Abstract
Probiotics hold promise as a potential therapy for colorectal cancer (CRC), but encounter obstacles related to tumor specificity, drug penetration, and dosage adjustability. In this study, genetic circuits based on the E. coli Nissle 1917 (EcN) chassis were developed to sense indicators of tumor microenvironment and control the expression of therapeutic payloads. Integration of XOR gate amplify gene switch into EcN biosensors resulted in a 1.8-2.3-fold increase in signal output, as confirmed by mathematical model fitting. Co-culturing programmable EcNs with CRC cells demonstrated a significant reduction in cellular viability ranging from 30% to 50%. This approach was further validated in a mouse subcutaneous tumor model, revealing 47%-52% inhibition of tumor growth upon administration of therapeutic strains. Additionally, in a mouse tumorigenesis model induced by AOM and DSS, the use of synthetic bacterial consortium (SynCon) equipped with multiple sensing modules led to approximately 1.2-fold increased colon length and 2.4-fold decreased polyp count. Gut microbiota analysis suggested that SynCon maintained the abundance of butyrate-producing bacteria Lactobacillaceae NK4A136, whereas reducing the level of gut inflammation-related bacteria Bacteroides. Taken together, engineered EcNs confer the advantage of specific recognition of CRC, while SynCon serves to augment the synergistic effect of this approach.
Similar content being viewed by others

Introduction
Malignant neoplasms pose a huge challenge on global health, affecting the health and life quality of individuals worldwide1. Colorectal cancer (CRC) stands out as one of the most prevalent life-threatening cancers, with genetic and environmental risk factors contributing to its onset2. The survival rate for advanced stage colorectal cancer remains low3 despite the availability of treatment options, such as minimally invasive surgery, chemotherapy, radiation therapy, and immunotherapy4. The drawbacks of these regimens mainly include nonspecific toxicity to rapidly dividing normal cells5, multidrug-resistant cell formation6 and cytokine storm7. Even targeted therapies (e.g. Bevacizumab or Aflibercept) with low side effects often come with limited practical treatment options8. Furthermore, the occurrence of metastasis and its recurrence severely limits conventional treatment options for CRC9. Hence, there is a dire need to develop novel therapies to complement or substitute traditional therapies for CRC treatment.
Due to inhibition of intratumor immune surveillance and availability of necrotic tumor core nutrients, the ability of bacteria to selectively homing tumors promotes novel models of cancer treatment and diagnosis10,11. Natural bacteria, such as Coley toxin, have been used to treat patients with malignant tumors since 100 years ago12. The Bacillus Calmette-Guérin, the anti-tuberculosis vaccine, is also used to treat non-muscular invasive bladder cancer13. Some anaerobic bacteria, such as Salmonella14,15 and Clostridium16,17, can selectively invade oxygen-deprived areas of tumors and destroy cancer cells. However, the risk of toxicity and infection has hindered the clinical application of natural bacteria as anticancer agents18,19.
Probiotics have been used to ameliorate human diseases, with successful applications in mitigating hyperuricemia20,21 and alleviating heavy metal toxicity22. Nevertheless, the inadequacy of innate therapeutic potency of natural probiotic remains an obstacle to their applications on CRC treatments23,24. Synthetic gene circuits have been designed to improve the application potential of bacteriotherapy25,26,27. In an impressive study, utilizing a synchronous bacterial lysis cycle for S. typhimurium modification continuously delivered therapeutic drugs in tumor regions28. Engineered E. coli harboring myrosinase and tumor-targeting adhesion protein HlpA could prevent carcinogenesis and promote CRC regression through cruciferous vegetable diet29. However, the vast majority of engineered bacteria are adopted constitutive expression systems to deliver payloads; Some payloads are toxic to normal cells30,31,32,33, which increases the exposure risk to non-targeted organs23,34.
E. coli Nissle 1917 (EcN) are generally considered safe and beneficial to host health35. Furthermore, oxygen36, pH37, and lactate38 can be used as indicators of tumor microenvironment (TME) uniqueness. By employing genetic circuit programming to perceive these physiological characteristics, it may enhance EcN’s specific recognition of tumors39 and strictly control the production of payloads40. On the other hand, single cellular chassis often faces limitations such as foreign DNA burden, metabolic crosstalk, and intra-cellular resource competition41. Synthetic bacterial consortium (SynCon) can alleviate the metabolic load on individual chassis42 and achieve division of labor among multiple strains43. We further hypothesized that SynCon could enhance the therapeutic efficacy of CRC through synergies. To verify these concepts, the performance of biosensors was accessed in simulators and cell culture mediums. Subsequently, in vitro co-culture assays were conducted to evaluate the cytotoxicity of modified EcNs towards tumor cells. Further, the therapeutic effects of the engineered EcNs and SynCons were validated using the CT26 homograft mouse model and azoxymethane (AOM)/dextran sulfate sodium (DSS) induced colitis-associated mouse tumorigenesis, respectively. Considering the notable interaction between gut microbiota and individual’s response to medications, the distribution of microbiota during the administration of SynCons in AOM/DSS model were also examined to elucidate the intricate interactions among specific microbial constituents.
Result
Engineered biosensors can recognize the tumor microenvironment
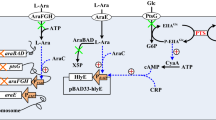
A dual-plasmid architecture was devised to sense the TME and control the expression of therapeutic payloads (Fig. 1a and Supplementary Fig. 1). The genetic circuit consisted of two main parts: the sensing module and the working module. The sensing module I was comprised of L-lactate/H+/hypoxic inducible promoters (pLldR, pCadC and pPepT) and the serine integrase coding gene TP901, whereas the sensing module II was additionally coupled with the lysis gene φX174E downstream of TP901. The working module introduced coding genes of mRFP or therapeutic payloads downstream of XOR gate. The XOR gate consisted of the recognition site of serine integrase and a terminator sequence, and formed an amplifying gene switch (XOR Switch) with TP901. When environmental factors are present, the induction module produces TP901, which recognizes and flips the termination sequences of the XOR gate, allowing expression of downstream products. In addition, sensing module II encoded φX174E and triggered bacterial lysis. As for ten mutants of the lactate-responsive operon pLldR (Supplementary Fig. 2), the mutant pLldR10 was selected for further study owing to its highest fold change and the lowest noise. Besides, pCadC44 and pPepT45 were chosen based on previous studies. As expected39, mRFP reporter system suggested pLldR and pCadC is regulated by lactate and H+, respectively, while pPepT synthesized more fluorescent proteins under anoxic conditions (Fig. 1b left). Among them, the pLldR maintained the maximum transcriptional strength. Subsequently, the effect of XOR Switch on promoter transcription levels were tested. At 10 mM lactate, pLldR transcribed mRFP to produce a normalized fluorescence density of 70 A.U., which is increased to 120 A.U. with the addition of XOR Switch (Fig. 1b left). Similarly, the XOR Switch enhanced the transcription levels of pCadC and pPepT by approximately 1.9-fold and 2.8-fold, respectively. The characteristics of the lysis biosensors (sensing module II) were also tested. Under the same culture conditions, the bacterial density of biosensors supplemented with φX174E gradually decreased with increasing induction intensity (Fig. 1b, right). The fluorescence intensity of pLldR induced lysis biosensor peaked at 1 mM lactate (25 A.U), and then decreased to 18 A.U at 10 mM lactate. The tracking analysis of bacterial population dynamics observed the periodic variation of lysis biosensor (Supplementary Fig. 3). As a pivotal stride towards in vivo characterization of biosensors, the capacity of bacterial biosensors to perceive cellular metabolic activity was assessed within the supernatant of cell cultures (Fig. 1c, left). The fluorescence levels of pLldR and pCadC-controlled biosensors were increased with lactate and H+ concentration, while the fluorescence signal of pPepT based biosensor showed an increasing trend with decreasing oxygen content (Fig. 1c, middle). Similarly, three lysis biosensors shared the same responses trend with induction signals, but exhibited the lower fluorescence intensity than biosensors without the lysis gene (Fig. 1c, right). Furthermore, the OD600 of lysis biosensors exhibited a concomitant decline as the corresponding induction signal in the cell culture supernatants intensified. Fluorescence imaging further confirmed the six engineered biosensors can detect and react to specific biochemical signals in the host environment (Fig. 1d and Supplementary Fig. 4).
a Construction diagram of engineered bacteria. Programmable bacterial circuits can sense specific environmental signals and increase the transmission function of biosensors by amplifying gene switch. When lactate, H+ are present, or in anoxic condition, serine integrase TP901 driven by inductive promoter pLldR, pCadC, and pPepT can reverse the terminator orientation of the XOR gate, enabling the strong promoter pP7 transcript downstream genes. When φX174E is added to the circuit, engineered EcN would lyse itself with above environmental factors induction. b Relationship between environmental change and biosensor fluorescence or population intensity. Biosensor strains harboring mRFP were grown in specified environmental condition (0–10 mM lactate, pH 5.3, 5.8, 6.3 and 7.3, and 0% or 20% oxygen) for 48 h and their induction fluorescence signal were assayed (n = 3 biological replicates. Data are shown as mean ± s.e.m). c Biosensor strains responded to physiological cues. Cell culture medium supernatant from CT26 cancer cell line was collected every 12 h over 5 d and then cultured with biosensor strains. The hypoxic biosensor co-cultured with cell medium supernatant was grown under conditions with or without oxygen. After incubation at 37 °C for 12 h, mRFP fluorescence intensity of lactase (red), pH (green) and hypoxic (blue) biosensor strains was measured and recorded (n = 3 biological replicates. Data are shown as mean ± s.e.m). d Fluorescence microscopy observation. The fluorescence signals of lactate, pH and hypoxic biosensors under induced (10 mM, pH 5.3 and 0% O2, respectively) and noninduced (0 mM, pH 7.3 and 20% O2, respectively) conditions were observed using Olympus Bx53 (magnification: 40x). Bacteria were cultured in LB medium at 37 °C for 12 h. The scale bar marked in the lower right corner of the image represents 5 μm.
Biosensor behavior can be predicted using mathematical models
To bridge the temporal gap between bacterial cloning and animal experimentation, it is necessary to engage in predictive modeling of bacterial therapy prior to in vivo testing (Fig. 2a). Based on the Hill equation28,46, which describes promoter activity through repressor or activator occupancy, a system of ordinary differential equations was built upon prior work39. The equations detail the response of six biosensors to ambient lactate, oxygen, and H+ via XOR Switch. In actual measurements, the peak of biosensors fluorescence was observed at approximately 20 hours after induction, and a detectable signal persisted for at least 48 hours (Fig. 2b-g down). The reaction time for the lactate and pH biosensors could be extended up to 132 hours. These observations were consistent with the results predicted by the computer model (Fig. 2b-g up). After the parameter variables of φX174E were introduced, the total amount of fluorescent protein produced by the model was no longer linearly related to the inducer concentration (Fig. 2e-g up). In the course of time, the results of model fitting present fluctuations. This variability can be ascribed to the influence of lysis genes on the bacterial population (Supplementary Fig. 5)28,47. The time progression curve predicted by the simplified model aligns only approximately with actual measurements, possibly due to external environmental disturbances (Fig. 2e-g down). Nevertheless, the predictability of biosensors in terms of their community size and expression level is necessary for the successful implementation of the platform in vivo’s complex and fluctuating conditions.
a Fluorescent changes of biosensor strains under varying environmental signals were measured using microplate reader or predicted in silico modelling. b–d Biosensor strains were modelled (up) under regulation of pLldR (lactate response), pCadC (pH response) and pPepT (hypoxic response) promoters and compared with (down) in vitro experimental results (n = 3, ± s.e.m). The fluorescent results of mRFP were normalized by OD600. e–g In presence of lysis gene, in vitro mRFP fluorescence of biosensor strains were compared with mathematic modeling prediction (n = 3, ± s.e.m). Detailed equations and parameters used in this study could be obtained in supplementary materials.
Therapeutic strains exhibit inhibitory effects on tumor cell activity in vitro
Next, colorectal cancer cells were co-incubated with therapeutic strains expressing hemolysin (Fig. 3a). A loss of 50%-65% activity was observed in CT26 cells exposed to therapeutic strains harboring hlyE (encoding hemolysin) and environmental response promoters, under induction conditions (Fig. 3b-d and Supplementary Fig. 6). The stronger cytotoxicity mediated by pLldR than that by pCadC and pPepT is consistent with fluorescence measurements (Fig. 1) and mathematical model fitting (Fig. 2). Furthermore, the XOR Switch enabled therapeutic strains to produce more intense cytotoxicity (25-45%). Despite being attenuated, the incorporation of the lysis gene retains the therapeutic strains’ ability to inhibit CT26 cell activity. In contrast to the inducible system, the activity of CT26 cells only remained 25% when exposed to constitutive therapeutic EcN under the control of plac promoter48, while remained 75% under the treatment of equivalent doses of wild-type EcN. Comparable results were obtained by co-culture with RKO and SW480 cells, indicating the broad applicability of this approach (Supplementary Fig. 6). Besides, therapeutic strains exhibited time-dependent and dose-dependent cell mortality on RKO and SW480 cell (Supplementary Fig. 7). Live and dead cell staining also revealed therapeutic EcNs accelerated the death of CT26 cells (Fig. 3e and Supplementary Fig. 8).
a Diagram of the co-culture of therapeutic strains and cancer cells. Cancer cells were inoculated in 96-well plates for adherence, then bacteria cultures were seeded for diffusion of released therapeutic. b–d CT26 cell viability after 3 h co-culture with 30 μL therapeutic strains controlled by pLldR, pCadC and pPepT promoters. “plac” is a constitutive expression promoter, and HlyE represents hemolysin. “Switch” refers to the XOR gate amplifying gene switch, while “lysis” represents the cell lysis element encoded by φX174E (n = 3, ± s.e.m; One-way ANOVA with Tukey post-test; ** p < 0.01, *** p < 0.001, and **** p < 0.0001). e After co-culturing with 30 μL of therapeutic strains for 3 hours, the CT26 cell line was stained with Calcein/PI. The live cells (green fluorescence, stained by Calcein) and dead cells (red fluorescence, stained by PI) were observed using a fluorescence microscope (40x magnification). The oxygen concentration under normoxic conditions is 20%, while under anoxic conditions, it is 0%. The scale bar marked in the lower right corner of the image represents 5 μm.
Therapeutic strains suppress the growth of subcutaneous tumors in mice
Using a subcutaneous model of CRC in Balb/c mice, the therapeutic potential of programable EcNs were evaluated via intratumoral injection (Fig. 4a). To track bacterial population in mouse homograft tumors, luxCDABE was utilized as in vivo reporter gene49 (Fig. 4b). Consistent with in vitro measurements (Fig. 1), the luminescence intensity of reporter strain with lysis gene was about 50% compared to that only harboring inducible promoter, demonstrating a notable reduction in bacterial population within tumors (Fig. 4c, d). Compared to the PBS group, the wild-type EcN (WT) group exhibited a 22% reduction in tumor size (p = 0.001, One-way ANOVA with Tukey post-test). Relative to the WT group, therapeutic strains harboring inducible promoters and hlyE significantly reduced subcutaneous tumor size (Fig. 4e, Supplementary Figs. 9 and 10). Specifically, the pLldR-controlled strain achieved a 54.77% reduction (p < 0.001, One-way ANOVA with Tukey post-test), while the pCadC and pPepT strains reduced tumor sizes by 47.95% (p < 0.001, One-way ANOVA with Tukey post-test) and 42.51% (p < 0.001, One-way ANOVA with Tukey post-test), respectively. Western blot analysis confirmed that HlyE can be detected in tumor tissues (Supplementary Fig. 11). The tumor’s response to bacteria modified with the lysis gene was also notably superior to that observed with unmodified bacteria (Fig. 4f). In the WT group, the average volume of subcutaneous tumors was 1337 mm³. In contrast, tumors treated with lysing strains had an average volume ranging between 638 and 830 mm³. Since the lysis module enables engineered bacteria to release various payloads in tumor grafts50, as a prototype validation, two additional therapeutic strains based on pLldR-regulated lysis strains were constructed to activate host immune response (mouse CCL21)51 or initiate programmed cell death in tumor cells (CDD-iRGD)52. Relative to the WT group, hemolytic or immune-recruiting strains reduced tumor growth by 61.02% and 52.26% (p < 0.001, One-way ANOVA with Tukey post-test), while apoptotic strains achieved only a 26.6% reduction (p < 0.001, One-way ANOVA with Tukey post-test, Fig. 4f).
a Therapeutic schedule of engineered strains administration in a CT26 mouse subcutaneous tumor model. b In vivo imaging of mice with dual hind flank tumors injected with luciferase reporter strains using chemiluminescence imager (Vilber, fx6). c Integrated luminescence density of the luxCDABE reporter strains in a single tumor after injection for 2d was analyzed and calculated using software imageJ 1.53. d Dimension of subcutaneous tumor in mice after injection of luciferase reporter strains (n = 4, ± s.e.m; One-way ANOVA with Tukey post-test; ns: no significant, **p < 0.01, ***p < 0.001). e, f Average tumor volume over time for subcutaneous tumor bearing mice injected with wild-type or engineered EcNs. When average tumor volume reached 150 mm³, the engineered bacterial strain was injected intratumorally on day 0 (start of bacterial therapy), 4, 7 and 11, indicated by red arrows (n = 10 tumors, error bars represent s.e.m; One-way ANOVA with Tukey post-test; **p < 0.01, ***p < 0.001). CCL is an abbreviation for CCL21 and CDD is an abbreviation of fusion protein CDD_iRGD. g Photograph of harvested tumor tissues after different treatments. Scale bar: 2 cm. h Average body weight relative to PBS group for mice bearing subcutaneous tumors injected with the therapeutic strain expressing HlyE under control of pLldR (emerald), strain with constitutively expressing HlyE (orange), or the no-plasmid control strain (sapphire). From the date of tumor cell injection, the engineered strains were injected on day 6, 10, 11 and 15 (n = 5 mice, error bars show s.e.m). i H&E staining and TUNEL staining of the same tumor sections; Scale bars (100 μm) labeled at bottom right of images; TUNEL staining positive rate shown in upper left of images (black). In the TUNEL stained sections, brown represented positive cells. The ratio of TUNEL-positive cells to total cells was analyzed using Aipathwell software. The red box marks the magnified region, highlighting the representative area of positive cells, and is displayed below.
EcN that constitutively expresses hemolysin (plac-hlyE) showed a stronger tumor suppressive effect than those with promoter-controlled hlyE expression (Fig. 4g and Supplementary Fig. 9). However, intratumoral administration of plac-hlyE significantly reduced mouse weight to 90% of its day 0 value by the experiment’s end, underscoring the potential systemic impacts of bacterial therapy (Fig. 4h). In contrast, treatment with inducible strains shared comparable body weight changes with unmodified bacteria (Supplementary Fig. 12), suggesting their safety for therapeutic use. Although additional investigations are necessary to comprehensively investigate the impact of these bacteria on host health, these preliminary experiments indicate that using inducible promoters to regulate the payload may alleviate the burden of bacterial injections. Post-mortem histopathological analysis was conducted on residual tumors of mice. TUNEL staining showed increased apoptotic activity in tumor treated with therapeutic strains (Fig. 4i and Supplementary Fig. 13). In addition to the tumor tissue, these therapeutic EcNs were also distributed in the liver and spleen (Supplementary Fig. 14).
Oral administration of synthetic bacterial consortiums ameliorates the AOM/DSS-induced mouse colorectal cancer
To explore the feasibility for applying designed circuit in the in situ tumor context, we examined the efficacy of programmable bacteria in an AOM/DSS induced colitis-related mouse CRC model (Fig. 5a). Since a mixture of engineered strains containing different payloads showed synergistic therapeutic efficacy in mouse models of CRC liver metastasis28, three synthetic bacterial consortiums (SynCon) were tested. SynCon1 comprises three strains, wherein each strain harbors one of the inducible promoters and regulates hemolysin expression. In contrast to SynCon1, the three therapeutic strains of SynCon2 incorporated XOR Switch through the sensing module I (Fig. 1a). The constituents of SynCon3 bear sensing module II and XOR Switch, facilitating both signal amplification and cellular lysis. Oral administration of EcNs effectively colonized mice’s intestinal tract, as evidenced by a stable population ranging from 1% to 3% (Fig. 5b, Supplementary Figs. 15a and 16). SynCon displayed an approximately equal distribution of its constituent members, accounting for about 12-15% of the total EcN (Supplementary Figs. 15b). Moreover, the recombinant plasmids based on pSB1A3 exhibited a retention rate of approximately 40% within the mouse gastrointestinal tract, whereas those based on pSB4C5 demonstrated a retention rate of around 30% (Supplementary Fig. 17a). A sharp decrease in the bacterial population was also observed during each DSS treatment (Fig. 5b). Although AOM/DSS-induced mice from PBS, WT and pLldR-hlyE groups experienced significant weight loss, the administration of SynCon partially restored their weights (Fig. 5c and Supplementary Fig. 18). Compared to PBS group, EcN treatment not only relieved symptoms of severe bleeding and perianal bleeding (Fig. 5d and Supplementary Fig. 19a), but also improved fecal inconsistency induced by AOM/DSS (Fig. 5e and Supplementary Fig. 20). In contrast to the single strain pLldR-hlyE, SynCons intervention significantly reduced the number of polyps (Supplementary Fig. 21a). In terms of colon length, only SynCon2 showed a significant increase in colon length (p = 0.001, One-way ANOVA with Tukey post-test, Supplementary Fig. 21b). Although no differences were observed in occult blood score (Supplementary Fig. 21c), the average fecal consistency score in the SynCon intervention group was lower than that in the pLldR-hlyE group (Supplementary Fig. 21d) during the last two weeks.
a Schematic diagram of animal treatment scheme. Colon carcinogenesis of mice (n = 10) was induced by intraperitoneal injection of azoxymethane (AOM), followed administration of 2% w/v dextran sodium sulfate (DSS) and regular water (repeated three times over 68 days). Mice were orally given therapeutic strain combinations (1 × 109 c.f.u for each mouse) 6 times a week. b Temporal dynamics of the EcN population during the whole experimental period (Black dashed lines represent the DSS treatment episodes). WT refers to wild-type EcN. SynCon1 consists of a mixture of three bacterial strains, each carrying one of inducible promoters that control the expression of hemolysin. In comparison to SynCon1, SynCon2 includes three therapeutic strains with additional XOR Switch. In SynCon3, the three bacterial strains carry sensing module II to achieve bacterial lysis. c Average body weight changes of each group mice were measured weekly. d Rectal bleeding scores were evaluated by hemoccult testing during treatment duration. e Fecal consistency of AOM/DSS model mice treated with different regimens. f Kaplan-Meier survival curves of mice in different groups. g Total number of polyps and h Colon length of each group were tested after 68 days (One-way ANOVA with Tukey post-test; *p < 0.05 and **** p < 0.0001). i Macroscopic appearance of colons in AOM/DSS model mice. Black dots denote visible tumors. Scale bar (2 cm) labeled at bottom right of images. j Representative TUNEL staining images of the colon tissues. The distal colon of the AOM/DSS mouse model was stained using TUNEL and DAPI. TUNEL staining detected apoptotic cells, which were labeled with green fluorescence, while cell nuclei were labeled with blue fluorescence using DAPI staining. Aipathwell image analysis software was used to calculate the ratio of TUNEL-positive cells, and the result was labeled in the right corner of the image. The upper images display the scanned results of colon sections. The red box denotes the enlarged area and is presented in the lower image. Within the magnified image, the colon’s muscle layer is situated on the left, while the mucosal layer is on the right, with boundaries delineated by dashed white line.
Kaplan-Meier survival curves also showed that the SynCons group increased the survival rate of AOM/DSS treated mice (Fig. 5f). However, the survival rate of 5-FU group in phase I was dramatically decreased compared to Ctrl group (Supplementary Fig. 19b, p = 0.012, Log-rank test). Furthermore, the mortality rate of the PBS group reached 30% (Fig. 5f). The number of colon polypoid tumors in SynCon1, SynCon2 and SynCon3 was reduced by 24%, 48% and 40% compared with PBS group, respectively (Fig. 5g). Among them, SynCon2 showed a comparable number of polyps with 5-FU group, slightly lower than that of plac-hlyE (Supplementary Fig. 19d). SynCon2 and SynCon3 groups also increased the colon length of model mice compared with the PBS group (Fig. 5h, i, Supplementary Fig. 22). Hematoxylin and eosin (H&E) staining showed that the colon section from the Ctrl group displayed intact surface epithelium, intestinal glands, stroma, and submucosal layer (Supplementary Fig. 23). In contrast, the PBS group mice showed infiltrating inflammatory cells and atrophied crypts. Meanwhile, probiotic intervention reduced the colonic developmental abnormalities and structural damage. While local crypt atrophy and loss were still observed in the WT, plac-hlyE, and SynCon1 groups, the structural characteristics of SynCon2 and SynCon3 resembled those of the Ctrl group. However, 5-FU treatment did not show evidence of further improvement in inflammatory cell infiltration. Distinct from the lower pro-inflammatory cytokines in SynCons, plac-hlyE treated mice exhibited higher serum levels of LPS, TNF-α, IL-6, and IL-1β (Supplementary Fig. 24). Their mRNA levels of these inflammatory agents in colon tissue revealed similar trends (Supplementary Fig. 25), suggesting the potential oral risks of constitutive expression of the therapeutic strain. In addition, SynCons inhibited tumor proliferation and improved intestinal barrier via regulating the expression levels of tumor proliferation markers (Supplementary Fig. 26) and intestinal barrier-related proteins (Supplementary Fig. 27). Loss of control over cell apoptosis can allow cancer cells to survive for a longer period53. ELISA analysis showed that compared to the PBS group, SynCons intervention increased the levels of the tumoral apoptosis factors p53 and Bcl-2 associated X protein (Bax) and decreased the expression level of the anti-apoptotic factor B-cell lymphoma 2 (Bcl-2) (Supplementary Fig. 28). TUNEL staining results also demonstrated the SynCons enhance the apoptosis level of tumor cells (Fig. 5j). Collectively, SynCon2 has the best therapeutic efficacy and safety profile compared to the high mortality of 5-FU and the high inflammation level of plac-hlyE.
Synthetic bacterial consortiums modulate AOM/DSS-induced gut microbiota dysbiosis
The possible effect of SynCon2 and SynCon3 on intestinal microbiota was further investigated in light of the beneficial therapeutic outcomes for CRC treatment. In addition to the decline in Chao index and Shannon index of plac-hlyE group, the α-diversity in the SynCons and 5-FU was considerable with Ctrl group (Fig. 6a). The administration of either a single EcN strain or SynCons significantly enhanced the relative abundance of Escherichia, with colonization rates of 5% colonization for SynCon3 and 10% for other groups (Fig. 6b). The dominant families were Lactobacillaceae, Muribaculaceae, Lachnospiraceae and Prevotellaceae (Fig. 6c). Likewise, a significant increase in Enterobacteriaceae was observed in the EcN treatment group. Compared to the PBS group, all EcN intervention groups showed an increase in the abundance of Lactobacillus, Rikenella, and Clostridia_vadinBB60_group (Supplementary Fig. 29). Lactobacillus is commonly regarded as a beneficial bacterium54, while Clostridia are a predominant group for butyrate production55. Additionally, the WT group exhibited an upregulation in the abundance of Alistipes, Rikenellaceae_RC9_gut_group, and Odoribacter. SynCon2 exhibited an increase in the abundance of Prevotellaceae_UCG-001. SynCon3 enhanced the abundance of Akkermansia, Alistipes, Odoribacter, and Paramuribaculum. Akkermansia muciniphila can metabolize mucin and is postulated as a prospective probiotic56. Rikenellaceae_RC9_gut_group57, Alistipes58, Odoribacter59, and Rikenella60 are considered short-chain fatty acids producers. Linear discriminant analysis Effect Size (LEfSe)61 indicated that Ligilactobacillus, Bacteroides, Muribaculum were the most significant characteristics of the PBS group, while Lachnospiraceae_NK4A136_group and Rikenella decreased significantly compared with Ctrl group (Fig. 6d and Supplementary Table 1). The SynCon intervention groups contributed to restore the abundance of these genera (Supplementary Fig. 30).
a The alterations in alpha diversity indices of gut microbiota were assessed, with species richness and evenness represented by the Chao1 and Shannon indices, respectively (n = 5, ± s.e.m; One-way ANOVA with Tukey post-test; *p < 0.05, **** p < 0.0001). b Relative abundance of Escherichia (n = 5, ± s.e.m; One-way ANOVA with Tukey post-test; ***** p < 0.0001). c Relative abundance of microbial composition at family level. Proportions are the average of five samples. d At the genus level, the heatmap exhibited the differences in gut microbiota observed among the groups. e Spearman’s correlation analysis of microbiota and physiological indexes. Correlations with adjusted p < 0.05 by the Benjamini-Hochberg FDR method are marked with * symbols. f Heatmaps of the differential metabolites between Ctrl, PBS and SynCon3 group. NK4A214_group*: Ruminococcus_NK4A214_group; NK4A136_group*: Lachnospiraceae_NK4A136_group; UCG-001*: Prevotellaceae_UCG_001.
Paired spearman rank correlation coefficients were employed to identify interactions among members of the intestinal consortium and visualized as heat map (Supplementary Fig. 31). The diagram reveals two competing clusters: one composed of Rikenella and Lactobacillus, and one composed of Muribaculum, Bacteroides and Parasutteralla. In addition, Lachnospiraceae_NK4A136_group was negatively correlated with Prevotellaceae_UCG-001, Escherichia and Ligilactobacillus. Spearman’s correlation analysis of gut microbiota and physiological indexes showed that Ligilactobacillus was positively correlated with tumor proliferating factors NF-κB and β-catenin, anti-apoptotic marker Bcl-2, inflammatory factors IL-1β, IL-6 and TNF-α, and negatively correlated with gut barrier related protein MUC2. Bacteroides, on the other hand, was positively correlated with NF-κB and β-catenin, while negatively correlated with pro-apoptosis marker p53, Bax, anti-inflammatory related cytokines IL-10 and IL-4, and gut barrier related proteins TFF3 and ZO-1. In contrast, Lachnospiraceae_NK4A136_group was negatively correlated with NF-κB and β-catenin, IL-1β, IL-6, and TNF-α (Fig. 6e). Compared to the PBS group, the Ctrl group exhibited a downregulation of 2,647 metabolites and an upregulation of 3,666 metabolites (Supplementary Fig. 32a). Relative to the PBS group, 4,066 metabolites were upregulated in the SynCon3 group, while 3,281 metabolites were downregulated (Supplementary Fig. 32b). Both Ctrl vs. PBS and PBS vs. SynCon3 comparisons showed differential metabolites enriched in the glycerophospholipid and linoleic acid pathways (Supplementary Fig. 33). Compared to the Ctrl group, the PBS group showed increased abundance of Pfaffoside_A, Tetrahydrocortisone, D-Erythrose, D-Urobilinogen, and Zalcitabine (as visualized in Heatmap A) and decreased abundance of Ophiobolin_F, Heterodendrin, N-Acetylglucosamine_4-sulfate, Taurolithocholate_sulfate, and Belladine (Supplementary Fig. 34a). Compared to the PBS group, the SynCon3 intervention resulted in elevated abundance of Ammoresinol, Delcosine, Leucomycin_A6, N-Oleoyl_dopamine, and alpha-Phocaecholic_acid, and a reduction in the abundance of Methyl_acetyl_ricinoleate, alpha-Ergocryptine, 1,2-Didecanoylglycerol, Leukotriene_F4, and Mupirocin (Supplementary Fig. 34b). The top 5 differential metabolites were examined among three groups. Further analysis of the top 5 differential metabolites showed that SynCon3 supplementation reversed the changes in Pfaffoside_A, D-Erythrose, D-Urobilinogen, Zalcitabine, Ophiobolin_F, Heterodendrin, N-Acetylglucosamine_4-sulfate, and Taurolithocholate_sulfate observed in the PBS group (Fig. 6f and Supplementary Fig. 35).
Discussion
Due to the Warburg effect, a large amount of lactate is released into the TME, leading to a decrease in pH. On the other hand, the rapid tumor proliferation results in a depletion of oxygen39. Therefore, lactate, pH, and hypoxic-inducible promoters pLldR, pCadC, and pPepT were chosen as the sensing elements for the engineered strains to distinguish unique organ environments. The expression of mRFP by pPepT is exclusively observed in anoxic environments. Similarly, gene circuits reliant on pPepT are triggered when oxygen levels plummeting below 0.5%, thus constraining the dissemination of Salmonella bacteria from organs45. Transcriptional activation of pLldR and pCadC was observed under 10 mM lactate and pH 5.3, as reported by Chien et al., resulted in a remarkable reduction in the escape rate of E. coli from solid tumors, from 100 to 10−3 and 10−2, respectively39. Serine integrases based amplification gene switches have been used in the design of biosensing systems62. These genetic devices enable bacteria biosensors to achieve reliable detection, multiplex logic, and signal amplification63. Consistent with our study, the amplifying gene switch magnified the output signals of NO and glucose promoters by 1.5 to 2.6-fold64. By introducing different replication initiation sites (Ori) and antibiotic resistance genes, we constructed a dual-plasmid system harboring sensing module and therapeutic module separately. Such system can avoid crosstalk or competition of different modules, thereby improving its stability and reliability65,66. The established mathematical model can accurately describe the fundamental characteristics and dynamics of TME-induced engineered bacterial strains (Fig. 2). The validated model can be used for predictions under various conditions and indirectly forecast the expression levels of therapeutic proteins in the engineered strains28. This, in turn, provides guidance for subsequent therapeutic strategies at the animal level. Furthermore, the mathematical model employed in this study holds promise as a reference for the design of similar biological systems.
The potential protein payload must be released from the bacterial vector and possesses high cytotoxicity against malignant cancer cells. HlyE is a small hemolysin exported from E.coli via extravascular vesicle67, which has been widely used for cancer therapy due to its high cytotoxic activity48,68. In vitro co-culture confirmed that wild-type EcN had no effect on the viability of CRC cells. Under induction conditions, EcNs modified with environmental response promoters allowed for ex vivo hemolysin release. Cell death efficiency was positively correlated with bacteria population size and exposure time, which was consistent with the amplitude of payload release. Bacterial lysis diminishes the cytotoxicity of genetically modified EcNs, which can be ascribed to the decline in population and effective payload content. As a living drug, programmable EcNs exhibited autonomous control in subcutaneous tumor model, resulting in approximately 60% inhibition of tumor growth via sensing TME and releasing hemolysin. Recent finding revealed that acid promoter adiA can regulate the expression of cytolysin, supporting approximately 79% of tumor regression69. The scalability of payload capacity can enhance the therapeutic efficacy of engineered EcN based platform. Given that most proteins are not readily secreted by EcNs70,71, many efforts have been directed towards relying on secretory tags to translocate recombinant proteins from the cytoplasm29. However, the compatibility of secretory tags and payloads remains a long-standing challenge72. φX174E has been employed in multiple studies47,73, and developed for bacterial host lysis and in vivo drug delivery28. For example, synchronous lysis circuits mediated by quorum sensing allowed bacteria to release different payloads of hemolysin, immune factors and apoptotic peptides28. In another example, attenuated strains of S. typhimurium were designed to self-destruct using φX174E when sensing an invasion of tumor cells, thereby reducing 2.3 fold tumor load74. Parallel to the aforementioned studies, the TME-regulated φX174E facilitated tumor regression of approximately 45%-50% in subcutaneous tumor models through the release of CCL21 and CCD.
SynCons based on cell interaction programming have shown promise and potential applications in human health supervision75,76,77. Oral inoculation of 17 Clostridium strains in mouse models enhanced Treg cell abundance and attenuated colitis and allergic diarrhea78. A population control circuit consisting of three strains was implemented to release therapeutic payloads in TME, demonstrating 1.6-4.0 times greater inhibition of subcutaneous tumors in mice than that of single strains28. Our study also observed that SynCons exhibited superior therapeutic efficacy in the AOMDSS model as compared to single strain. This could be attributed to the existence of multiple specific promoters in SynCons, which enhances the likelihood of triggering expression. Moreover, additional XOR Switch has also contributed to the amelioration of CRC. Within the gastrointestinal milieu, the proportionate distribution of SynCon members aligned with their in vitro compositions, thus culminating in optimal therapeutic efficacy through synergistic interactions. Notably, the effectiveness of oral probiotics was considerably lower than that of intratumoral administration (Figs. 4 and 5). This discrepancy may be attributed to the difficulty of reaching the lesion site. Oral administration of unshielded bacteria are prone to destruction by environmental factors such as stomach acid and the intestinal milieu79. Encapsulating bacteria with alginate-chitosan-alginate (ACA) has been shown to enhance their activity in gastrointestinal tract49,80. In the complex microbial environment of the intestine, plasmid stability in engineered bacteria is a key issue. The plasmid retention rate of the engineered EcN in this study was about 45% (Supplementary Fig. 15b). This loss of plasmid might be due to the expression burden of the synthetic circuit81. L. paracasei BL23 only retained 35-60% plasmid in the rat intestine82. Danino et al. also reported plasmid loss in S. typhimurium83. To minimize plasmid loss in vivo without antibiotic selection, studies have utilized plasmid stabilization systems, such as hok/sok84 and alp7 cassettes28, or integrated genetic circuits into the host genome85. Considering the smaller metabolic burden, the hok/sok cassette was tested. The loss rate of pSB1A3 skeleton plasmid was reduced from 60% to 20% in the intestinal environment after integrating this cassette (Supplementary Fig. 16b). Considering the observed EcN escape in liver and spleen (Supplementary Fig. 14), bacterial chemotaxis towards tumor cells should also be further considered. To this end, the specific adhesion effect of HlpA expressing EcN on RKO and SW480 cells was verified (Supplementary Fig. 36)29. As previously reported, 5-FU exhibited promising therapeutic efficacy while also demonstrating severe toxicity26. One theory is that standard chemotherapy is used in the vascularized area, bacteria act synergistically in the ischemic tumor compartment86. Therefore, the interaction between programmed SynCons and chemotherapeutic agents needs to be further explored. The addition of φX174E reduced the efficacy of SynCons against CRC, possibly attributed to a reduced population and effective payload concentration87. Nevertheless, lysis genes can enable the transient release of therapeutics and rapid elimination of bacterial delivery agents upon completion of treatment, thereby minimizing potential host side effects88. For example, Salmonella-based drug delivery system can activate flhDC to drive cell invasion and induce lysis to release therapeutic proteins into tumor cells74. Given these findings, future efforts should focus on fine-tuning the expression of lysis genes to precisely control the spatiotemporal release of therapeutic agents in vivo. Some genetic circuits, such as focused ultrasound based63 or near-infrared light-sensitive based non-invasive control elements89, as well as protein degradation element SsrA90, may offer solutions to achieve this delicate balance.
CRC models induced by AOM/DSS exhibit perturbed gut microbiota structure49. The SynCons modulated the α-diversity of gut microbiota structure by promoting gut remediation. The observed improvements in body weight, survival, and colon length among the probiotic-treated mice lend support to this notion. Growing attention has been received to the mutual modification between microbiota and chemotherapeutic drugs91,92. Compared with PBS, 5-FU reduced Ligilactobacillus and Bacteroides, while increasing Pseudoflavonifractor93. In addition to its anti-cancer effects, the rapid weight loss, decreased survival, and shortened colon length in the 5-FU treatment group were consistent with previous studies, indicating the ambivalent nature of chemotherapy26,94. In the AOM/DSS model, LEfSe results between groups solicit specific microbial taxa. Consistent with previous studies, AOM/DSS treatment was associated with increased abundance of Bacteroides95,96,97, Muribaculum96,97, and Parabacteroides97, while Lachnospiraceae_NK4A136_group96 and Rnikenella97 showed decreased abundance. Of these taxa, the presence of Bacteroides is known to be positively correlated with intestinal inflammation and the occurrence of colorectal cancer98,99. The decrease of butyrate-producing bacteria Lachnospiraceae_NK4A136_group100 in the PBS group may compromise intestinal barrier integrity and increase the risk of intestinal inflammation49,101. Conversely, the SynCon2 and SynCon3 facilitated the growth of Lachnospiraceae_NK4A136_group while reducing the abundance of Bacteroides. Moreover, Wang et al. reported a positive correlation between Bacteroides and the inflammatory cytokines TNF-α and IL-1β, while Lachnospiraceae_NK4A136 was negatively correlated with these two markers95. This further elucidates the negative or positive roles of Lachnospiraceae_NK4A136 and Bacteroides in the development of CRC.
Radiotherapy, chemotherapy, or their combination can induce various adverse effects102, such as gastrointestinal and neurotoxicity. Probiotics offer a minimally invasive approach by restoring gut microbiota dysbiosis during CRC therapy. However, the efficacy of natural probiotics as therapeutic agents remains limited. A crucial design of this biotherapeutic system is coupling of TME response promoters and therapeutic payloads expression, which generates more precise organotropism and extends the safety of bacteria-based platforms in precision therapy. However, the dual-plasmid system contains redundant elements unrelated to the therapeutic target. In the future, integrating them into a single plasmid or incorporating them into the bacterial genome using CRISPR may enhance the stability and reliability of engineered bacteria in clinical settings. The unintended release of engineered bacteria outside the designated environment should also be prevented by introducing lethal genes. Nonetheless, the U.S. Food and Drug Administration (FDA) permits the use of investigational drugs obtained outside of clinical trials in emergency situations103. This study may offer an additional option for patients with advanced stage colorectal cancer.
Conclusion
We designed an EcN-based platform for CRC therapy, whereby the TME triggers the expression of payloads. In vitro experiments demonstrated that the XOR Switch augmented the output of the TME response promoter, enabling therapeutic strains to effectively suppress the activity of CRC cells. By virtue of their facultative anaerobic nature and the controlled expression of therapeutic payload, genetically encoded EcNs inhibited subcutaneous tumor growth without significant adverse effects. In the AOM/DSS model, SynCons harboring multiplexed biosensors exhibited improved efficacy and avoided off-target toxicity. Although bacterial therapies are still in the preliminary stage of research, the approach described here could enhance the use of bacteria as a sophisticated diagnostic device, thereby improving current oncology therapies. For example, we envision using Boolean logic gates to achieve more precise activation, which can distinguish microenvironments of various tumor type64. As the advancement of biomedical engineering technologies, engineered probiotics are poised to serve as a precise and robust tool for desired human disease, offering a safe and effective means for topical treatment regimens targeting focal sites.
Methods
Host strains and culturing
E. coli Nissle 1917 was purchased from Biobw (China, Beijing) and E. coli DH5α was previously stored in lab. All bacteria were cultured in LB broth containing appropriate antibiotics (100 μg mL−1 ampicillin, 50 μg mL−1 kanamycin, and 10 μg mL−1 tetracycline) at 37 °C.
Plasmids and engineered bacteria library construction
The recombinant plasmids were verified by sequencing and then transformed into EcN to construct the engineered strains (Supplementary Table 2-4 and Supplemental Methods: Molecular biology).
Mathematical model. Ordinary differential equations based on hill equation and logistic equation were used to describe the fluorescence changes and bacterial growth of the biosensor. Specific equations and parameters can be found in Supplemental Methods: Modeling.
Protein quantitative analysis
Protein expression levels in colon and serum samples were analyzed using enzyme-linked immunosorbent assay (ELISA) kits. For detailed information on the assay kit and the detected biomarkers, please refer to Supplemental Methods: Biomarker analysis.
Western blot analysis
Mouse primary antibody against His-Tag and HRP-conjugated Goat Anti-Mouse IgG secondary antibody were utilized to detect protein expression in engineered strains within tumor tissues (Supplemental Methods: Protein expression analysis).
Characterization of biosensor strains in vitro
Each variant strain was cultured in LB medium overnight with appropriate antibiotic (37 °C, 150 rpm), and then used for induction experiments in the next morning. The cultures of variant strain were transferred to 96-well plates (3 replicates) and diluted with 125 μL LB broth to OD600 = 0.1. As for lactate, pH and hypoxic biosensor assays, the corresponding EcNs were detected in LB medium with different L-lactate (Sigma-Aldrich) concentrations (0, 0.1, 1, 5, and 10 mM), pH values (5.5, 5.8, 6.3 and 7.3), and oxygen conditions (normoxic, 20%;anoxic, 0%) at 37°C for overnight incubation, respectively. To realize anoxic condition, the tested EcNs were statically cultured in anaerobic bags containing oxygen indicator (Hopebiol, China). After 16-20 h of growth, their absorbance (OD600) and mRFP fluorescence intensity (excitation λ: 584 nm/10 nm, emission λ: 607/10 nm) data were measured using a microplate reader (Thermo Fisher Scientific, USA). After subtraction of background fluorescence signal (untransformed EcN), the normalized fluorescence of biosensor strains was obtained by dividing raw mRFP pixel intensity by OD600 value. All triplicate values were averaged. To capture fluorescent images, Olympus BX53 (40 x) was used to observe and photograph the biosensor bacteria after incubation for 12 hours under induced (10 mM lactate; pH 7.3; 0% O2) or non-induced conditions.
Cell culture
CT-26 mouse colon cancer cell, RKO human colon adenocarcinoma cell, and SW480 human colon cancer cell were purchased from Procell (Wuhan, China). All cells were cultured at 37 °C with 5% CO2 in DMEM (Basal Media, Shanghai, China), supplemented with 10% fetal bovine serum (Gemini, USA), and 1% penicillin/streptomycin (Gibco, USA).
Biosensor strains were tested in supernatant of cell cultures
All cell lines were inoculated in 25 cm2 culture bottle containing 6 mL complete medium (6 replicates) with an initial cell count of 105. For the next 5 days, the cell cultures were removed from flasks twice a day and transferred to 15 mL sterile centrifuge tubes. After centrifugation at 200 r.c.f. for 5 min, the culture medium supernatant was cryopreserved at −80 °C. Then biosensor strains were inoculated into 24-well plates containing 800 μL LB medium (supplemented with corresponding antibiotics). After that, 200 μL of stored cell cultures supernatant was thawed and incubated with the above strains. pH test paper was used to detect the pH of cell cultures supernatant. Lactate concentration of cell cultures supernatant was measured using LA Assay Kit (BC2235, Solarbio). The hypoxic biosensor was tested in a constant temperature incubator under hypoxic or normoxic condition. All bacteria were cultured for 12-16 hours, and the fluorescence and absorbance of the biosensor strains were determined using a microplate reader (Thermo Fisher Scientific, USA).
Co-culture of therapeutic strains with colorectal cancer cells
Cancer cells were inoculated in 96-well plates containing 100 μL complete DMEM. When cancer cells adhered, 10 μL of therapeutic strains and control strains (initial OD600 = 0.6) were inoculated, respectively. The supernatant of the cell culture on day 3 was used as the inducer. Co-cultivation was carried out for 3 h at 37 °C30. After that, the media containing bacteria were removed and replaced with fresh DMEM. The cell viability of colorectal cancer cell was monitored using CCK8 kit (C0038, Beyotime, China) on the microplate reader. The Calcein/PI kit (C2015S, Biotime) was used to label live and dead cells of CT26 after different treatments. The staining results were visualized with Olympus BX53, green and red fluorescence represent live and dead cells, respectively.
Anticancer drugs preparation
Therapeutic strains were grown overnight (37 °C, 150 r.p.m) in 5 mL LB. Then, the bacteria culture was transferred to 300 mL LB medium for expansion culture. After centrifugation at 8000 r.c.f for 10 min, the cell pellets were harvested and suspended with sterilized PBS (pH 7.4). The bacterial density was 5 × 109 c.f.u per mL for gavage and 5 × 107 c.f.u per mL for subcutaneous tumor injection28.
Mouse strains and growth conditions
Female Balb/c (6-week-old, 18-22 g, for subcutaneous tumor model) and C57bL-6J mice (6-week-old, 18-22 g, for AOM/DSS induced CRC model) were purchased from Lanzhou University Animal Center. Mice were fed in a pathogen-free facility with 12/12 hours light/dark cycle and constant temperature (20 ± 3°C) and humidity (40 ± 20%) (Laboratory Animal Center of the School of Life Sciences, Lanzhou University). During study period, the animals were given free access to sterilized water and dry pellet feed. The animals were euthanized according to the study schedule or when the tumor size reached 2 cm26.
Subcutaneous tumor model
CT26 colorectal cells were used to construct subcutaneous tumor mouse model. At the exponential growth stage, CT26 cells were harvested and suspended in DEME medium (no phenol red) to achieve a concentration of 5×107 cell per mL. Cells were implanted at bilateral subcutaneous hind flank, with a volume of 100 μL (i.e., 5 × 106 cell) per flank. Once the average tumor size reached an average of about 150 mm3 (i.e., 5.3 mm), the mice were randomly assigned (n = 5 for each group). Then, tumor injection was performed with 20 μL 0.9% normal saline or (i.e., 1 × 106 c.f.u) therapeutic strains at 0, 4, 7 and 11 days. Mice body weight and tumor volume were recorded every 2 days. The inhibition rate (IR%) of anticancer drugs on tumor growth was calculated as: IR (%) = (1 − T/C) × 100, where C is the average tumor weight of the control group and T is the average tumor weight of the tested drug groups39.
AOM/DSS-induced CRC mouse model
On the first day of the experiment, 6-week-old C57BL-6 mice (about 18-22 g) were intraperitoneally injected with 12.5 mg/kg body weight AOM (Sigma-Aldrich). After 5 days, mice were given drinking water containing 2% (wt/vol) DSS (molecular weight 36-50 kDa, YEASEN, China) for 7 consecutive days, followed by regular drinking water for 14 days. Repeat the cycle for three times. Mice were orally given 0.9% normal saline or 5 × 108 c.f.u EcN (i.e., 100 μL bacteria suspension), five times a week for 68 days. The positive control group was intraperitoneal injected 40 mg/kg 5-FU twice a week. Rectal occult blood or gross blood, food intake and body weight of each mouse were reordered weekly. The bleeding situation was analyzed using fecal occult blood test kit (C027, Nanjing Jiancheng Bioengineering Institute, China). The negative result was scored as 0. Slight positive was scored as 1. Strong positive was scored as 2. Fecal consistency was classified as follows: 0 for normal, 1 for soft and sticky. Weight change during the study period was calculated as a percentage change in body weight relative to baseline measurements. On day 68, the mice were euthanized, and colon length and polyp number were measured.
Biodistribution
After intratumor injection of bacteria, mice were euthanized to obtain the liver, spleen, and tumor. The harvested organs were weighed and homogenized using an automatic grinder (Tissuelyser-24L, Jingxin, Shanghai, China). Homogenates underwent sequential dilution and spread onto LB agar plates with antibiotic selection at 37 °C overnight. Colonies were enumerated and quantified as c.f.u. per gram of tissue39.
Quantification of EcN population in gut microbiota
The colonization of recombinant EcN in AOM/DSS induced CRC model mice was analyzed by absolute quantitative method104 For experimental procedures and primer information, please refer to Supplemental Methods: Absolute quantitative analysis and Supplemental Table 5.
Colon gene expression analysis
Quantitative reverse transcription PCR (qRT-PCR) was employed to quantify mRNA expression of colon genes. For experimental procedures and primer information, please refer to Supplemental Methods: Quantitation of gene expression and Supplemental Table 6.
Bioluminescent assay
After 3 days of intratumoral injection of luxCDABE-labeled programmed EcNs, mice were anesthetized with 1% (m:v) pentobarbital sodium saline solution (Intraperitoneally, 40 mg/kg body weight)39. The luminous signals were measured using chemiluminescent imager (Fx6, Vilber, France) to track the number and distribution of bacteria in subcutaneous tumor105. During the entire imaging trial period, tumour volume was quantified to follow physical tumour growth28. To further determine the survival rate of probiotics in the digestive tract, C57BL/6 mice were orally given EcN labeled with luxCDABE (1 × 109 c.f.u), and their GI tracts were imaged with Vilber 3 h later. Bioluminescence signals in the region of interest were quantified by Image 2.1 software.
16 S rRNA sequencing and bioinformatics analysis
Stool samples from AOM/DSS induced CRC mice were collected and frozen at -80 °C. After the samples were slowly thawed, DNA was extracted using Stool DNA extraction kits (Omega, USA). The 16 S rRNA V3-V4 regions were amplified using specific primers and sequencing was conducted on Illumina MiSeq platform (LC-BioTechnology, China). The QIIME 2 pipeline was utilized for data processing. To determine α diversity, the Shannon index and Chao index were analyzed using Mothur v.1.31.0. LEfSe (p < 0.05 and logarithmic LDA score was set at 3.0) was performed using the online tool at https://www.omicstudio.cn. Heatmaps were plotted using R package version 3.6.3.
Fecal untargeted metabolome analysis using LC-MS
50 mg of thawed faecal samples were mixed with 500 μL of pre-cooled methanol/water (1:1, v/v), homogenized with ultrasonic treatment at 4 °C for 30 min. After adding acetonitrile, the solutions were incubated at −20 °C for 1 h, then centrifuged and freeze-dried. The pre-processed samples, QC samples, and blanks were stored at −80 °C prior to LC-MS analysis. Chromatographic separations were performed using a Thermo Scientific UltiMate 3000 HPLC and mass spectrometry data were acquired using a Q-Exactive. The data was processed using Compound Discoverer 3.1.0 and metabolites were annotated based on HMDB and KEGG database. Differential metabolites were selected using two-tailed Student’s t-tests.
Ethics Statement
The animal experimental proposal was approved and supervised by the Animal Ethics Committee of School of Life Sciences, Lanzhou University and carried out in accordance with ethical guidelines of the 1975 Declaration of Helsinki (permission number: EAF2022053).
Statistical analysis
The study data were statistically analyzed using GraphPad Prism 8.0, and results are presented as means ± s.e.m. Statistical tests included Student’s t-test (two-tailed, unpaired) for two-group comparisons, One-way ANOVA with Tukey post-test for multiple-group comparisons, and Kaplan-Meier analysis with log-rank test for survivability analysis. Significance was denoted as *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The sequencing data for 16 S rRNA have been deposited in the Sequence Read Archive (SRA) of the National Center for Biotechnology Information (NCBI) under the accession number PRJNA970984. The raw data of fecal metabolites were stored in Figshare with the https://doi.org/10.6084/m9.figshare.24844692.v1. All relevant data are available from the authors.
References
Soleimanpour, S., Hasanian, S. M., Avan, A., Yaghoubi, A. & Khazaei, M. Bacteriotherapy in gastrointestinal cancer. Life Sci. 254, 117754 (2020).
The Lancet, O. Colorectal cancer: a disease of the young? Lancet Oncol. 18, 413 (2017).
Rawla, P., Sunkara, T. & Barsouk, A. Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Prz. gastroenterol. 14, 89–103 (2019).
Kuipers, E. J. et al. Colorectal cancer. Nat. Rev. Dis. Prim. 1, 15065 (2015).
Weng, J. et al. Exploring immunotherapy in colorectal cancer. J. Hematol. Oncol. 15, 95 (2022).
Cao, C., Yan, T. D., Black, D. & Morris, D. L. A systematic review and meta-analysis of cytoreductive surgery with perioperative intraperitoneal chemotherapy for peritoneal carcinomatosis of colorectal origin. Ann. Surg. Oncol. 16, 2152–2165 (2009).
Johdi, N. A. & Sukor, N. F. Colorectal Cancer Immunotherapy: Options and Strategies. Front Immunol. 11, 1624 (2020).
Ohhara, Y. et al. Role of targeted therapy in metastatic colorectal cancer. World J. Gastrointest. Oncol. 8, 642–655, (2016).
Piawah, S. & Venook, A. P. Targeted therapy for colorectal cancer metastases: A review of current methods of molecularly targeted therapy and the use of tumor biomarkers in the treatment of metastatic colorectal cancer. Cancer 125, 4139–4147 (2019).
Urbaniak, C. et al. Microbiota of human breast tissue. Appl. Environ. Microbiol. 80, 3007–3014 (2014).
Forbes, N. S. Engineering the perfect (bacterial) cancer therapy. Nat. Rev. Cancer 10, 785–794 (2010).
Malmgren, R. A. & Flanigan, C. C. Localization of the vegetative form of Clostridium tetani in mouse tumors following intravenous spore administration. Cancer Res. 15, 473–478 (1955).
Gontero, P. et al. The role of bacillus Calmette-Guérin in the treatment of non-muscle-invasive bladder cancer. Eur. Urol. 57, 410–429 (2010).
Van Mellaert, L., Barbé, S. & Anné, J. Clostridium spores as anti-tumour agents. Trends Microbiol. 14, 190–196 (2006).
Lee, C. H., Wu, C. L. & Shiau, A. L. Endostatin gene therapy delivered by Salmonella choleraesuis in murine tumor models. J. Gene Med. 6, 1382–1393 (2004).
Tomita, Y. et al. Association of Probiotic Clostridium butyricum Therapy with Survival and Response to Immune Checkpoint Blockade in Patients with Lung Cancer. Cancer Immunol. Res. 8, 1236–1242 (2020).
Dang, L. H. et al. Targeting vascular and avascular compartments of tumors with C. novyi-NT and anti-microtubule agents. Cancer Biol. Ther. 3, 326–337 (2004).
Roberts, N. J. et al. Intratumoral injection of Clostridium novyi-NT spores induces antitumor responses. Sci. Transl. Med. 6, 249ra111 (2014).
Fritz, S. E. et al. A phase I clinical study to evaluate safety of orally administered, genetically engineered Salmonella enterica serovar Typhimurium for canine osteosarcoma. Vet. Med Sci. 2, 179–190 (2016).
Zhao, S. et al. Probiotic Limosilactobacillus fermentum GR-3 ameliorates human hyperuricemia via degrading and promoting excretion of uric acid. iScience 25, 105198 (2022).
Wu, Y. et al. Limosilactobacillus fermentum JL-3 isolated from “Jiangshui” ameliorates hyperuricemia by degrading uric acid. Gut Microbes 13, 1–18 (2021).
Feng, P. et al. Tibet plateau probiotic mitigates chromate toxicity in mice by alleviating oxidative stress in gut microbiota. Commun. Biol. 3, 242 (2020).
Sieow, B. F.-L., Wun, K. S., Yong, W. P., Hwang, I. Y. & Chang, M. W. Tweak to Treat: Reprograming Bacteria for Cancer Treatment. Trends Cancer 7, 447–464 (2021).
Staedtke, V., Roberts, N. J., Bai, R.-Y. & Zhou, S. Clostridium novyi-NT in cancer therapy. Genes Dis. 3, 144–152 (2016).
Chiang, C. J. & Hong, Y. H. In situ delivery of biobutyrate by probiotic Escherichia coli for cancer therapy. Sci. Rep. 11, 18172 (2021).
Chung, Y. et al. A synthetic probiotic engineered for colorectal cancer therapy modulates gut microbiota. Microbiome 9, 122 (2021).
Bohlul, E., Hasanlou, F., Taromchi, A. H. & Nadri, S. TRAIL-expressing recombinant Lactococcus lactis induces apoptosis in human colon adenocarcinoma SW480 and HCT116 cells. J. Appl Microbiol. 126, 1558–1567 (2019).
Din, M. O. et al. Synchronized cycles of bacterial lysis for in vivo delivery. Nature 536, 81–85 (2016).
Ho, C. L. et al. Engineered commensal microbes for diet-mediated colorectal-cancer chemoprevention. Nat. Biomed. Eng. 2, 27–37 (2018).
Li, R. et al. Expressing cytotoxic compounds in Escherichia coli Nissle 1917 for tumor-targeting therapy. Res. Microbiol. 170, 74–79 (2019).
Lim, D. et al. Anti-tumor activity of an immunotoxin (TGFα-PE38) delivered by attenuated Salmonella typhimurium. Oncotarget 8, 37550–37560 (2017).
Liu, X., Jiang, S., Piao, L. & Yuan, F. Radiotherapy combined with an engineered Salmonella typhimurium inhibits tumor growth in a mouse model of colon cancer. Exp. Anim. 65, 413–418 (2016).
Zhang, Y. et al. Escherichia coli Nissle 1917 targets and restrains mouse B16 melanoma and 4T1 breast tumors through expression of azurin protein. Appl. Environ. Microbiol. 78, 7603–7610 (2012).
Rong, L., Lei, Q. & Zhang, X. Z. Engineering Living Bacteria for Cancer Therapy. ACS Appl Bio Mater. 3, 8136–8145 (2020).
Sonnenborn, U. & Schulze, J. The non-pathogenic Escherichia coli strain Nissle 1917 – features of a versatile probiotic. Microb. Ecol. Health Dis. 21, 122–158 (2009).
Li, Y. C. et al. Melatonin and hyperbaric oxygen therapies suppress colorectal carcinogenesis through pleiotropic effects and multifaceted mechanisms. Int J. Biol. Sci. 17, 3728–3744 (2021).
Stine, Z. E., Schug, Z. T., Salvino, J. M. & Dang, C. V. Targeting cancer metabolism in the era of precision oncology. Nat. Rev. Drug Discov. 21, 141–162 (2022).
Vander Heiden, M. G., Cantley, L. C. & Thompson, C. B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324, 1029–1033 (2009).
Chien, T. et al. Enhancing the tropism of bacteria via genetically programmed biosensors. Nat. Biomed. Eng. 6, 94–104 (2022).
Duong, M. T.-Q., Qin, Y., You, S.-H. & Min, J.-J. Bacteria-cancer interactions: bacteria-based cancer therapy. Exp. Mol. Med. 51, 1–15 (2019).
Jia, X. et al. Design, analysis and application of synthetic microbial consortia. Synth. Syst. Biotechnol. 1, 109–117 (2016).
Brenner, K., You, L. & Arnold, F. H. Engineering microbial consortia: a new frontier in synthetic biology. Trends Biotechnol. 26, 483–489 (2008).
Tan, Y. et al. Engineered Live Biotherapeutics: Progress and Challenges. Biotechnol. J. 15, e2000155 (2020).
Viala, J. P. et al. Sensing and adaptation to low pH mediated by inducible amino acid decarboxylases in Salmonella. PLoS One 6, e22397 (2011).
Yu, B. et al. Explicit hypoxia targeting with tumor suppression by creating an “obligate” anaerobic Salmonella Typhimurium strain. Sci. Rep. 2, 436 (2012).
Chou, T. C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharm. Rev. 58, 621–681 (2006).
Miano, A., Liao, M. J. & Hasty, J. Inducible cell-to-cell signaling for tunable dynamics in microbial communities. Nat. Commun. 11, 1193 (2020).
Chiang, C.-J. & Huang, P.-H. Metabolic engineering of probiotic Escherichia coli for cytolytic therapy of tumors. Sci. Rep. 11, 5853 (2021).
Zhou, J. et al. Programmable probiotics modulate inflammation and gut microbiota for inflammatory bowel disease treatment after effective oral delivery. Nat. Commun. 13, 3432 (2022).
Chowdhury, S. et al. Programmable bacteria induce durable tumor regression and systemic antitumor immunity. Nat. Med 25, 1057–1063 (2019).
Luo, H. et al. Coexpression of IL7 and CCL21 Increases Efficacy of CAR-T Cells in Solid Tumors without Requiring Preconditioned Lymphodepletion. Clin. Cancer Res. 26, 5494–5505 (2020).
Chen, R. et al. Application of a proapoptotic peptide to intratumorally spreading cancer therapy. Cancer Res 73, 1352–1361 (2013).
Pfeffer, C. M. & Singh, A. T. K. Apoptosis: A Target for Anticancer Therapy. Int. J. Mol. Sci. 19, https://doi.org/10.3390/ijms19020448 (2018).
Zhang, Z., Lv, J., Pan, L. & Zhang, Y. Roles and applications of probiotic Lactobacillus strains. Appl Microbiol Biotechnol. 102, 8135–8143 (2018).
Dürre, P. Physiology and Sporulation in Clostridium. Microbiol. Spectr. 2, Tbs-0010-2012, https://doi.org/10.1128/microbiolspec.TBS-0010-2012 (2014).
Cani, P. D., Depommier, C., Derrien, M., Everard, A. & de Vos, W. M. Akkermansia muciniphila: paradigm for next-generation beneficial microorganisms. Nat. Rev. Gastroenterol. Hepatol. 19, 625–637 (2022).
Ahmad, A. A. et al. Age-dependent variations in rumen bacterial community of Mongolian cattle from weaning to adulthood. BMC Microbiol. 22, 213 (2022).
Broadfield, L. A. et al. Metformin-induced reductions in tumor growth involves modulation of the gut microbiome. Mol. Metab. 61, 101498 (2022).
Deng, L., Yang, Y. & Xu, G. Empagliflozin ameliorates type 2 diabetes mellitus-related diabetic nephropathy via altering the gut microbiota. Biochim Biophys. Acta Mol. Cell Biol. Lipids 1867, 159234 (2022).
Shi, H. et al. Dietary fucoidan of Acaudina molpadioides alters gut microbiota and mitigates intestinal mucosal injury induced by cyclophosphamide. Food Funct. 8, 3383–3393 (2017).
Avuthu, N. & Guda, C. Meta-Analysis of Altered Gut Microbiota Reveals Microbial and Metabolic Biomarkers for Colorectal Cancer. Microbiol Spectr. 10, e0001322 (2022).
Bonnet, J., Yin, P., Ortiz, M. E., Subsoontorn, P. & Endy, D. Amplifying Genetic Logic Gates. Science 340, 599–603 (2013).
Abedi, M. H. et al. Ultrasound-controllable engineered bacteria for cancer immunotherapy. Nat. Commun. 13, 1585 (2022).
Courbet, A., Endy, D., Renard, E., Molina, F. & Bonnet, J. Detection of pathological biomarkers in human clinical samples via amplifying genetic switches and logic gates. Sci. Transl. Med. 7, 289ra283 (2015).
Han, C. et al. A highly effective and adjustable dual plasmid system for O-GlcNAcylated recombinant protein production in E. coli. J. Biochem 157, 477–484 (2015).
Liu, W. et al. A Dual-Plasmid CRISPR/Cas System for Mycotoxin Elimination in Polykaryotic Industrial Fungi. ACS Synth. Biol. 9, 2087–2095 (2020).
Wai, S. N. et al. Vesicle-mediated export and assembly of pore-forming oligomers of the enterobacterial ClyA cytotoxin. Cell 115, 25–35 (2003).
Ryan, R. M. et al. Bacterial delivery of a novel cytolysin to hypoxic areas of solid tumors. Gene Ther. 16, 329–339 (2009).
Qin, W. et al. Bacteria-Elicited Specific Thrombosis Utilizing Acid-Induced Cytolysin A Expression to Enable Potent Tumor Therapy. Adv. Sci. (Weinh.) 9, e2105086 (2022).
Choi, J. H. & Lee, S. Y. Secretory and extracellular production of recombinant proteins using Escherichia coli. Appl Microbiol Biotechnol. 64, 625–635 (2004).
Gurbatri, C. R., Arpaia, N. & Danino, T. Engineering bacteria as interactive cancer therapies. Science 378, 858–864 (2022).
Freudl, R. Signal peptides for recombinant protein secretion in bacterial expression systems. Microb. Cell Factories 17, 52 (2018).
Diao, W. et al. Reprogramming microbial populations using a programmed lysis system to improve chemical production. Nat. Commun. 12, 6886 (2021).
Raman, V. et al. Intracellular delivery of protein drugs with an autonomously lysing bacterial system reduces tumor growth and metastases. Nat. Commun. 12, 6116 (2021).
Bittihn, P., Din, M. O., Tsimring, L. S. & Hasty, J. Rational engineering of synthetic microbial systems: from single cells to consortia. Curr. Opin. Microbiol 45, 92–99 (2018).
Qian, X. et al. Biotechnological potential and applications of microbial consortia. Biotechnol. Adv. 40, 107500 (2020).
Deter, H. S. & Lu, T. Engineering microbial consortia with rationally designed cellular interactions. Curr. Opin. Biotechnol. 76, 102730 (2022).
Atarashi, K. et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 500, 232–236 (2013).
Sarao, L. K. & Arora, M. Probiotics, prebiotics, and microencapsulation: A review. Crit. Rev. Food Sci. Nutr. 57, 344–371 (2017).
Lin, J. et al. In Vitro and in Vivo characterization of alginate-chitosan-alginate artificial microcapsules for therapeutic oral delivery of live bacterial cells. J. Biosci. Bioeng. 105, 660–665 (2008).
Ceroni, F., Algar, R., Stan, G. B. & Ellis, T. Quantifying cellular capacity identifies gene expression designs with reduced burden. Nat. Methods 12, 415–418 (2015).
Lin, Y. et al. Oral Delivery of Pentameric Glucagon-Like Peptide-1 by Recombinant Lactobacillus in Diabetic Rats. PLoS One 11, e0162733 (2016).
Danino, T., Lo, J., Prindle, A., Hasty, J. & Bhatia, S. N. In Vivo Gene Expression Dynamics of Tumor-Targeted Bacteria. ACS Synth. Biol. 1, 465–470 (2012).
Sun, B. Y. et al. Engineering Escherichia coli for l-homoserine production. J. Basic Microbiol. 63, 168–178 (2023).
Isabella, V. M. et al. Development of a synthetic live bacterial therapeutic for the human metabolic disease phenylketonuria. Nat. Biotechnol. 36, 857–864 (2018).
Dang, L. H., Bettegowda, C., Huso, D. L., Kinzler, K. W. & Vogelstein, B. Combination bacteriolytic therapy for the treatment of experimental tumors. Proc. Natl Acad. Sci. USA 98, 15155–15160 (2001).
Ma, Y. et al. Construction and In Vitro Evaluation of a Tumor Acidic pH-Targeting Drug Delivery System Based on Escherichia coli Nissle 1917 Bacterial Ghosts. Bioengineering (Basel) 9, https://doi.org/10.3390/bioengineering9090433 (2022).
Alexander, L. M. et al. Exploiting Prophage-Mediated Lysis for Biotherapeutic Release by Lactobacillus reuteri. Appl Environ. Microbiol 85, e02335–18 (2019).
Fu, S. et al. Programming the lifestyles of engineered bacteria for cancer therapy. Natl Sci. Rev. 10, nwad031 (2023).
Chai, Q., Wang, Z., Webb, S. R., Dutch, R. E. & Wei, Y. The ssrA-Tag Facilitated Degradation of an Integral Membrane Protein. Biochemistry 55, 2301–2304 (2016).
Ma, W. et al. Gut Microbiota Shapes the Efficiency of Cancer Therapy. Front Microbiol 10, 1050 (2019).
Zhang, X., Han, Y., Huang, W., Jin, M. & Gao, Z. The influence of the gut microbiota on the bioavailability of oral drugs. Acta Pharm. Sin. B 11, 1789–1812 (2021).
Borda-Molina, D., Vital, M., Sommerfeld, V., Rodehutscord, M. & Camarinha-Silva, A. Insights into Broilers’ Gut Microbiota Fed with Phosphorus, Calcium, and Phytase Supplemented Diets. Front Microbiol 7, 2033 (2016).
Yoshimi, K. et al. Use of a chemically induced-colon carcinogenesis-prone Apc-mutant rat in a chemotherapeutic bioassay. BMC Cancer 12, 448 (2012).
Wang, M. et al. Amelioration of AOM/DSS-Induced Murine Colitis-Associated Cancer by Evodiamine Intervention is Primarily Associated with Gut Microbiota-Metabolism-Inflammatory Signaling Axis. Front Pharm. 12, 797605 (2021).
Chen, H. et al. Berberine inhibits intestinal carcinogenesis by suppressing intestinal pro-inflammatory genes and oncogenic factors through modulating gut microbiota. BMC Cancer 22, 566 (2022).
Xu, H. M. et al. Selection strategy of dextran sulfate sodium-induced acute or chronic colitis mouse models based on gut microbial profile. BMC Microbiol 21, 279 (2021).
Zackular, J. P. et al. The gut microbiome modulates colon tumorigenesis. mBio 4, e00692–00613 (2013).
Zou, S., Fang, L. & Lee, M. H. Dysbiosis of gut microbiota in promoting the development of colorectal cancer. Gastroenterol. Rep. (Oxf.) 6, 1–12 (2018).
Ma, L. et al. Spermidine improves gut barrier integrity and gut microbiota function in diet-induced obese mice. Gut Microbes 12, 1–19 (2020).
Li, D. P., Cui, M., Tan, F., Liu, X. Y. & Yao, P. High Red Meat Intake Exacerbates Dextran Sulfate-Induced Colitis by Altering Gut Microbiota in Mice. Front Nutr. 8, 646819 (2021).
Ebrahimzadeh, S. et al. Colorectal cancer treatment using bacteria: focus on molecular mechanisms. BMC Microbiol. 21, 218 (2021).
Gould, P., Salam, T., Kimberly, L., Bateman-House, A. & Fernandez Lynch, H. Perspectives of Academic Oncologists About Offering Expanded Access to Investigational Drugs. JAMA Netw. Open 5, e2239766 (2022).
Brankatschk, R., Bodenhausen, N., Zeyer, J. & Bürgmann, H. Simple absolute quantification method correcting for quantitative PCR efficiency variations for microbial community samples. Appl Environ. Microbiol. 78, 4481–4489 (2012).
Danino, T. et al. Programmable probiotics for detection of cancer in urine. Sci. Transl. Med. 7, 289ra284–289ra284 (2015).
Acknowledgements
This work was supported by National Natural Science Foundation of China (No: 32070117 and 32200080), the Gansu Province Science and Technology Planning Project (21YF5WA115). We also thank to Core Facility of School of Life Sciences, Lanzhou University for technical support and especially appreciate Mrs. Li Xie and Mrs. Liang Peng for their kindly help.
Author information
Authors and Affiliations
Contributions
T.Z. conceived the study, conducted molecular biology experiments, prepared original daft, and analyzed the output data. J.W. helped established subcutaneous tumor model and conducted data analysis. H.T. assisted to build AOM/DSS model. D.L., B.-H.J., W.J. and Y.W. contributed to manuscript revisions. Y.Z. and A.K. contributed to data analysis. H.H. and X.L. contributed substantially to revisions. X.L. also provided financial support and supervision. All the authors read and approved the final manuscript. J.W. and T.Z. are “co-first author”.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhou, T., Wu, J., Tang, H. et al. Enhancing tumor-specific recognition of programmable synthetic bacterial consortium for precision therapy of colorectal cancer. npj Biofilms Microbiomes 10, 6 (2024). https://doi.org/10.1038/s41522-024-00479-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41522-024-00479-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.