Abstract
With improvements in survival for patients with metastatic cancer, long-term local control of brain metastases has become an increasingly important clinical priority. While consensus guidelines recommend surgery followed by stereotactic radiosurgery (SRS) for lesions >3 cm, smaller lesions (≤3 cm) treated with SRS alone elicit variable responses. To determine factors influencing this variable response to SRS, we analyzed outcomes of brain metastases ≤3 cm diameter in patients with no prior systemic therapy treated with frame-based single-fraction SRS. Following SRS, 259 out of 1733 (15%) treated lesions demonstrated MRI findings concerning for local treatment failure (LTF), of which 202 /1733 (12%) demonstrated LTF and 54/1733 (3%) had an adverse radiation effect. Multivariate analysis demonstrated tumor size (>1.5 cm) and melanoma histology were associated with higher LTF rates. Our results demonstrate that brain metastases ≤3 cm are not uniformly responsive to SRS and suggest that prospective studies to evaluate the effect of SRS alone or in combination with surgery on brain metastases ≤3 cm matched by tumor size and histology are warranted. These studies will help establish multi-disciplinary treatment guidelines that improve local control while minimizing radiation necrosis during treatment of brain metastasis ≤3 cm.
Similar content being viewed by others
Introduction
Brain metastasis remains a common manifestation of systemic cancer and remains a poor prognostic factor for cancer patients1. With improvement in overall survival of patients with brain metastasis being driven by more effective systemic treatment regimens, the incidence of brain metastasis has increased considerably in recent years1. While these new systemic therapies have demonstrated impressive clinical activity against primary cancers outside of the central nervous system (CNS), the blood-brain-barrier (BBB) limits the efficacy of systemic treatments within CNS2, resulting in an unmet and urgent need for optimal local disease control strategies including surgery with stereotactic radiosurgery (SRS) or SRS alone in the management of brain metastases.
Several clinical trials have evaluated local management strategies for brain metastasis. The EORTC 22952–26001 study evaluated the impact of adjuvant whole brain radiation therapy (WBRT) after SRS or surgery for patients with 1–3 brain metastases3. Results showed that WBRT reduced intracranial relapses and neurological death but did not improve functional independence or overall survival. The RTOG 9508 study compared WBRT alone or WBRT followed by SRS boost for patients with 1–3 newly diagnosed brain metastases4. Results showed that WBRT and SRS boost improved functional outcomes and survival for patients with a single unresectable brain metastasis. An MD Anderson-led prospective trial compared observation to SRS after surgical resection of 1–3 brain metastasis with a post-operative cavity size that was <4 cm in maximum diameter5. Results showed that SRS of the surgical cavity in patients with complete resection of 1–3 brain metastasis significantly lowers local recurrence compared to post-operative observation. These results indicate that for patients presenting with 1–3 brain metastasis with large lesions >3 cm, surgery followed by SRS provides good functional outcomes and optimizes local disease control.
SRS alone is recommended for brain metastasis up to 3 cm maximum diameter (or 14 cm3)6. Our previous study which analyzed local disease control rates for brain metastases in 135 patients (N = 153 lesions) who received SRS at MD Anderson between 1991 and 2001 demonstrated that the 1- and 2-year local control rates (LCRs) for tumors greater than 0.5 cm3 (or 1 cm diameter) were significantly lower (56% and 24%, respectively) than for lesions smaller than 0.5 cm3 (86% and 78%, respectively; P = 0.0016)7. These results indicate that lesions less than 0.5 cm3 (or 1 cm diameter) are sensitive to SRS but lesions greater than 0.5 cm3 (or 1 cm diameter) are less sensitive. The response of brain metastasis 1–3 cm maximum diameter to SRS is variable, likely due to other factors independent of size8,9. Therefore, the local management of brain metastasis ≤3 cm has evolved to incorporate other factors including cumulative tumor volume of intracranial disease (not limited to specific number of lesions), tumor location (eloquent v. non-eloquent) and primary tumor histology10. There are, however, no formal treatment guidelines that incorporate these factors into the management of patients with brain metastasis ≤3 cm.
Here, we retrospectively analyzed one of the largest single-institution cohorts of patients with 1–3 treatment-naive brain metastasis who received framed SRS over a 25-year period. The purpose of this study was to identify variables or factors that influence the time to local treatment failure (TTF) and LCRs after SRS for brain metastasis in patients without prior or ongoing systemic treatment. These variables could then inform the design of prospective clinical trials seeking more effective radiotherapeutic and surgical treatment strategies that enhance local disease control and minimize variability in the outcomes of brain metastasis ≤3 cm treated with SRS.
Results
Demographics and treatments
Inclusion and exclusion criteria for patients and lesions includes in analysis are listed in Table 1. Specific criteria for determining response to SRS are show in in Table 2. Baseline patient characteristics, radiation treatment parameters and sequence of radiation therapy are listed in Tables 3 and 4. Among 1095 patients with 1733 lesions, 507 (46%) were female, and 588 (54%) were male. The median patient age was 62 years (range 16–95). Of 1095 patients, 616 had only one SRS-treated lesion, while 320 patients had two and 159 had three SRS-treated lesions. The most common primary tumor type was non-small cell lung cancer (36%), followed by melanoma (21%), breast cancer (12%), and renal cell carcinoma (6%). Seventy-four percent of lesions received single fraction Gamma Knife SRS with a mean periphery dose of 20 Gy and a range of 13.5–24 Gy. Twenty-six percent of lesions received single fraction LINAC SRS with a mean periphery dose of 18 Gy and a range of 8–22 Gy (one calvarial metastasis received 8 Gy). There were no fractionated treatments. Median target tumor diameter was 1.3 cm (range 0.28–2.96 cm).
Outcomes following SRS for treatment-naïve brain metastasis
Outcomes of the 1733 treatment-naïve lesions treated with SRS in eligible patients were analyzed (Fig. 1). Following SRS, 259 (15% of all treated lesions) showed imaging findings concerning for LTF. Of these, 202 lesions (11% of all treated lesions) were deemed LTF based on specific criteria (Table 2). LTF was diagnosed after concerning radiographic findings led to surgical resection with pathology showing viable tumor only or mixed viable tumor and radiation necrosis (RN; n = 110 or 6% of all treated lesions) or clinical/radiographic signs necessitating a change in management (n = 92 or 5% of all treated lesions). ARE was identified in 57 lesions (4% of all treated lesions). Pure RN without viable tumor on pathology after surgery occurred in 22 lesions (1.3% of all treated lesions). 48 lesions (3% of treated lesions) had mixed pathology with both viable tumor and RN seen on pathology after surgery. Radiographic AREs that were deemed to be RN on ABTI and/or were responsive to steroids or Bevacizumab occurred in 18 lesions (1% of all treated lesions). In sum, radiographic and pathology proven RN occurred in 88 lesions (5% of all treated lesions). Hemorrhage or edema requiring surgical resection within 60 days of SRS occurred in 19 lesions (1% of all treated lesions). There were 26 patients with 36 lesions (2% of all treated lesions) with concerning imaging findings who were functionally not fit for further treatment or who chose not to proceed with further treatment. Perfusion data was available for 3 out of 36 lesions, all of which were consistent with a viable tumor signature. 17 out of the 26 patients with concerning imaging findings went on to hospice care after declining clinical intervention for the suspected intracranial progression.
Outcomes following Stereotactic Radiosurgery (SRS) for treatment-naive brain metastasis. SRS treated lesions that met inclusion criteria (n = 1733) were categorized as Local Treatment Failure (LTF) or Adverse Radiation Effect (ARE) according to specific criteria in Table 2. Source data provided as a Source data file.
Univariate and multivariate analysis for factors influencing TTF
Based on univariate analysis, year of SRS treatment (P < 0.0001), SRS dose (P < 0.0001), tumor size, primary tumor histology and SRS modality (LINAC v. GK SRS; P < 0.0001) significantly influenced TTF (Table 5). Multivariate analysis, however, showed that age, year of SRS, tumor size and primary tumor histology significantly influenced TTF (Table 5). The TTF ratio (ratio of TTF specified size range versus TTF of SRS susceptible lesions ≤0.5 cm; see methods for details) for lesions >0.5 and 1 cm, >1 and 1.5 cm, in diameter was shorter compared to lesions ≤0.5 cm, but this did not reach statistical significance (Table 5). However, the TTF ratios were significantly lower for lesions >1.5 and 2 cm (TTF ratio 0.31; 95% CI, 0.21–0.44; P = 0.014), >2 and 2.5 cm (TTF ratio 0.22; 95% CI, 0.16–0.32; P = 0.0005), >2.5 and 3 cm (TTF ratio 0.12; 95% CI, 0.07–0.20; P = 0.0003; Table 5). Multivariate analysis also showed that melanoma had a significantly shorter TTF relative to NSCLC and RCC (Table 5).
Local control rates after SRS
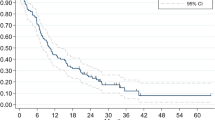
The 1- and 2- year local control rate (LCR) for all lesions treated with SRS were 82% and 78%, respectively with lesions censored at time of last imaging follow-up and at WBRT administration if it occurred before treatment failure of SRS treated lesion. The 1- and 2- year LCRs for lesions ≤0.5 cm were 93% and 90.5% respectively (Fig. 2 and Table 6). The 1-and 2-year LCRs for lesions in diameter ranges of 0.5–1, 1–1.5, 1.5–2, 2–2.5, 2.5–3 cm are 92.1 and 91%, 85.8 and 80.9%, 80.4 and 66.5%, 69.9 and 61.7% and 55.1 and 34.5% respectively (Fig. 2 and Table 6). Amongst tumor histologies, melanoma and breast cancers had a lower 2-year LCRs at 67.4% and 68.5% respectively (Table 7; Supplementary Fig. S1). Renal cell carcinoma (RCC) and non-small cell lung cancer (NSCLC) had a higher 2-year LCRs at 93.4% and 84.7% respectively (Table 7; Supplementary Fig. S1). LCR based on age and sex are shown in Supplementary Figs. S2 and S3.
Kaplan–Meier curves showing percent local control of brain metastasis lesions over time after Stereotactic Radiosurgery (SRS) stratified by diameter (cm). x-axis is censored at 5 years post-SRS as only 5 lesions under surveillance failed SRS after 5 years. Source data provided as a Source data file.
Illustrative cases
We demonstrate the differential response of non-small cell lung and solitary melanoma brain metastatic lesions of similar sizes to GK SRS in an 83-year-old male patient with a history of NSCLC presenting with a solitary left parietal lesion of 1.42 cm diameter (or 1.5 cm3) and a 62-year-old female patient with history of melanoma presenting with a solitary right frontal lesion of 1.45 cm diameter (or 1.6 cm3), respectively (Fig. 3). Both lesions were treated with GK SRS at a dose of 20 Gy. At 1-year follow-up, the NSCLC lesion showed a positive treatment response with near complete regression of the treated lesion. In contrast, the treated melanoma lesion showed treatment failure with an increase in lesion size at the 1-year follow-up.
Discussion
The American Society of Clinical Oncology, Society for Neuro-Oncology and the American Society for Therapeutic Radiology and Oncology recommend single-fraction SRS for patients with brain metastases measuring 3 cm diameter (14 cm3)6,11,12. Fractionated SRS (e.g., 27 Gy in 3 fractions or 30 Gy in 5 fractions) is conditionally recommended for lesions ≥3 to 5 cm diameter (14–65 cm3)12. A meta-analysis of 24 SRS brain metastasis clinical trials showed that relative to single-fraction SRS, fractionated SRS reduces the risk of RN for lesions between 2–3 cm (4–14 cm3) but not for lesions >3 cm (>14 cm3)13. There was no significant difference in the 1-year local disease control between single- versus multifraction SRS for lesions over 2 cm. For lesions >4 cm diameter (>30 cm3), surgical resection is recommended followed by single fraction SRS. Based on these guidelines, many centers including the University of Texas MD Anderson Cancer Center perform single-fraction SRS for lesions up to 3 cm (14 cm3) and multifraction SRS for lesions >3 cm14. There remains significant variability, however, in the response of lesions 1–3 cm in diameter to single-fraction SRS, with some lesions demonstrating durable clinical responses and others failing treatment7. We hypothesized that to improve local control, all tumor intrinsic factors that significantly influence susceptibility of lesions to SRS need to be formally incorporated into the treatment guidelines for brain metastasis.
While prior studies have demonstrated the influence of tumor size on response to SRS7,15, very few studies are powered to evaluate the influence of other tumor intrinsic factors on the response of treatment-naive lesions to SRS. This is an important consideration, because evaluating SRS in this patient population would directly evaluate the biological response of brain metastases to SRS without the influence of previous or ongoing systemic treatments. To identify tumor intrinsic properties influencing response to SRS, we performed a retrospective analysis of SRS-treated brain metastases in patients with treatment-naive brain lesions. We find that in treatment-naïve brain lesions that 3 cm, tumor size and primary histology significantly influence long term local control after SRS as initial local therapy. This indicates that the current recommendation for treatment of brain metastases based solely on size is not sufficient to identify lesions or patients who will respond best to treatment. Consistent with our prior report7, lesions 1.5 cm demonstrated higher 2-year LCRs (over 81%), while lesions over 1.5 cm had significantly lower 2-year LCRs (66.5%). Even within the former group (lesions 1.5 cm), histology plays a role in the susceptibility to SRS as exemplified in the case illustration comparing the response of melanoma to NSCLC brain metastasis and in preclinical predictive models validated in tumor cell lines16.
Predictive models using cell lines and in vivo data have indicated that multifraction SRS may be equally effective for local control when compared with single fraction SRS for radioresistant lesions16. One study found that for melanoma brain metastasis, three 8-Gy SRS fractions (EQD2) would have similar tumor control as that of a single fraction 20 Gy SRS16. At MD Anderson, single fraction SRS dosing is modified based on tumor volume in accordance with cavity volumes outlined in N107C17. For lesions between 1.5–3 cm, treatment is not uniform with some cases receiving single fraction SRS and others multifraction SRS. For lesions larger than 3 cm in patients who are not good surgical candidates, fractionation is typical, most often with 27 Gy in 3 fractions. In larger tumors, multifraction treatments are done to minimize the risk of toxicity or RN18. It is unclear, however, whether multifraction SRS can achieve local control in radioresistant histologies between 1.5–3 cm while limiting the incidence of RN. The SAFESTEREO study is an ongoing phase II prospective and randomized study that is comparing the incidence of adverse local events in patients with brain metastasis treated with 1 or 3 fractions versus 5 fractions (NCT05346367)19. Results from this prospective study will hopefully elucidate whether multifraction SRS could achieve effective local control in radioresistant histologies between 1.5–3 cm (identified in our study as the vulnerable range), with decreased incidence of RN. Another prospective study that remains in the concept phase is a histology-specific prospective trial comparing single fraction SRS to multifraction SRS (9 Gy × 3 fractions; NRG Oncology). Here, patients with radioresistant histologies like melanoma who have lesions between 1.5 cm and 3 cm will be randomized to receive single fraction SRS or multifraction SRS. The primary outcomes are incidence of RN and LTF. Altogether, these trials will identify radiotherapy-based approaches that improve local control while minimizing RN in radioresistant histologies between 1.5–3 cm.
A second approach to improve local control for radioresistant histologies is surgical resection combined with RT or SRS. Currently, there is limited consensus on the need for surgery for lesions ≤3 cm maximum diameter and hence there is significant variability in the management of lesions within this range with a propensity to perform surgery for larger lesions especially if associated with symptomatic mass effect and/or edema20. Therefore, a prospective randomized clinical trial is needed to determine whether there is a role for surgery in the management of radioresistant histologies that are 3 cm. In this trial, patients with radioresistant histologies between 1.5 cm and 3 cm are randomized to either surgery followed by single fraction or multifraction SRS to the cavity or single fraction SRS-only to the lesion (standard of care for lesions <3 cm). Another ongoing approach that could uncover the role of surgery for management of radioresistant brain metastasis is surgery followed by Cs-131 collagen tile brachytherapy. In a phase 1/2 human clinical trial, post-operative Cs-131 collagen tile brachytherapy for newly-diagnosed brain metastasis (n = 24 patients and histologies including lung, renal, melanoma and cervical) was associated with no local recurrences or RN21. At MD Anderson, there is an ongoing phase 3 randomized controlled trial of post-surgical stereotactic radiotherapy (SRT, standard of care) versus surgically targeted radiation therapy (STaRT) with Cs-131 collagen tile brachytherapy for treatment of newly-diagnosed metastatic brain tumors or ROADS (Radiation One and Done Study; NCT 04365374). We anticipate that data from this study will also provide insights into whether Cs-131 collagen tile brachytherapy following surgical resection may provide more effective local control while minimizing RN especially for radioresistant histologies. Pre-operative SRS is another strategy that may improve local control while minimizing RN and incidence of leptomeningeal disease (LMD). To this end, there are several ongoing phase III randomized studies including the NRG-BN012 (NCT05438212) and an MD Anderson study that are comparing preoperative SRS to postoperative SRS for newly-diagnosed brain metastasis22. We expect that these ongoing prospective studies will provide a framework for designing future prospective studies focused on identifying the optimal synergistic approach using surgery and radiotherapy for radioresistant histologies between 1.5–3 cm.
There are also systemic avenues to enhance response of brain metastasis to RT. Administration of radiosensitizers have also been proposed to overcome the intrinsic resistance mechanisms of brain metastasis to RT23. It has been shown that in preclinical models, competitive inhibitors of the DNA Damage repair (DDR) genes ATM and ATR Kinase sensitizes NSCLC brain metastasis to radiation therapy24. Histology (squamous cell carcinoma or adenocarcinoma) influenced the types of DDR alterations in NSCLC indicating that histology may also play a role in determining the susceptibility of NSCLC to radiosensitizers. Following pre-clinical studies, we anticipate that human clinical trials will assess the role of radiosensitizers in the response of radioresistant histologies to SRS. There are reports of synergy between RT and systemically administered immune check point inhibitors (ICI) in preclinical and clinical studies for primary and metastatic brain tumors25,26,27. This approach with ICIs leverages the potential immunostimulatory effect of RT on the tumor micro-environment28. In NSCLC, the addition of ICI to RT boosts the infiltration of anti-tumor immune cells which enhance local control27. The synergy between RT and ICI, however, also appears to be histology-specific and ICI-specific indicating that tumor-specific micro-environment factors may influence susceptibility to these treatments26,29. The use of RT with ICI, may increase the risk of RN30,31. In patients with melanoma brain metastasis treated with SRS and anti-CTLA-4 and/or anti-PD-1 at MD Anderson Cancer Center, a multivariate analysis showed that use of chemotherapy within 6 months of SRS and number of lesions treated were predictive of increased RN risk (HR 2.20, 95% CI 1.22–3.97, p = 0.009; HR 1.09, 95% CI 1.03–1.15, p = 0.002)31. Ultimately, prospective randomized clinical trials are needed to determine the influence of tumor histology, prior treatments and number of lesions treated on the response (Local control v. RN) of brain metastasis with radioresistant histologies to SRS and ICI.
Distinguishing RN (or pseudoprogression) from true local tumor progression without a pathologic evaluation poses a significant challenge, as both can present with similar clinical features and radiographic findings32. Imaging technology is still in the early stages of reliably differentiating RN from true local progression33. Additionally, the presence of viable tumor on pathologic evaluation following early surgical resection (within 60 days post-SRS) may not reliably indicate treatment failure in cases such as hemorrhagic conversion. We addressed this challenge by diagnosing LTF based on a combination of radiographic findings, clinical management, and extensive patient follow up and outcome assessment. We also utilized perfusion weighted ABTI, when available, to distinguish RN from true progression (Supplementary Fig. S4). In prospective clinical trials, LITT was shown to be effective for symptomatic RN and allowed for a decreased dependency on steroids34. Therefore, we performed LITT for symptomatic RN to minimize complications from long term steroids or bevacizumab use (Supplementary Fig. S4). In the case of early salvage resection showing mixed tumor and RN on pathology, we differentiated local progression from ARE after a multidisciplinary characterization of radiographic features and the presence of sustained tumor progression despite the administration of steroids or bevacizumab. The 5% overall incidence of RN across all treated lesions (pure, mixed, clinical and radiographic; see Table 2) in this study is consistent with prior reports of a RN rate of 5–26% per lesion35,36,37. Additionally, SRS is associated with a lower incidence of RN in tumors <2 cm37 and over 85% of the treated lesions in this study were <2 cm. These results support the relatively lower RN rate noted in this study.
From a clinical perspective, although size, histology and location are part of the discussion during management of patients with brain metastasis38, there are currently no standardized or high level guidelines that incorporate these factors a decision-making algorithm that weights risk of local failure with risk of RN. Until prospective studies discussed above are completed, we are developing an MD Anderson brain metastasis nomogram that allows for determination of risk of local failure and RN based on a combination of factors including tumor size and histology. This will allow for better patient selection for treatment with SRS or surgery with SRS. Our hope is that as the overall survival of patients with metastatic cancer continues to improve, refined selection of the most appropriate local therapy approach for brain metastasis will improve outcomes and the quality of life of patients with brain metastases.
Study limitations
This is a single institution study that is subject to selection bias. It is also a retrospective study, and our findings need to be validated in prospective and randomized clinical trials. Although there are some minor differences between findings from our study and prior studies assessing the susceptibilities of brain metastasis to SRS, the main findings from our study regarding the radioresistance of melanoma are consistent with published literature. One consideration that may contribute to the differences between our study and prior studies may lie in the fact that our cohort is exclusively treatment-naïve while other studies pool lesions from patients receiving a diverse array of local or systemic treatments. Our multivariate analysis also showed that year of treatment influenced TTF. Therefore, it is possible that other factors such as the availability of more effective brain penetrant agents and immunotherapy administered after SRS may have improved outcomes in the post-2009 era. As our retrospective analysis does not include frameless SRS cases, it is unclear if a frameless approach influences the results described in the study. Finally, despite our thorough criteria characterizing local tumor progression, it remains challenging to reliably differentiate pseudoprogression from tumor progression in certain cases especially in the patients with suspected intracranial progression based on MRI findings that ultimately were not functionally fit for further treatment or declined clinical intervention, although these cases represented a small fraction of treated lesions in our cohort (2% of all treated lesions).
In summary, treatment-naive brain metastases that are ≤3 cm diameter or 14 cm3 (recommended cut-off for single-fraction SRS) are not uniformly responsive to SRS. Within this group of brain metastases, we find that tumor size (>1.5 cm diameter or 1.8 cm3) and primary histology (melanoma) have significantly shorter TTF and consequently lower 1- and 2-year local control rates after SRS relative to lesions <1.5 cm. Since our results are not influenced by prior or ongoing systemic treatments, these findings indicate that tumor intrinsic properties significantly influence susceptibility to SRS. To establish standardized and multidisciplinary clinical guidelines regarding the optimal brain metastatic lesion size and histologic criteria that portend a favorable LC and low risk of RN after SRS or surgery with SRS, prospective clinical trials enrolling patients matched by tumor size and histology are warranted.
Methods
Study design and patient characteristics
The Institutional Review Board (IRB) at the University of Texas MD Anderson Cancer Center approved this retrospective study and a waiver of informed consent, which included a chart review of 3000 patients with metastatic brain lesions treated with the frame-based LINAC and Gamma Knife (GK) SRS from June 1, 1993 to June 30, 2018. Patients with a maximum of 3 treatment-naive lesions were included (Table 1). We included patients with prior surgical resection of a different brain metastasis. We excluded patients who had whole brain radiation therapy (WBRT) and/or chemotherapy prior to SRS and those with no postoperative imaging. We excluded lesions that were >3 cm in maximum dimension (Table 1). LINAC SRS prescription doses were delivered to the 81–95% isodose line while GK SRS prescription doses were delivered to the 50% isodose line, per our standard practice at MD Anderson Cancer Center during the study time period7,39. Local treatment failure (LTF) was defined either radiographically or by pathology after surgery for radiographically suspected LTF (Table 2). Radiographic progression of an SRS-treated lesion was defined as a sustained increase in tumor size on serial imaging, or, when available, increased perfusion suggestive of viable tumor on advanced brain tumor imaging (ABTI). Such changes were resistant to treatment with steroids or bevacizumab and required a change in the clinical management, including WBRT for local and distant recurrence, systemic treatment, or targeted therapy (repeat SRS or laser interstitial thermal therapy (LITT)). To distinguish tumor progression from pseudoprogression, perfusion data was reviewed from the ABTI if available. Patients with concerning intracranial imaging findings, who did not have an ABTI but were offered further treatment such as WBRT and chose not to proceed with further treatment or had poor functional status were deemed as having LTF based on the clinical decision to offer further treatment for a suspected LTF (Fig. 1; Pseudoprogression cannot be ruled out entirely in patients who chose not to proceed with treatment). For patients who underwent salvage surgical resection, pathology showing viable tumor was deemed LTF. Adverse radiation effects (ARE) were documented and included clinically or radiographically defined ARE requiring steroids or bevacizumab, decreased perfusion suggestive of radiation necrosis (RN) on ABTI when available, pure RN on pathology following surgical resection, and post-SRS hemorrhage or peri-tumoral edema requiring surgical resection within the first 60 days postoperatively. Patients with concerning imaging findings who did not have a follow-up clinical encounter to address such changes were excluded. Cases with concerning imaging findings that resolved spontaneously without intervention were not deemed as LTF or ARE.
Patient characteristics, radiation treatment parameters and sequence of radiation therapy are listed in Tables 3 and 4. Follow-up data was obtained for 1095 patients and censored at last imaging follow-up or ARE. At the time of analysis, the mean duration of follow-up from time of SRS was 19 months for all patients. Follow-up imaging was obtained at the discretion of the team treating the patient (neurosurgeon, medical oncologist, and radiation oncologist). Since the goal of the project was to identify LTF, we did not include distant failure as an endpoint.
Statistical analysis
TTF was summarized by Kaplan–Meier method for discrete variables, with each lesion treated as an independent sample (within-patient clustering was subsequently accommodated within appropriate models)40. Follow-up was censored at last imaging follow-up or ARE. Since additional radiation therapy to the brain via WBRT may alter the outcomes following SRS, patients were censored at time of post-SRS WBRT if local failure of the SRS treated lesion had not occurred when WBRT was given. TTF was modeled by accelerated failure time models with log-logistic distribution selected per Akaike Information Criteria among Weibull, hat exponential, Gaussian, logistic, log-normal, and log-logistic distributions, and verified by residual plot overlaid on the distribution as well as deviance residual plots for covariates, and clustering on patients to control for repeated events. Rather than the reported hazard ratios typical of Cox models, accelerated failure time models report the TTF ratio which compares the TTF across the size ranges (versus 0.5 cm) or across multiple histologies. The TTF ratio is approximately interpretable as the inverse of hazard ratios. A multi-variable accelerated failure time model of TTF was found by exhaustive variable selection by comparing the Akaike Information Criterion of models of all combinations of variables (age, year of treatment, primary tumor histology, discrete diameter, LINAC vs. Gamma knife SRS, KPS, SRS dose). The resulting optimal model assessed the association between TTF and the variables of age, year of treatment, primary tumor histology, discrete diameter, and post-SRS WBRT status. Model-adjusted differences among the levels of discrete variables in TTF were assessed by Tukey test. Age, Sex, year of treatment, primary tumor histology, tumor size, SRS modality (LINAC vs. GK SRS), pre-SRS KPS, and SRS dose were assessed in a univariate analysis. Statistical analyses were performed using R statistical software41. All statistical tests utilized two-sided alpha = 0.05 for a 95% level of statistical confidence. Survival modeling was performed using the “survival” package42,43. Assessment of differences among discrete variable levels in the accelerated failure time model were estimated using the emmeans package which includes adjusted means weighted proportionally to covariate marginal frequencies44.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Although de-identified source data are provided with this paper, the complete clinical data that support the findings of this study are not openly available given IRB restrictions on human clinical data. Anonymized data are available from the corresponding author upon reasonable request and IRB approval. Following IRB approval, the de-identified clinical data will be made available within 2 weeks. Source data are provided with this paper.
References
Ostrom, Q. T., Wright, C. H. & Barnholtz-Sloan, J. S. Brain metastases: epidemiology. Handb. Clin. Neurol. 149, 27–42 (2018).
Arvanitis, C. D., Ferraro, G. B. & Jain, R. K. The blood-brain barrier and blood-tumour barrier in brain tumours and metastases. Nat. Rev. Cancer 20, 26–41 (2020).
Kocher, M. et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J. Clin. Oncol. 29, 134–141 (2011).
Andrews, D. W. et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 363, 1665–1672 (2004).
Mahajan, A. et al. Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases: a single-centre, randomised, controlled, phase 3 trial. Lancet Oncol. 18, 1040–1048 (2017).
Vogelbaum, M. A. et al. Treatment for Brain Metastases: ASCO-SNO-ASTRO Guideline. J. Clin. Oncol. 40, 492–516 (2022).
Chang, E. L. et al. The role of tumor size in the radiosurgical management of patients with ambiguous brain metastases. Neurosurgery 53, 272–280 (2003).
Moraes, F. Y. et al. Outcomes following stereotactic radiosurgery for small to medium-sized brain metastases are exceptionally dependent upon tumor size and prescribed dose. Neuro Oncol. 21, 242–251 (2019).
Khan, M. et al. Tumor Primary Site and Histology Subtypes Role in Radiotherapeutic Management of Brain Metastases. Front Oncol. 10, 781 (2020).
Linskey, M. E. et al. The role of stereotactic radiosurgery in the management of patients with newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J. Neurooncol. 96, 45–68 (2010).
Schiff, D. et al. Radiation Therapy for Brain Metastases: ASCO Guideline Endorsement of ASTRO Guideline. J. Clin. Oncol. 40, 2271–2276 (2022).
Gondi, V. et al. Radiation Therapy for Brain Metastases: An ASTRO Clinical Practice Guideline. Pr. Radiat. Oncol. 12, 265–282 (2022).
Lehrer, E. J. et al. Single versus Multifraction Stereotactic Radiosurgery for Large Brain Metastases: An International Meta-analysis of 24 Trials. Int J. Radiat. Oncol. Biol. Phys. 103, 618–630 (2019).
Minniti, G. et al. Single-Fraction Versus Multifraction (3 x 9 Gy) Stereotactic Radiosurgery for Large (>2 cm) Brain Metastases: A Comparative Analysis of Local Control and Risk of Radiation-Induced Brain Necrosis. Int J. Radiat. Oncol. Biol. Phys. 95, 1142–1148 (2016).
Soliman, H., Das, S., Larson, D. A. & Sahgal, A. Stereotactic radiosurgery (SRS) in the modern management of patients with brain metastases. Oncotarget 7, 12318–12330 (2016).
Li, S. et al. A unified multi-activation (UMA) model of cell survival curves over the entire dose range for calculating equivalent doses in stereotactic body radiation therapy (SBRT), high dose rate brachytherapy (HDRB), and stereotactic radiosurgery (SRS). Med. Phys. 48, 2038–2049 (2021).
Brown, P. D. et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC.3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 18, 1049–1060 (2017).
Kjellberg, R. N., Masamitsu, A. Stereotactic Bragg peak proton radiosurgery results. In Modern Stereotactic Neurosurgery, (ed. Lunsford, L. D.) 463–470 (Martinus Nijhoff, 1988).
Crouzen, J. A. et al. SAFESTEREO: phase II randomized trial to compare stereotactic radiosurgery with fractionated stereotactic radiosurgery for brain metastases. BMC Cancer 23, 273 (2023).
Sankey, E. W. et al. Operative and peri-operative considerations in the management of brain metastasis. Cancer Med. 8, 6809–6831 (2019).
Wernicke, A. G. et al. Phase I/II study of resection and intraoperative cesium-131 radioisotope brachytherapy in patients with newly diagnosed brain metastases. J. Neurosurg. 121, 338–348 (2014).
Yeboa, D. N. et al. MD Anderson Phase III Randomized Preoperative Stereotactic Radiosurgery (SRS) vs. Postoperative SRS for Brain Metastases Trial. Int. J. Radiat. Oncol.*Biol.*Phys. 117, e160–e161 (2023).
Beg, U. et al. Current Landscape and Future Prospects of Radiation Sensitizers for Malignant Brain Tumors: A Systematic Review. World Neurosurg. 151, e839–e856 (2021).
Baschnagel, A. M. et al. ATR Inhibitor M6620 (VX-970) Enhances the Effect of Radiation in Non-Small Cell Lung Cancer Brain Metastasis Patient-Derived Xenografts. Mol. Cancer Ther. 20, 2129–2139 (2021).
Ene, C. I. et al. Anti-PD-L1 antibody direct activation of macrophages contributes to a radiation-induced abscopal response in glioblastoma. Neuro Oncol. 22, 639–651 (2020).
Ko, E. C. & Formenti, S. C. Radiation therapy to enhance tumor immunotherapy: a novel application for an established modality. Int J. Radiat. Biol. 95, 936–939 (2019).
Formenti, S. C. et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat. Med 24, 1845–1851 (2018).
Takahashi, J. & Nagasawa, S. Immunostimulatory Effects of Radiotherapy for Local and Systemic Control of Melanoma: A Review. Int. J. Mol. Sci. 21 https://doi.org/10.3390/ijms21239324 (2020).
Singh, C., Qian, J. M., Yu, J. B. & Chiang, V. L. Local tumor response and survival outcomes after combined stereotactic radiosurgery and immunotherapy in non-small cell lung cancer with brain metastases. J. Neurosurg. 132, 512–517 (2019).
Kim, P. H. et al. Immune checkpoint inhibitor therapy may increase the incidence of treatment-related necrosis after stereotactic radiosurgery for brain metastases: a systematic review and meta-analysis. Eur. Radio. 31, 4114–4129 (2021).
Fang, P. et al. Radiation necrosis with stereotactic radiosurgery combined with CTLA-4 blockade and PD-1 inhibition for treatment of intracranial disease in metastatic melanoma. J. Neurooncol 133, 595–602 (2017).
Salari, E., Elsamaloty, H., Ray, A., Hadziahmetovic, M. & Parsai, E. I. Differentiating Radiation Necrosis and Metastatic Progression in Brain Tumors Using Radiomics and Machine Learning. Am. J. Clin. Oncol. 46, 486–495 (2023).
Nichelli, L. & Casagranda, S. Current emerging MRI tools for radionecrosis and pseudoprogression diagnosis. Curr. Opin. Oncol. 33, 597–607 (2021).
Ahluwalia, M. et al. Laser ablation after stereotactic radiosurgery: a multicenter prospective study in patients with metastatic brain tumors and radiation necrosis. J. Neurosurg. 130, 804–811 (2018).
Kohutek, Z. A. et al. Long-term risk of radionecrosis and imaging changes after stereotactic radiosurgery for brain metastases. J. Neurooncol 125, 149–156 (2015).
Minniti, G. et al. Stereotactic radiosurgery for brain metastases: analysis of outcome and risk of brain radionecrosis. Radiat. Oncol. 6, 48 (2011).
Sneed, P. K. et al. Adverse radiation effect after stereotactic radiosurgery for brain metastases: incidence, time course, and risk factors. J. Neurosurg. 123, 373–386 (2015).
Barbour, A. B. et al. Radiation Therapy Practice Patterns for Brain Metastases in the United States in the Stereotactic Radiosurgery Era. Adv. Radiat. Oncol. 5, 43–52 (2020).
Chang, E. L. et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 10, 1037–1044 (2009).
Kaplan, E. L. Nonparametric estimation from incomplete observations. J. Am. Stat. Assoc. 53, 457–481 (1958).
RCoreTeam. A language and environment for statistical computing (R Foundation for Statistical Computing, 2022).
Therneau, T. M. A Package for Survival Analysis in R. R package version 3.4-0. https://CRAN.R-project.org/package=survival (2022).
Terry, M., Therneau, P. M. G. Modeling Survival Data: Extending the Cox Model (Springer, 2000).
Lenth, R. V. emmeans: Estimated Marginal Means, aka Least-Squares Means. R package version 1.8.4-1. https://CRAN.R-project.org/package=emmeans. (2023).
Acknowledgements
C.I.E. is supported by the MD Anderson Cancer Center Physician Scientist Program and the National Cancer Institute/ National Institutes of Health, Early Surgeon Scientist Program. We thank Preeti Ramadoss, Ph.D., for critical review and editing of the manuscript.
Author information
Authors and Affiliations
Contributions
C.I.E., D.S. and R.E.S. conceptualized the study. C.I.E., C.A.F., A.H. performed chart review. C.R.A. was the statistician. R.E.S., G.R., S.D.F., A.B.H., I.E.M., J.S.W., S.S.P., F.F.L., D.N.Y., J.L., T.H.B., B.Y.S.K., S.L.M., M.F.M., T.M.B., performed stereotactic radiosurgery cases. C.A.B., C.M.W., A.J.G., C.C., M.C.T., S.P., T.A.S. reviewed and edited the manuscript. J.T.H. and G.N.F. performed pathological analysis on tissue from surgery. C.I.E., C.A.F., T.B, D.S. and R.E.S. analyzed and interpreted the results. C.I.E., D.S., T.B., R.E.S. supervised the project. C.I.E., C.A.F., R.E.S. wrote the manuscript. All authors reviewed and edited the work. All authors have read and agreed to the submitted version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Shidong Li, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ene, C.I., Abi Faraj, C., Beckham, T.H. et al. Response of treatment-naive brain metastases to stereotactic radiosurgery. Nat Commun 15, 3728 (2024). https://doi.org/10.1038/s41467-024-47998-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-47998-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.