study (NCT04539938) will assess the use of fam-trastuzumab deruxtecan in combination with tucatinib.
Other HER2 targeted TKIs are being investigated. Epertinib (S-22611) is a reversible inhibitor of EGFR, HER2, and HER4 that has shown good results in combination with trastuzumab with or without capecitabine in a phase 1/2 study that included patients with CNS disease23. Varlitinib (ASLAN001) is another reversible inhibitor of EGFR, HER2, and HER4 that is being studied in combination with chemotherapy for advanced solid tumors in the NCT02396108 trial22. Poziotinib is an irreversible inhibitor of EGFR, HER2, and HER4 that has also been studied in BC93. Ongoing studies will help determine their efficacy and unique toxicity profiles as well as the role of this pan-HER TKI in the treatment of HER + BC.
Some remaining questions include the duration of HER2-directed TKI in patients with early stage BC and for those with metastatic disease that achieve a complete response and a good quality of life. The role of TKI in de-escalation of therapy has also not been fully elucidated. Another remaining question is treatment sequencing, as it is possible that some drugs allow for better responses used as first line. Also, combinations with immunotherapeutic agents are a promising area of research in HER2 positive BC and the role that TKIs play in this arena is unknown.
The treatment paradigm of HER2 + BC is evolving rapidly, leading to improved prognosis and quality of life of patients diagnosed with early and metastatic disease. However, there are still many unanswered questions and there are ongoing trials to try to find the answers to further optimize the treatment and outcomes of this patient population for best quality of life.
Data availability
Source data for all figures and tables are provided in the paper. No new data sets have been generated or analyzed for this article.
References
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 70, 7–30 (2020).
Ferlay J. E. M. et al. Global Cancer Observatory: Cancer Today (WHO, 2018).
Institute, N. C. Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Mortality - All COD, Aggregated With State, Total U.S. (1969-2017) (NIH, 2019).
Slamon, D. J. et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235, 177–182 (1987).
Slamon, D. J. et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 244, 707–712 (1989).
Spector, N. L. & Blackwell, K. L. Understanding the mechanisms behind trastuzumab therapy for human epidermal growth factor receptor 2-positive breast cancer. J. Clin. Oncol. 27, 5838–5847 (2009).
Yarden, Y. & Sliwkowski, M. X. Untangling the ErbB signalling network. Nat. Rev. Mol. Cell Biol. 2, 127–137 (2001).
Mitri, Z., Constantine, T. & O’Regan, R. The HER2 receptor in breast cancer: pathophysiology, clinical use, and new advances in therapy. Chemother. Res Pract. 2012, 743193 (2012).
Bose, R. et al. Activating HER2 mutations in HER2 gene amplification negative breast cancer. Cancer Discov. 3, 224–237 (2013).
Connell, C. M. & Doherty, G. J. Activating HER2 mutations as emerging targets in multiple solid cancers. ESMO Open 2, e000279 (2017).
Genentech. Trastuzumab [package insert]. 2010. US Food and Drug Administration website. (2010). https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/103792s5250lbl.pdf. Accessed 17 March, 2021.
HopeS. R. et al. Abstract GS1-02: Phase 3 SOPHIA study of margetuximab + chemotherapy vs trastuzumab + chemotherapy in patients with HER2+ metastatic breast cancer after prior anti-HER2 therapies: second interim overall survival analysis. Cancer Res. https://doi.org/10.1158/1538-7445.SABCS19-GS1-02 (2020).
Boehringer Ingelheim Pharmaceuticals. Afatinib [package insert]. 2018. US Food and Drug Administration website. (2018). https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/201292s014lbl.pdf. Accessed 17 March, 2021.
Cortes, J. et al. Afatinib alone or afatinib plus vinorelbine versus investigator’s choice of treatment for HER2-positive breast cancer with progressive brain metastases after trastuzumab, lapatinib, or both (LUX-Breast 3): a randomised, open-label, multicentre, phase 2 trial. Lancet Oncol. 16, 1700–1710 (2015).
Harbeck, N. et al. Afatinib plus vinorelbine versus trastuzumab plus vinorelbine in patients with HER2-overexpressing metastatic breast cancer who had progressed on one previous trastuzumab treatment (LUX-Breast 1): an open-label, randomised, phase 3 trial. Lancet Oncol. 17, 357–366 (2016).
Eskens, F. A. et al. A phase I dose escalation study of BIBW 2992, an irreversible dual inhibitor of epidermal growth factor receptor 1 (EGFR) and 2 (HER2) tyrosine kinase in a 2-week on, 2-week off schedule in patients with advanced solid tumours. Br. J. Cancer 98, 80–85 (2008).
Lin, N. U. et al. A phase II study of afatinib (BIBW 2992), an irreversible ErbB family blocker, in patients with HER2-positive metastatic breast cancer progressing after trastuzumab. Breast Cancer Res. Treat. 133, 1057–1065 (2012).
Ma, F. et al. Pyrotinib or lapatinib combined with capecitabine in HER2-positive metastatic breast cancer with prior taxanes, anthracyclines, and/or trastuzumab: a randomized, phase ii study. J. Clin. Oncol. 37, 2610–2619 (2019).
Murthy, R. K. et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N. Engl. J. Med. 382, 597–609 (2020).
Saura, C. et al. Neratinib plus capecitabine versus lapatinib plus capecitabine in her2-positive metastatic breast cancer previously treated with >/= 2 HER2-directed regimens: phase III NALA trial. J. Clin. Oncol. 38, 3138–3149 (2020).
Moulder, S. L. et al. Phase I study of ONT-380, a HER2 inhibitor, in patients with HER2(+)-advanced solid tumors, with an expansion cohort in HER2(+) metastatic breast cancer (MBC). Clin. Cancer Res. 23, 3529–3536 (2017).
clinicaltrials.gov. Dose-confirmation Study of ASLAN001 Combined With Weekly Paclitaxel and Carboplatin in Advanced Solid Tumours, Followed by a Study in Patients With Stage I-III HER2 Positive Breast Cancer. NCT identifier NCT02396108 (NIH, 2017).
Macpherson, I. R. et al. A phase I/II study of epertinib plus trastuzumab with or without chemotherapy in patients with HER2-positive metastatic breast cancer. Breast Cancer Res. 22, 1 (2019).
Pohlmann, P. R., Mayer, I. A. & Mernaugh, R. Resistance to trastuzumab in breast cancer. Clin. Cancer Res. 15, 7479–7491 (2009).
Goutsouliak, K. et al. Towards personalized treatment for early stage HER2-positive breast cancer. Nat. Rev. Clin. Oncol. 17, 233–250 (2020).
Xia, W. et al. Anti-tumor activity of GW572016: a dual tyrosine kinase inhibitor blocks EGF activation of EGFR/erbB2 and downstream Erk1/2 and AKT pathways. Oncogene 21, 6255–6263 (2002).
Baselga, J. et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet 379, 633–640 (2012).
Carey, L. A. et al. Molecular heterogeneity and response to neoadjuvant human epidermal growth factor receptor 2 targeting in CALGB 40601, a randomized phase III trial of paclitaxel plus trastuzumab with or without lapatinib. J. Clin. Oncol. 34, 542–549 (2016).
Robidoux, A. et al. Lapatinib as a component of neoadjuvant therapy for HER2-positive operable breast cancer (NSABP protocol B-41): an open-label, randomised phase 3 trial. Lancet Oncol. 14, 1183–1192 (2013).
Untch, M. et al. Lapatinib versus trastuzumab in combination with neoadjuvant anthracycline-taxane-based chemotherapy (GeparQuinto, GBG 44): a randomised phase 3 trial. Lancet Oncol. 13, 135–144 (2012).
Fernandez-Martinez, A. et al. Survival, pathologic response, and genomics in CALGB 40601 (Alliance), a neoadjuvant phase III trial of paclitaxel-trastuzumab with or without lapatinib in her2-positive breast cancer. J. Clin. Oncol. https://doi.org/10.1200/JCO.20.01276 (2020).
Schettini, F. et al. HER2-enriched subtype and pathological complete response in HER2-positive breast cancer: A systematic review and meta-analysis. Cancer Treat. Rev. 84, 101965 (2020).
Prat, A. et al. HER2-enriched subtype and ERBB2 expression in HER2-positive breast cancer treated with dual HER2 blockade. J. Natl Cancer Inst. 112, 46–54 (2020).
Swain, S. M. et al. Pathologic complete response and outcomes by intrinsic subtypes in NSABP B-41, a randomized neoadjuvant trial of chemotherapy with trastuzumab, lapatinib, or the combination. Breast Cancer Res. Treat. 178, 389–399 (2019).
Goss, P. E. et al. Adjuvant lapatinib for women with early-stage HER2-positive breast cancer: a randomised, controlled, phase 3 trial. Lancet Oncol. 14, 88–96 (2013).
Piccart-Gebhart, M. et al. Adjuvant lapatinib and trastuzumab for early human epidermal growth factor receptor 2-positive breast cancer: results from the randomized phase III adjuvant lapatinib and/or trastuzumab treatment optimization trial. J. Clin. Oncol. 34, 1034–1042 (2016).
Rabindran, S. K. et al. Antitumor activity of HKI-272, an orally active, irreversible inhibitor of the HER-2 tyrosine kinase. Cancer Res. 64, 3958–3965 (2004).
Park, J. W. et al. Adaptive randomization of neratinib in early breast cancer. N. Engl. J. Med. 375, 11–22 (2016).
Chan, A. et al. Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 17, 367–377 (2016).
Chan A., et al. Final efficacy results of neratinib in HER2-positive hormone receptor-positive early- stage breast cancer from the phase III ExteNET trial. Clin. Breast Cancer https://doi.org/10.1016/j.clbc.2020.09.014. (2020).
Barcenas, C. H. et al. Improved tolerability of neratinib in patients with HER2-positive early-stage breast cancer: the CONTROL trial. Ann. Oncol. 31, 1223–1230 (2020).
clinicaltrials.gov. T-DM1 and Tucatinib Compared With T-DM1 Alone in Preventing Relapses in People With High Risk HER2-Positive Breast Cancer, the CompassHER2 RD Trial. Identifier NCT04457596 (NIH, 2020).
clinicaltrials.gov. I-SPY TRIAL: Neoadjuvant and Personalized Adaptive Novel Agents to Treat Breast Cancer (I-SPY). Identifier: NCT01042379 (NIH, 2010).
Storniolo, A. M. et al. Phase I dose escalation and pharmacokinetic study of lapatinib in combination with trastuzumab in patients with advanced ErbB2-positive breast cancer. J. Clin. Oncol. 26, 3317–3323 (2008).
Gomez, H. L. et al. Efficacy and safety of lapatinib as first-line therapy for ErbB2-amplified locally advanced or metastatic breast cancer. J. Clin. Oncol. 26, 2999–3005 (2008).
Geyer, C. E. et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N. Engl. J. Med. 355, 2733–2743 (2006).
Johnston, S. et al. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. J. Clin. Oncol. 27, 5538–5546 (2009).
Gelmon, K. A. et al. Lapatinib or trastuzumab plus taxane therapy for human epidermal growth factor receptor 2-positive advanced breast cancer: final results of NCIC CTG MA.31. J. Clin. Oncol. 33, 1574–1583 (2015).
GlaxoSmithKline. Lapatinib [package insert] 2010. US Food and Drug Administration Website. (2010). https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/022059s007lbl.pdf. Accesed 17 March, 2021.
Awada, A. et al. Neratinib plus paclitaxel vs trastuzumab plus paclitaxel in previously untreated metastatic ERBB2-positive breast cancer: the nefert-t randomized clinical trial. JAMA Oncol. 2, 1557–1564 (2016).
Kulukian, A. et al. Preclinical activity of HER2-selective tyrosine kinase inhibitor tucatinib as a single agent or in combination with trastuzumab or docetaxel in solid tumor models. Mol. Cancer Ther. 19, 976–987 (2020).
N. A. O’Brien et al. The small molecule inhibitor of HER2, tucatinib, has potent and highly selective activity in preclinical modes of HER2-driven cancer. Cancer Res. https://doi.org/10.1158/1538-7445.SABCS18-P6-17-11 (2019).
Hamilton, E. et al. in Presented at the virtual San Antonio Breast cancer Symposium, December 8–11, 2020. Asbtract (2020).
Borges, V. F. et al. Tucatinib combined with ado-trastuzumab emtansine in advanced ERBB2/HER2-positive metastatic breast cancer: a phase 1b clinical trial. JAMA Oncol. 4, 1214–1220 (2018).
Jiang, Z. et al. Pyrotinib combined with capecitabine in women with HER2+ metastatic breast cancer previously treated with trastuzumab and taxanes: a randomized phase III study. J. Clin. Oncol. 37, 1001–1001 (2019).
Xu, B. et al. Pyrotinib plus capecitabine versus lapatinib plus capecitabine for the treatment of HER2-positive metastatic breast cancer (PHOEBE): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol. https://doi.org/10.1016/S1470-2045(20)30702-6 (2021).
Bendell, J. C. et al. Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer 97, 2972–2977 (2003).
Lai, R., Dang, C. T., Malkin, M. G. & Abrey, L. E. The risk of central nervous system metastases after trastuzumab therapy in patients with breast carcinoma. Cancer 101, 810–816 (2004).
Lin, N. U. et al. Pertuzumab (P) plus high-dose trastuzumab (H) for the treatment of central nervous system (CNS) progression after radiotherapy (RT) in patients (pts) with HER2-positive metastatic breast cancer (MBC): primary eficacy analysis results from the phase II PATRICIA study. [Abstracts: 2019 San Antonio Breast Cancer Symposium; December 10-14, 2019; San Antonio] Cancer Res. 80, P1-18-03 (2020).
Montemurro, F. et al. Safety of trastuzumab emtansine (T-DM1) in patients with HER2-positive advanced breast cancer: Primary results from the KAMILLA study cohort 1. Eur. J. Cancer 109, 92–102 (2019).
Montemurro, F. et al. Trastuzumab emtansine (T-DM1) in patients with HER2-positive metastatic breast cancer and brain metastases: exploratory final analysis of cohort 1 from KAMILLA, a single-arm phase IIIb clinical trial(). Ann. Oncol. 31, 1350–1358 (2020).
Lin, N. U. et al. Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin. Cancer Res. 15, 1452–1459 (2009).
Bachelot, T. et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. Lancet Oncol. 14, 64–71 (2013).
Krop, I. E. et al. Trastuzumab emtansine (T-DM1) versus lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer and central nervous system metastases: a retrospective, exploratory analysis in EMILIA. Ann. Oncol. 26, 113–119 (2015).
Pivot, X. et al. CEREBEL (EGF111438): a phase III, randomized, open-label study of lapatinib plus capecitabine versus trastuzumab plus capecitabine in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J. Clin. Oncol. 33, 1564–1573 (2015).
Freedman, R. A. et al. TBCRC 022: a phase II trial of neratinib and capecitabine for patients with human epidermal growth factor receptor 2-positive breast cancer and brain metastases. J. Clin. Oncol. 37, 1081–1089 (2019).
Lin, N. U. et al. Intracranial efficacy and survival with tucatinib plus trastuzumab and capecitabine for previously treated HER2-positive breast cancer with brain metastases in the HER2CLIMB trial. J. Clin. Oncol. 38, 2610–2619 (2020).
Seagen (tucatinib) [package insert]. 2020. US Food and Drug Administration website. (2020). https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/213411s000lbl.pdf. Accessed 17 March, 2021.
Smyth, L. M. et al. Update on the phase II SUMMIT trial: Neratinib 1 fulvestrant for HER2- mutant, HR-positive, metastatic breast cancer. Ann. Oncol. 30, III10–III11 (2019).
Jhaveri, K. S. C. & Guerrero-Zotano, A. in Presented at the virtual San Antonio Breast cancer Symposium, December 8-11, 2020. Asbtract (2020).
Xu, X. et al. HER2 reactivation through acquisition of the HER2 L755S mutation as a mechanism of acquired resistance to HER2-targeted therapy in HER2(+) breast cancer. Clin. Cancer Res. 23, 5123–5134 (2017).
Scaltriti, M. et al. Expression of p95HER2, a truncated form of the HER2 receptor, and response to anti-HER2 therapies in breast cancer. J. Natl Cancer Inst. 99, 628–638 (2007).
Veeraraghavan, J. et al. HER2 L755S mutation is associated with acquired resistance to lapatinib and neratinib, and confers cross-resistance to tucatinib in HER2-positive breast cancer models. Cancer Res. https://doi.org/10.1158/1538-7445.AM2020-1911 (2020).
Ritter, C. A. et al. Human breast cancer cells selected for resistance to trastuzumab in vivo overexpress epidermal growth factor receptor and ErbB ligands and remain dependent on the ErbB receptor network. Clin. Cancer Res. 13, 4909–4919 (2007).
Liu, L. et al. Novel mechanism of lapatinib resistance in HER2-positive breast tumor cells: activation of AXL. Cancer Res. 69, 6871–6878 (2009).
Sergina, N. V. et al. Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3. Nature 445, 437–441 (2007).
Berns, K. et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell 12, 395–402 (2007).
Nagata, Y. et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell 6, 117–127 (2004).
Goel, S. et al. Overcoming therapeutic resistance in HER2-positive breast cancers with CDK4/6 inhibitors. Cancer Cell 29, 255–269 (2016).
Giuliano, M. et al. Upregulation of ER signaling as an adaptive mechanism of cell survival in HER2-positive breast tumors treated with anti-HER2 therapy. Clin. Cancer Res. 21, 3995–4003 (2015).
Denkert, C. et al. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J. Clin. Oncol. 33, 983–991 (2015).
Kim, R. S. et al. Stromal tumor-infiltrating lymphocytes in NRG oncology/NSABP B-31 adjuvant trial for early-stage HER2-positive breast cancer. J. Natl Cancer Inst. 111, 867–871 (2019).
Luen, S. J., Savas, P., Fox, S. B., Salgado, R. & Loi, S. Tumour-infiltrating lymphocytes and the emerging role of immunotherapy in breast cancer. Pathology 49, 141–155 (2017).
Liu, S. et al. Role of cytotoxic tumor-infiltrating lymphocytes in predicting outcomes in metastatic HER2-positive breast cancer: a secondary analysis of a randomized clinical trial. JAMA Oncol. 3, e172085 (2017).
Emens, L. A. et al. Trastuzumab emtansine plus atezolizumab versus trastuzumab emtansine plus placebo in previously treated, HER2-positive advanced breast cancer (KATE2): a phase 2, multicentre, randomised, double-blind trial. Lancet Oncol. 21, 1283–1295 (2020).
Loi, S. et al. Pembrolizumab plus trastuzumab in trastuzumab-resistant, advanced, HER2-positive breast cancer (PANACEA): a single-arm, multicentre, phase 1b-2 trial. Lancet Oncol. 20, 371–382 (2019).
Puma. Neratinib [Package intesert]. 2021. US Food and Drug Administration website. (2020). https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208051s005s006lbl.pdf. Accessed 17 March 2021.
Administration, F. F. A. D. Highlights Of Prescribing Information - TUKYSATM (tucatinib) (2020).
Modi, S. et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N. Engl. J. Med. https://doi.org/10.1056/NEJMoa1914510 (2019).
Rugo, H. S. et al. Efficacy of margetuximab vs trastuzumab in patients with pretreated erbb2-positive advanced breast cancer: a phase 3 randomized clinical trial. JAMA Oncol, https://doi.org/10.1001/jamaoncol.2020.7932 (2021).
Posada, M. M. et al. Predicting clinical effects of CYP3A4 modulators on abemaciclib and active metabolites exposure using physiologically based pharmacokinetic modeling. J. Clin. Pharmacol. 60, 915–930 (2020).
Topletz-Erickson, A. R. et al. Tucatinib inhibits CYP3A, CYP2C8 and P-gp-mediated elimination and is impacted by CYP2C8 inhibition in healthy volunteers. Cancer Res. https://doi.org/10.1158/1538-7445.AM2020-3016 (2020).
Park, Y. H. et al. A phase II trial of the pan-HER inhibitor poziotinib, in patients with HER2-positive metastatic breast cancer who had received at least two prior HER2-directed regimens: results of the NOV120101-203 trial. Int. J. Cancer 143, 3240–3247 (2018).
Acknowledgements
This study was supported by the Breast Cancer Research Foundation (BCRF-20-156 2020-2021 and BCRF-19-156 2019-2020).
Author information
Authors and Affiliations
Contributions
I.S. and S.M.S. conceived the work and wrote the manuscript. All the authors read and approved the final version of the manuscript and are accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
I.S has nothing to disclose. S.M.S.: Member, independent data monitoring committee: AstraZeneca. Consulting: Athenex, Daiichi-Sankyo, Silverback Therapeutics, Exact Sciences (Genomic Health), Beijing Medical Foundation, Natera, AstraZeneca, Genentech/Roche. Nonpromotional speaking: Genentech/Roche. Research to institution: Genentech/Roche, Kailos Genetics. Third party writing assistance: Genentech/Roche.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schlam, I., Swain, S.M. HER2-positive breast cancer and tyrosine kinase inhibitors: the time is now. npj Breast Cancer 7, 56 (2021). https://doi.org/10.1038/s41523-021-00265-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41523-021-00265-1
This article is cited by
-
Unlocking the power of precision medicine: exploring the role of biomarkers in cancer management
Future Journal of Pharmaceutical Sciences (2024)
-
Management of patients with advanced-stage HER2-positive breast cancer: current evidence and future perspectives
Nature Reviews Clinical Oncology (2024)
-
Elucidating molecular mechanisms and therapeutic synergy: irreversible HER2-TKI plus T-Dxd for enhanced anti-HER2 treatment of gastric cancer
Gastric Cancer (2024)
-
Addressing Residual Disease in HER2-Positive and Triple-Negative Breast Cancer: What Is Next?
Current Oncology Reports (2024)
-
Pyrotinib-based therapeutic approaches for HER2-positive breast cancer: the time is now
Breast Cancer Research (2023)