Abstract
Current thermochemical methods to generate H2 include gasification and steam reforming of coal and natural gas, in which anthropogenic CO2 emission is inevitable. If biomass is used as a source of H2, the process can be considered carbon-neutral. Seaweeds are among the less studied types of biomass with great potential because they do not require freshwater. Unfortunately, reaction pathways to thermochemically convert salty and wet biomass into H2 are limited. In this study, a catalytic alkaline thermal treatment of brown seaweed is investigated to produce high purity H2 with substantially suppressed CO2 formation making the overall biomass conversion not only carbon-neutral but also potentially carbon-negative. High-purity 69.69 mmol-H2/(dry-ash-free)g-brown seaweed is produced with a conversion as high as 71%. The hydroxide is involved in both H2 production and in situ CO2 capture, while the Ni/ZrO2 catalyst enhanced the secondary H2 formation via steam methane reforming and water-gas shift reactions.
Similar content being viewed by others
Introduction
Given the rapid development of the global economy and ever-increasing population, the world’s energy demand will increase by 30% by 2040 according to the International Energy Agency (IEA)1. Global CO2 emissions from energy use are expected to increase up to 35.7 GT per year, which is far from the necessary level required to avoid severe climate change2,3. Thus, further use of renewable low-carbon and carbon-neutral energy sources (e.g., solar photovoltaic, wind, hydropower, and biomass) is urgently needed4,5.
Not all biomass can be considered sustainable resources since their cultivation, harvesting, treatment, processing, and transportation can lead to greater environmental impacts than benefits depending on the paths have been taken. For example, the transportation of low energy density biomass for long distance using fossil fuels would make biomass less attractive as sustainable resources. Nevertheless, biomass is one of the renewable resources that have a potential to be not only carbon-neutral but even be carbon-negative if combined with carbon capture and storage (CCS) scheme6,7. According to the U.S. Energy Information Administration (EIA), biomass contributed 5% of the U.S. primary energy supply in 2018 and is expected to replace more than 30% of U.S. petroleum consumption by 20308. Most of the biomass is terrestrial. Their limited availability compared to fossil resources, the associated land use change, and the need for freshwater for cultivation and growth are often used to argue against the large-scale long-term impact of bioenergy9,10.
Seaweed is among less investigated types of bioenergy sources that is available worldwide, compared to the global oil distribution (Fig. 1a)11,12,13. Seaweeds and salt-tolerant algae can also be farmed in regions where algal biomass is currently cultivated (light green areas in Fig. 1a) by using brine instead of freshwater. Seaweeds have a global production of 216.6 thousands metric tons per year and have mainly been used for human foods, cosmetics, fertilizers, and chemical feedstock9,14. Interestingly, compared to terrestrial biomass, seaweed has a high average CO2 sequestration rate (36.7 ton hectare−1 year−1, seven times greater than that of conventional lignocellulosic biomass), rapid growth rate (harvested up to six times per year even without fertilizer), and does not compete with land-based food crops if grown in the sea15,16.
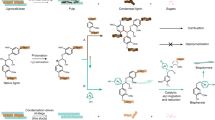
a Global distribution of seaweed (green) and algae (light green) compared to major oil reserves (red). b Alkaline thermal treatment (ATT) of seaweed innovatively produces high purity bio-H2 from wet and salty biomass feedstock with a carbon capture and storage potential. Seaweed absorbs CO2 from atmosphere and stores the solar energy through photosynthesis without needing fresh water. The catalytic ATT involves the conversion of seaweeds in the presence of hydroxide and gas phase reforming catalyst. The overall reaction leads to bio-H2 as the carbon-free energy carrier, while carbon in biomass is stored as solid carbonate.
A few papers have reported on the use of seaweed as an energy source. Most of these studies have mainly focused on the biological conversion of seaweed;17,18,19 only a limited number of studies have focused on their thermochemical conversion10,20,21. Thermochemical conversion pathways of biomass (e.g., gasification) are attractive because of rapid reaction kinetics but are challenged by the need for dry feedstock. Thus, seaweeds and algae that have a high-moisture content (80–90%), generally need to be dried before their conversion22,23. Recently, subcritical and supercritical water gasification has been studied for wet biomass, e.g., algae and waste, but challenges remain given the high energy consumption due to high reactor pressure, low fuel (e.g., biocrude, CH4, H2, etc) yield, and salt precipitation24,25. Thus, an effective energy conversion technology that can convert wet and salty biomass into efficient energy fuels and energy carriers (e.g., H2) with high purity with reduced environmental footprints continues to be desired.
Extensive studies26,27 have demonstrated that alkali metals and alkali earth metals can enhance gasification, including promoted gasification rate, increased char conversion, reduced soot and tar formation. Umerki et al28 found that K can suppress the polycyclic aromatic hydrocarbon formations and promoted light tar decomposition. However, these reactions often require high reactor temperatures and generate low yield H2 as well as large amount CO2.
In this study, an alkaline thermal treatment (ATT) reaction is investigated by converting brown seaweeds in the presence of hydroxide (i.e., NaOH) and a gas-reforming Ni/ZrO2 catalyst. This particular reaction is less studied but very interesting in terms of its moderate reaction conditions (i.e., ambient pressure and temperature < 500 °C) that would allow the development of distributed biomass conversion systems without the need of a skilled operator. As shown in Fig. 1b, the overall ATT reaction is designed to push all the energy towards the H2 product, while the carbon in the seaweed is captured and stored as solid carbonates. If the purity of produced H2 is high enough to eliminate any subsequent gas cleaning steps, the overall biomass conversion technology would have a great potential to be sustainable. The biomass carbon captured in a form of solid carbonate can be stored with long-term stability in geologic formations, and if so, the overall ATT technology could achieve net carbon-negativity leading to a BioEnergy with Carbon Capture and Storage (BECCS) potential.
Results
H2 production from seaweed via ATT
Seaweed can have a wide range of moisture and salt content23,29. As a result, a dry ash-free (daf) mass, m1, provided in Eq. (1) is generally used to fairly compare different studies.
where m0 is the mass of biomass, a is the moisture content, and b is the ash content in total solid (TS). Brown seaweed procured for this study also contains a high ash content of 28.3 wt.% in total solid with a low moisture content of 7.8 wt.% (detailed composition is provided in Supplementary Table 1). In terms of carbon, brown seaweed is very rich in carbohydrates, mainly 10–40 wt.% alginates (C6H8O6), 2–34 wt.% laminarin (C6H10O5), 5–25 wt.% mannitol (C6H14O6), and 5–20 wt.% fucoidan (C7H14O7S)10.
Some interesting studies have been reported on the gasification24,29,30 and supercritical water (SCW) reaction31,32,33 of seaweed to enhance H2 production. SCW has been suggested as an effective means for producing H2 due to the ability of dissociated water to behave as an organic solvent31. The concept of using a base in the ATT reaction scheme can be analogous to the role of the OH− ion from dissociated water in SCW34,35. Recent studies have demonstrated that the presence of NaOH in the ATT reaction greatly enhances H2 production from model biomass compounds such as glucose and cellulose under moderate reaction conditions (<500 °C) while capturing carbon in the form of solid carbonates34,35,36,37,38,39,40. The main differences between ATT and SCW/steam gasification include its relatively low reaction temperature and pressure, as well as a tolerance to salt and moisture.
The overall ATT reaction is described as follows with an example of cellulose:
In this reaction, NaOH participates in the fragmentation of cellulose and captures the produced CO2, thus generating high-purity H2 that can be directly used in various energy conversion systems such as a fuel cell without additional clean-up steps36,41. Since the ATT reaction involves water and salts, it inherently has a great potential to treat and utilize unconventional biomass with high salt and water content. However, ATT has not been demonstrated yet using a real biomass, particularly with seaweed. In this study, the ATT of brown seaweed is investigated and exemplified in the following ATT reactions:
The distinct conversion reaction of seaweed ATT is visibly shown in Fig. 2(a) (inset photos). The solid residue of steam gasification (SG) is as expected black char, whereas the solid product of the seaweed ATT reaction is a white powder illustrating the in situ fixation of biomass carbon in a solid carbonate form (white powder). In terms of H2 production, steam gasification generates a very low level of H2, and high levels of CO2 and many C2 hydrocarbon gases, indicating that it would be difficult to convert seaweed to pure H2 at low temperatures (e.g., 500 °C) via steam gasification. On the other hand, the ATT reaction results in a dramatically increased H2 production by 2573%. A small quantity of CH4 is also formed while both the evolution of C2 hydrocarbons and CO2 are suppressed. While sun-dried seaweeds are used for this study due to the ease of procurement and lab storage, the effect of water, supplied together with seaweed rather than from outside, on the ATT reaction is also investigated by pre-soaking seaweed to mimic real wet seaweed feedstock with 90% moisture content. The hydrated seaweed (ATT-WB) and dry seaweed (ATT) show very similar H2 formation behaviors, indicating that the ATT reaction can directly use both wet and dry seaweed feedstocks depending on how seaweed is harvested and transported. Once developed, the ATT reaction will be performed in a continuous mode feeding seaweed directly into a heated reactor system. Thus, any moisture from wet seaweed will quickly turn into steam and participate in the ATT reaction, for example as shown in Reaction (4) and (6).
a Major gaseous products generated via SG and ATT reactions performed at 500 °C and 1 atm under a steam atmosphere (temperature program given in the method section). The result is normalized to mmol/(daf)g-seaweed for the direct comparison to the previous seaweed conversion studies. b–d H2 production via CatATT-CC shown in a is compared to previous studies in terms of b—reaction temperature, c—reaction pressure and d—catalyst type.
Our prior studies on model biomass (e.g., cellulose) have shown that the Ni-based catalyst can be used to crack and reform minor products (e.g., light gases (CH4, C2H4, C2H6, etc) and condensable gases) to produce additional H236,37. The overall H2 production doubles (CatATT), while the CO2 emissions also significantly increases, suggesting catalytic steam reforming reactions. To capture secondary CO2, Ca(OH)2 is used (CatATT-CC (carbon capture)) at the end of the ATT reaction. The Ni-based catalyst is shown to be stable and can be continuously reused (data given in Supplementary Fig. 1). The reactor configuration describing the overall reaction sequence is given in Supplementary Fig. 2.
Compared to the previously reported thermochemical conversion of seaweed24,29,30,31,32,33,42, the present study truly stands out. Cat-ATT-CC has achieved a high-purity H2 production of 69.69 mmol (daf)g-seaweed-1, which is considerably greater than those reported in the literature (Fig. 2b–d). While other studies often needed high reaction temperature and pressure, and/or a noble metal catalyst, the current ATT reaction can operate at a relatively low temperature (<500 °C) and under ambient pressure using a recyclable Ni catalyst. More importantly, the ATT of biomass can be applied to wet and salty feedstock (e.g., seaweed, food waste, algae) without any pretreatment, while traditional thermochemical biomass conversion processes require an energy-intensive pretreatment of a drying step2,43,44. Therefore, this catalytic ATT reaction combining high-purity H2 production with in situ CO2 capture in a single step process under moderate reaction conditions (<500 °C, 1 atm) has a great potential to be a transformative approach to unconventional biomass conversion.
Carbon capture and storage potential of seaweed ATT
With ever-increasing concerns on climate change, the need for negative emission technologies has been widely argued45,46,47. There are only a few options that can achieve negative emission goals and BECCS is one of them. A dedicated carbon capture unit can be installed downstream on most of the traditional thermochemical biomass conversion system including those listed in Fig. 2b–d. However, a truly transformative technology would be an approach that can directly integrate biomass conversion with carbon capture.
To elucidate its CCS potential, the fate of seaweed carbon throughout the ATT reaction is explored. The distribution of carbon in gas, liquid and solid phases after the ATT reaction is shown in Fig. 3a and the composition of seaweed ash is listed in Supplementary Table 2. The carbon in procured seaweed is mostly organic carbon with trace amounts of inorganic carbon later found in seaweed ash. As expected, steam gasification converts the organic carbon into gases and tars as well as char, and only a small portion (~3%) of carbon is found as inorganic carbon, indicating a high carbon content in its main product stream. On the other hand, during the ATT of seaweed, the organic carbon in the seaweed is mostly converted to Na2CO3 (~80%) and significantly reduced amounts of carbon-containing gases and tars (~13%). These results indicate that ATT can in situ capture carbon from seaweed biomass as sodium carbonates, which is thermodynamically stable and chemically safe to handle. The carbon distributions between phases remain nearly the same for ATT cases with and without the Ni-catalyst since Ni-catalyst mainly enhances gas phase reforming leaving reformed carbon as CO2 in the gas phase. By adding the additional in situ CO2 capture using Ca(OH)2, the final gaseous product is nearly carbon-free and the majority of seaweed organic carbon is pushed towards solid carbonates (Na2CO3 and CaCO3). Ca(OH)2 is used as the secondary CO2 capture material due to its low cost but other CO2 capture materials can also be used. Figure 3b shows the formation of CaCO3 based on enhanced C and O spectra after CatATT-CC, confirming the formation of CaCO3 (CO2 + Ca(OH)2 → CaCO3 + H2O).
a Distribution of carbon from seaweed in different product streams illustrating the fate of carbon during the ATT reaction. Thermodynamically stable solid carbonates (red and orange) make up the most of the carbon fraction for CatATT-CC. b SEM-EDS of Ca(OH)2 before and after the CatATT-CC reaction confirming in situ carbon capture.
Another distinctive nature of seaweed as an energy source is a high ash content since the ash component of biomass can have a considerable effect on its conversion reactions. The ash composition of the seaweed used in this study is analyzed using the X-ray fluorescence (XRF) (Supplementary Table 2). It has a large amount of K, Na, Ca, and Mg, which account for 95.78% of the seaweed ash. This brown seaweed ash can be classified as a K type based on Vassilev’s chemical classification of biomass ash48. K type ashes can enhance the leaching behavior, low-temperature transformation, and emission of volatiles during thermochemical reactions of biomass36,48. Moreover, the alkali and alkaline earth species (e.g., K, Na, and Ca) can enhance the biomass conversion via altering surface active sites during catalysis49,50. They can also contribute to reduced tar formation, catalyzed tar decomposition, and hindered char formation49,51. In this study, ash does not significantly impact SG and ATT reactions.
The ATT reaction effectively fixes the seaweed organic carbon into solid carbonates directly preventing CO2 emission, while other biomass conversion processes often require an additional carbon capture unit. Therefore, the unique ATT reaction has a great potential of BECCS while directly utilizing wet and salty biomass to produce high-purity H2.
ATT reaction pathways generating high-purity H2
To understand how the ATT reaction of seaweed combines high-purity H2 production with CO2 capture, gas formation behaviors are further investigated as reaction temperature is scanned from ambient to 500 °C. As shown in Fig. 4a, the non-catalytic ATT, H2 formation occurs throughout 150–500 °C while the CH4 formation is observed at a slightly higher temperature range (250–500 °C) with a major peak at 410 °C. The formation of CO and CO2 is insignificant.
a–c Formation behaviors of major gaseous products during the ATT reaction of brown seaweed at various reaction temperatures. a ATT, b ATT with Ni-based catalyst, and c ATT with Ni-based catalyst and secondary CO2 capture using Ca(OH)2. d Pathways of H2 generation during the ATT reaction identified based on the results given in Fig. 2a.
When the Ni-catalyst is used downstream, the overall gas formation behaviors greatly change as seen in Fig. 4b. A large H2 formation peak at ~400–410 °C is detected with its formation rate reaching up to 2.0 mmol min−1(daf)g-seaweed−1. Within the same temperature range, both CH4 and CO2 concentrations are also greatly changed compared to the non-catalytic ATT case. The major peak of CH4 significantly decreases to below 0.2 mmol min−1(daf)g-seaweed−1, while CO2 formation greatly increases with the maximum 0.5 mmol min−1(daf)g-seaweed−1. The enhanced H2 and CO2 formation is clearly accompanied by the reduced CH4 formation. Therefore, gas reforming of CH4 and potentially light gases is the source of additional H2 formation observed in CatATT.
Ni-catalyst is well known for enhancing steam methane reforming (SMR) and water-gas shift (WGS)34,35.
From the results shown in Fig. 4a, b, 1 mol of CH4 generates ~4.19 mol of H2 and about 1.2 mol of CO2, which agree with the stoichiometries of reactions (7) and (8), in which 1 mol of CH4 generates 1 mol of CO and 3 mol of H2 via the SMR reaction; additionally, 1 mol of CO generates 1 mol of H2 and 1 mol of CO2 via the WGS reaction. Therefore, the secondary H2 formation is enhanced by the Ni-catalyst via the SMR and WGS reactions.
To capture the secondary CO2 generated during the WGS reaction and maintain the high-purity of H2, Ca(OH)2 was also placed in the system (CatATT-CC). As seen in Fig. 4c, the H2 formation behavior remains the same as the CatATT case, since the role of Ca(OH)2 is only CO2 capture. As designed, the CO2 peak disappears while maintaining the secondary H2 formation via SMR and WGS.
Based on these gas formation behaviors as well as the carbon distribution discussed earlier, it can be deduced that steam gasification given in reaction (6)29 is not significantly involved in H2 formation under temperatures below 500 °C.
On the other hand, NaOH is directly involved in the ATT reaction of seaweeds and controls the gaseous intermediates and products as well as in situ capturing CO2. NaOH greatly improves the biomass conversion by 25 times leading to the H2 production of 35.69 mmol H2/(daf)g-seaweed compared to the low-temperature steam gasification case. The minor gaseous products containing carbon (e.g., CO, and CH4) are then reformed by the Ni/ZrO2 catalyst to produce up to 70.52 mmol-H2/(daf)g-seaweed (combined primary and secondary H2 formation) via the SMR and WGS reactions. In addition to light gases, tar can also contribute to the secondary H2 formation via catalytic steam tar reforming—reaction (10)29,52, although its contribution is not as significant (<9%) (Fig. 4d):
Ca(OH)2 effectively captures all CO2 during various secondary H2 formation reactions, and thus, the final gaseous product is high-purity H2. In summary, reactions (3–10) provide five pathways to H2 formation from seaweed biomass and their relative contributions are summarized in Fig. 4d.
Regeneration of hydroxide via integrated mineral carbonation
NaOH exhibits an excellent performance in the ATT reaction of seaweed due to its high alkalinity and low melting point but the overall H2 production would not be net carbon-negative unless the produced carbonates can be safely stored for the long-term. CaCO3 produced from CO2 reaction with Ca(OH)2 can be permanently stored since it is insoluble and stable. Ca(OH)2 can be produced from alkaline industrial wastes and silicate minerals, and thus, it can be supplied with a low cost and a reduced carbon footprint53. On the other hand, Na2CO3 is highly soluble and NaOH is high in cost (USD921 per ton)36. Thus, in order to close the overall carbon loop and ultimately sequester seaweed carbon, it is highly desired to regenerate and re-use NaOH throughout the ATT reactions. The causticizing reaction (8) often used in the Kraft process of the pulp and paper industry can be employed to regenerate NaOH with Ca(OH)2 via the following metathesis reaction:
As illustrated in Fig. 5, if the source of Ca(OH)2 is either alkaline industrial wastes (e.g., steel slag and waste concrete) or silicate minerals (e.g., wollastonite)54,55,56,57, the net carbon cycle could be neutral or even negative leading to a greater BECCS potential.
The ATT reaction produces high-purity H2 while fixing the seaweed carbon into solid carbonates (Na2CO3). The dry solid residue containing Na2CO3 can be regenerated on-site or easily transported to a centralized location for hydroxide regeneration. Ca(OH)2 from silicate minerals and alkaline industry wastes such as steel slag can be used to regenerate NaOH via a metathesis reaction. The regenerated NaOH is used for the subsequent ATT reaction cycle and the produced CaCO3 can be used as an industry product or stored to achieve the overall carbon negativity of the seaweed conversion technology.
The performance of the regenerated NaOH in the CatATT-CC reaction is investigated and Fig. 6a shows that the regenerated NaOH can attain similar H2 production as that of the fresh NaOH. Due to the solubility limitation of Ca(OH)2, the regenerated NaOH stream still contains significant amounts of Na2CO3 and trace amounts of Ca(OH)2 but the overall performance of NaOH is not impacted. Na2CO3 may accumulate throughout the ATT cycles, but it will not influence the overall reaction. As shown in the inset of Fig. 6a, CH4 formation and CO2 capture behaviors also remain the same for the regenerated NaOH. The comparison between gas formation behaviors shown in Figs. 4c and 6b also confirms that NaOH can be regenerated and reused for the ATT of seaweed producing high-purity H2.
From these data, a preliminary biomass-to-H2 conversion efficiency of the biomass ATT is estimated and compared with that of the conventional gasification and waster-gas shift reactions found in literature58,59,60. In order to provide similar system boundaries, Ca(OH)2 sorbent is used to capture CO2 from the water-gas shift reactor (details are provided in Supplementary Note 1). Results show that the biomass ATT can produce more H2 than the combined process of gasification and water-gas shift reactions. This is a very promising result considering a potential learning curve in the development of the biomass ATT technology, which is still in the early stage of development. Based on the findings from this mechanistic study, the biomass ATT can be further optimized in terms of the overall H2 production in an isothermal continuous reactor system, while improving the overall BECCS potential.
Discussion
This study has shown that wet and salty seaweed can be directly used as a biomass feedstock to produce high-purity H2 without any pretreatment. The catalytic alkaline thermal treatment of brown seaweed has successfully produced 69.69 mmol H2/(daf)g-seaweed with in situ CO2 capture. The ATT reaction provides a considerably greater H2 production than those of other thermochemical methods under mild reaction conditions (<500 °C, 1 atm). NaOH can fragment the seaweed into relevant intermediates to increase the gaseous products including H2, while the Ni-based catalyst plays an important role in reforming gaseous intermediates to additional H2 via SMR and WGS reactions as well as tar reforming. The regeneration of NaOH and the stability of the Ni-based catalyst in the seaweed ATT reaction process show the effectiveness and feasibility of this innovative approach with a BECCS potential. The ATT of seaweed investigated in this study provides a novel pathway to utilize currently untapped unconventional biomass resources such as seaweed, food waste, and algae, which are often high in water and salt contents.
Methods
Biomass and reagents
Saccharina japonica, brown seaweed is obtained from Wando Island, South Korea and ground to <150 μm. While the proposed ATT reaction is developed for wet biomass, it would be difficult to store wet seaweed for long-term. Thus, the experiments are performed using readily-available sun-dried seaweed, which its salt content is retained in the biomass during the drying process (moisture content ~7.8%, salts content ~28%, by weight, respectively). NaOH and Ca(OH)2 are obtained from Sigma-Aldrich and used without further purification. The Ni/ZrO2 catalyst is prepared by dissolving 2752.6 mg of nickel(II) nitrate hexahydrate in 80 mL of ethanol. Then 5.0 g of ZrO2 powder (Alfa Aesar) is added to the solution. The mixture is stirred and heated to gradually impregnate the metal salt onto the ZrO2 support. The prepared catalyst is dried at 70 °C overnight and calcined in the air at 800 °C (heated at a ramping rate of 5 °C min−1) for 200 min. Finally, the catalysts are reduced under H2 atmosphere for 2 h at 500 °C. The prepared catalysts are analyzed using a NOVA 2200e for their specific surface area based on the Brunauer-Emmett-Teller (BET) method. The produced 10 wt.% Ni/ZrO2 catalyst has a specific surface area of 23.2 m2 g−1.
Reactor system
The horizontal reactor consists of an inner quartz tube (2.54 cm in O.D. × 56.00 cm in length) and an outer three-zone split-tube furnace (Mellen Co., SC12R), as shown in Supplementary Fig. 3. The brown seaweed sample mixed with NaOH is placed in a ceramic boat in zone 1 and a thermocouple is installed to monitor the temperature during the ATT reaction. For the cases incorporating the catalyst and/or Ca(OH)2, they are separately placed in zone 2 of the fixed bed reactor to investigate their isolated effects in enhancing the formation of H2 and capture of CO2 generated from catalytic reaction. The reactor outlet is connected to a Micro GC (Inficon 3000) for the continuous analysis of gaseous products while collecting all the produced gas in a tedlar bag installed downstream for the analysis of minor products produced at very low concentrations.
ATT reactions
Five distinct experimental combinations are designed to study the ATT reactions of brown seaweed and the roles of hydroxides and the catalyst (details given in Supplementary Table 3). In all cases, 1.0 g of mixed samples of a given molar ratio of brown seaweed and NaOH is loaded into a 10-mL ceramic boat, and placed in zone 1 of the horizontal reactor except for the steam gasification case performed without NaOH. The molar ratio of brown seaweed and NaOH is adjusted based on the reaction stoichiometry and all the results are normalized to dry ash-free (daf) grams of seaweed to enable accurate comparisons. For the experiments with catalysts and Ca(OH)2, 250.0 mg of 10 wt.% Ni/ZrO2 catalyst and (if used) 750.0 mg of Ca(OH)2 are separately placed in zone 2 of the fixed bed reactor. The carrier gas, N2, is introduced at 50 mL/min using a mass flow controller (Omega FMA5508) throughout the reaction. The reactor is first purged with N2 and then pre-heated to 100 °C (at a heating rate of 4 °C min−1) and maintained at that temperature for 30 min. After the pre-heating, water is injected into the hotbox at a rate of 0.023 mL min−1, where it is vaporized and carried by N2 to provide the steam for the ATT reaction. This creates a condition of an ATT reaction of wet biomass. While heating the reactor to 500 °C at a rate of 4 °C min−1, the H2 formation behavior of the ATT reaction of the brown seaweed is monitored. Subsequently, the reactor is maintained at 500 °C for 60 min before terminating each ATT experiment. The gaseous products from the reactor are passed through a condenser to separate the condensable compounds from light gases. The light gases are then analyzed online every 2.0 min using a Micro GC. The overall gas products are also collected in a tedlar bag for additional gas analysis.
Gas analysis
Gas samples are analyzed online via a gas chromatography (GC) (Inficon Micro-GC 3000). The Micro GC has two 10-m Molsieve columns for H2, O2, N2, CH4, and CO analyses, and an 8 m Plot U for CO2 and C2H6 analyses. The detection limits are 20 ppm for H2, and 1ppm for O2, N2, CH4, CO, and CO2. N2 is used as a reference gas to quantify different gases and all the gas production was normalized to moles.
Carbon analysis
The potential carbon-bearing product streams of the seaweed ATT reactions include carbon-containing gases (e.g., CO2, CO, CH4), liquid tar (C2+) and solid residue (organic and inorganic carbon (biochar and Na2CO3)). Both organic and inorganic contents of solid residues after the ATT reaction are determined using a UIC CM150 Coulometer with Total Carbon and Inorganic Carbon modules. The total carbon is calculated by combusting the residues in pure O2 at 900 °C, while the inorganic carbon is determined by dissolving the sample in perchloric acid. In both cases, the released amount of CO2 is measured to calculate the carbon content. The total carbon and inorganic carbon contents are used to determine the extent of the ATT reactions.
Solid analysis
The ash composition of brown seaweed is analyzed using a PanAlytical Axios Advanced 4 kW WD XRF, which determines the elemental concentrations based on the X-ray intensity of each element. The detection limit of XRF is 100 ppm. The morphologies and elemental compositions of the solid samples before and after the ATT reactions are investigated using a Zeiss Sigma VP Scanning Electron Microscope with an Energy Dispersive Spectrometer (SEM-EDS). By evaluating the X-ray energies of the different elements, quantitative analysis is accomplished for each solid sample.
Inductively coupled plasma optical emission spectrometer
The contents of Na and Ca cations in the solid samples obtained during the NaOH recycling step are analyzed using ICP-OES (ACTIVA-M, Horiba Jobin Yvon Inc., Edison, NJ). Based on the measured purity of the recycled hydroxide, its total mass added to the subsequent ATT reaction cycle is determined while maintaining the mass of NaOH constant.
Data availability
The authors declare that the main data supporting the findings of this study are available within the article and its Supplementary Information files. Extra data are available from the corresponding author upon request.
References
IEA. International Energy Agency in World Energy Outlook (2018).
Chu, S., Cui, Y. & Liu, N. The path towards sustainable energy. Nat. Mater. 16, 16 (2017).
BP., BP Statistical Review of World Energy (2017).
Chu, S. & Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 488, 294 (2012).
Sikarwar, V. S. et al. An overview of advances in biomass gasification. Energy Environ. Sci. 9, 2939–2977 (2016).
Ni, M., Leung, D. Y., Leung, M. K. & Sumathy, K. An overview of hydrogen production from biomass. Fuel Process. Technol. 87, 461–472 (2006).
Zhao, X. et al. Biomass-based chemical looping technologies: the good, the bad and the future. Energy Environ. Sci. 10, 1885–1910 (2017).
Perlack, R. D. et al. US billion-ton update: biomass supply for a bioenergy and bioproducts industry. US DOE (2011).
Ross, A., Jones, J., Kubacki, M. & Bridgeman, T. Classification of macroalgae as fuel and its thermochemical behaviour. Bioresour. Technol. 99, 6494–6504 (2008).
Ross, A., Anastasakis, K., Kubacki, M. & Jones, J. Investigation of the pyrolysis behaviour of brown algae before and after pre-treatment using PY-GC/MS and TGA. J. Anal. Appl. Pyrolysis 85, 3–10 (2009).
Wahl, M. et al. The responses of brown macroalgae to environmental change from local to global scales: direct versus ecologically mediated effects. Perspect. Phycol. 2, 11–29 (2015).
Hughes, A. D., Kelly, M. S., Black, K. D. & Stanley, M. S. Biogas from Macroalgae: is it time to revisit the idea? Biotechnol. Biofuels 5, 86 (2012).
Klemme, H. & Ulmishek, G. F. Effective petroleum source rocks of the world: stratigraphic distribution and controlling depositional factors (1). AAPG Bull. 75, 1809–1851 (1991).
Aziz, M. Power generation from algae employing enhanced process integration technology. Chem. Eng. Res. Des. 109, 297–306 (2016).
Lee, H. W. et al. Catalytic conversion of Laminaria japonica over microporous zeolites. Energy 66, 2–6 (2014).
Bayramoglu, G., Akbulut, A., Ozalp, V. C. & Arica, M. Y. Immobilized lipase on micro-porous biosilica for enzymatic transesterification of algal oil. Chem. Eng. Res. Des. 95, 12–21 (2015).
Neveux, N., Bolton, J. J., Bruhn, A., Roberts, D. A. & Ras, M. The bioremediation potential of seaweeds: Recycling nitrogen, phosphorus, and other waste products.). (John Wiley & Sons, Inc, Hoboken, 2018).
Choi, J. H., Kim, S.-S., Kim, J. & Woo, H. C. Fast pyrolysis of fermentation residue derived from Saccharina japonica for a hybrid biological and thermal process. Energy 170, 239–249 (2019).
Sadhukhan, J. et al. Novel macroalgae (seaweed) biorefinery systems for integrated chemical, protein, salt, nutrient and mineral extractions and environmental protection by green synthesis and life cycle sustainability assessments. Green. Chem. 21, 2635–2655 (2019).
Oliveira, J., Alves, M. & Costa, J. Design of experiments to assess pre-treatment and co-digestion strategies that optimize biogas production from macroalgae Gracilaria vermiculophylla. Bioresour. Technol. 162, 323–330 (2014).
Plis, A., Lasek, J., Skawińska, A. & Zuwała, J. Thermochemical and kinetic analysis of the pyrolysis process in Cladophora glomerata algae. J. Anal. Appl. Pyrolysis 115, 166–174 (2015).
Zhou, D., Zhang, L., Zhang, S., Fu, H. & Chen, J. Hydrothermal liquefaction of macroalgae Enteromorpha prolifera to bio-oil. Energy Fuels 24, 4054–4061 (2010).
Rajkumar, R., Yaakob, Z. & Takriff, M. S. Potential of micro and macro algae for biofuel production: a brief review. Bioresources 9, 1606–1633 (2013).
Kaewpanha, M. et al. Steam co-gasification of brown seaweed and land-based biomass. Fuel Process. Technol. 120, 106–112 (2014).
Elliott, D. C., Schmidt, A. J., Hart, T. R. & Billing, J. M. Conversion of a wet waste feedstock to biocrude by hydrothermal processing in a continuous-flow reactor: grape pomace. Biomass Convers. Biorefinery 7, 455–465 (2017).
Perander, M. et al. Catalytic effect of Ca and K on CO2 gasification of spruce wood char. Fuel 150, 464–472 (2015).
Kirtania, K. et al. Kinetic study of catalytic gasification of wood char impregnated with different alkali salts. Energy 118, 1055–1065 (2017).
Umeki, K., Häggström, G., Bach-Oller, A., Kirtania, K. & Furusjö, E. Reduction of tar and soot formation from entrained-flow gasification of woody biomass by alkali impregnation. Energy Fuels 31, 5104–5110 (2017).
Duman, G., Uddin, M. A. & Yanik, J. Hydrogen production from algal biomass via steam gasification. Bioresour. Technol. 166, 24–30 (2014).
Kaewpanha, M. et al. Steam reforming of tar derived from the steam pyrolysis of biomass over metal catalyst supported on zeolite. J. Taiwan Inst. Chem. Eng. 44, 1022–1026 (2013).
Cherad, R. et al. Macroalgae supercritical water gasification combined with nutrient recycling for microalgae cultivation. Environ. Prog. Sustain. Energy 32, 902–909 (2013).
Onwudili, J. A., Lea-Langton, A. R., Ross, A. B. & Williams, P. T. Catalytic hydrothermal gasification of algae for hydrogen production: composition of reaction products and potential for nutrient recycling. Bioresour. Technol. 127, 72–80 (2013).
Cherad, R., Onwudili, J. A., Williams, P. T. & Ross, A. B. A parametric study on supercritical water gasification of Laminaria hyperborea: a carbohydrate-rich macroalga. Bioresour. Technol. 169, 573–580 (2014).
Ishida, M., Otsuka, K., Takenaka, S. & Yamanaka, I. One-step production of CO- and CO2-free hydrogen from biomass. J. Chem. Technol. Biotechnol. 80, 281–284 (2005).
Ferguson, T. E., Park, Y., Petit, C. & Park, A.-H. A. Novel approach to hydrogen production with suppressed COx generation from a model biomass feedstock. Energy Fuels 26, 4486–4496 (2012).
Stonor, M. R., Ferguson, T. E., Chen, J. G. & Park, A.-H. A. Biomass conversion to H2 with substantially suppressed CO2 formation in the presence of Group I & Group II hydroxides and a Ni/ZrO2 catalyst. Energy Environ. Sci. 8, 1702–1706 (2015).
Stonor, M. R., Chen, J. G. & Park, A.-H. A. Bio-Energy with Carbon Capture and Storage (BECCS) potential: production of high purity H2 from cellulose via Alkaline thermal treatment with gas phase reforming of hydrocarbons over various metal catalysts. Int. J. Hydrog. Energy 42, 25903–25913 (2017).
Stonor, M. R., Ouassil, N., Chen, J. G. & Park, A.-H. A. Investigation of the role of Ca(OH)2 in the catalytic Alkaline Thermal Treatment of cellulose to produce H2 with integrated carbon capture. J. Energy Chem. 26, 984–1000 (2017).
Zhao, M. et al. Alkaline thermal treatment of cellulosic biomass for H2 production using Ca-based bifunctional materials. ACS Sustain. Chem. Eng. 7, 1202–1209 (2018).
Zhang K., et al. Kinetic and mechanistic investigation of catalytic alkaline thermal treatment of xylan producing high purity H2 with in-situ carbon capture. J. Ind. Eng. Chem. (Resumed). 85, 219–225 (2020).
Machell, G. & Richards, G. Mechanism of saccharinic acid formation. Part I. Competing reactions in the alkaline degradation of 4-O-methyl-D-glucose, maltose, amylose, and cellulose. J. Chem. Soc. 1924–1931 (1960).
Schumacher, M., Yanık, J., Sınağ, A. & Kruse, A. Hydrothermal conversion of seaweeds in a batch autoclave. J. Supercrit. Fluids 58, 131–135 (2011).
Parthasarathy, P. & Narayanan, K. S. Hydrogen production from steam gasification of biomass: influence of process parameters on hydrogen yield–a review. Renew. Energy 66, 570–579 (2014).
Nikolaidis, P. & Poullikkas, A. A comparative overview of hydrogen production processes. Renew. Sustain. Energy Rev. 67, 597–611 (2017).
Anderson, K. & Peters, G. The trouble with negative emissions. Science 354, 182–183 (2016).
Fuss, S. et al. Betting on negative emissions. Nat. Clim. Change 4, 850 (2014).
Smith, P. et al. Biophysical and economic limits to negative CO2 emissions. Nat. Clim. Change 6, 42 (2016).
Vassilev, S. V., Baxter, D., Andersen, L. K. & Vassileva, C. G. An overview of the composition and application of biomass ash. Part 1. Phase–mineral and chemical composition and classification. Fuel 105, 40–76 (2013).
Muangrat, R., Onwudili, J. A. & Williams, P. T. Influence of alkali catalysts on the production of hydrogen-rich gas from the hydrothermal gasification of food processing waste. Appl. Catal. B: Environ. 100, 440–449 (2010).
Wu, Y., Wang, J., Wu, S., Huang, S. & Gao, J. Potassium-catalyzed steam gasification of petroleum coke for H2 production: Reactivity, selectivity and gas release. Fuel Process. Technol. 92, 523–530 (2011).
Kuchonthara, P., Vitidsant, T. & Tsutsumi, A. Catalytic effects of potassium on lignin steam gasification with γ-Al2O3 as a bed material. Korean J. Chem. Eng. 25, 656–662 (2008).
Moon, J., Lee, J., Lee, U. & Hwang, J. Transient behavior of devolatilization and char reaction during steam gasification of biomass. Bioresour. Technol. 133, 429–436 (2013).
Fricker, K. J. & Park, A.-H. A. Investigation of the different carbonate phases and their formation kinetics during Mg(OH)2 slurry carbonation. Ind. Eng. Chem. Res. 53, 18170–18179 (2014).
Gadikota G., Park A.-H.A. Accelerated carbonation of Ca-and Mg-bearing minerals and industrial wastes using CO2. in Carbon Dioxide Utilisation. (Elsevier, 2015).
Gadikota G., Fricker K., Jang S.-H., Park A.-H.A. Carbonation of silicate minerals and industrial wastes and their potential use as sustainable construction materials. In Advances in CO 2 Capture, Sequestration, and Conversion. (ACS Publications, 2015).
Pan, S.-Y., Ling, T.-C., Park, A.-H. A. & Chiang, P.-C. An overview: Reaction mechanisms and modelling of CO2 utilization via mineralization. Aerosol Air Qual. Res. 18, 829–848 (2018).
Zhao, H., Park, Y., Lee, D. H. & Park, A.-H. A. Tuning the dissolution kinetics of wollastonite via chelating agents for CO2 sequestration with integrated synthesis of precipitated calcium carbonates. Phys. Chem. Chem. Phys. 15, 15185–15192 (2013).
Wang, Z. et al. Gasification of biomass with oxygen-enriched air in a pilot scale two-stage gasifier. Fuel 150, 386–393 (2015).
Montes-Hernández, G., Renard, F., Geoffroy, N., Charlet, L. & Pironon, J. Calcite precipitation from CO2–H2O–Ca (OH)2 slurry under high pressure of CO2. J. Cryst. Growth 308, 228–236 (2007).
Hwang, K.-R., Ihm, S.-K. & Park, J.-S. Enhanced CeO2-supported Pt catalyst for water–gas shift reaction. Fuel Process. Technol. 91, 729–736 (2010).
Acknowledgements
We acknowledge NSF CBET 1336567 and CBET 1231393 for financially supporting this work. This research was supported by C1 Gas Refinery Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (2015M3D3A1A01064929). We also would like to thank Prof. Hyon Hee Yoon at Gachon University in Korea and Prof. Zhihua Wang at Zhejiang University for their valuable discussions related to this project.
Author information
Authors and Affiliations
Contributions
A.-H.A.P. and W.-J.K. conceived this project. K.Z. performed the experiments and wrote the paper, while all the authors discussed the results and commented on the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Communications thanks the anonymous reviewers for their contributions to the peer review of this work. Peer review reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, K., Kim, WJ. & Park, AH.A. Alkaline thermal treatment of seaweed for high-purity hydrogen production with carbon capture and storage potential. Nat Commun 11, 3783 (2020). https://doi.org/10.1038/s41467-020-17627-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-020-17627-1
This article is cited by
-
Bioresource Upgrade for Sustainable Energy, Environment, and Biomedicine
Nano-Micro Letters (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.