Abstract
The pathogenesis of hypertension is multifactorial and highly complex. Basic research plays critical roles in elucidating the complex pathogenesis of hypertension and developing its treatment. This review covers recent topics in basic research related to hypertension in the following six parts: brain/autonomic nervous system, kidney, vascular system, potential treatments, extracellular vesicles, and gut microbiota. The brain receives afferent nerve inputs from peripheral organs, including the heart, kidneys, and adipose tissue, and humoral inputs from circulating factors such as proinflammatory cytokines and leptin, which are involved in the regulation of central sympathetic outflow. In the kidneys, changes in Wnt/β-catenin signaling have been reported in several hypertensive models. New findings on the renin-angiotensin-aldosterone system in the kidneys have also been reported. Sirtuin 6, which participates in various cellular functions, including DNA repair, has been shown to have protective effects on the vascular system. Skin water conservation, mediated by skin vasoconstriction and the accumulation of osmolytes such as sodium, has been found to contribute to hypertension. Studies of rivaroxaban and sodium-glucose cotransporter-2 inhibitors as drug repositioning candidates have been performed. Extracellular vesicles have been shown to be involved in novel diagnostic approaches and treatments for hypertension as well as other diseases. In gut microbiota studies, interactions between microbiota and antihypertensive drugs and potential pathophysiology linking microbiota and COVID-19 have been reported. It can be seen that inter-organ communication has received particular attention from these recent research topics. To truly understand the pathogenesis of hypertension and to develop treatments for conquering hypertension, interresearcher communication and collaboration should be further facilitated.

This mini-review focuses on recent topics on basic research in hypertension from the several points of view. The recent topics indicate that inter-organ communication has received particular attention. Interresearcher communication and collaboration should also be further facilitated to truly understand the complex pathogenesis of hypertension and to develop the treatments.
Similar content being viewed by others
Introduction
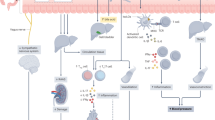
The pathogenesis of hypertension is multifactorial and highly complex. Basic research plays critical roles in elucidating the complex pathogenesis of hypertension and developing treatments for hypertension. It would be ideal to link all of the pathophysiology and the organ changes associated with hypertension and describe these associations systematically, but our understanding of the pathophysiology of hypertension has not been sufficient to reach that goal. Therefore, the present mini-review focuses on recent and emerging topics in basic research on hypertension from several points of view (Fig. 1).
Recent topics in basic research. Each ref. number indicates the reference paper cited in the text. LVH left ventricular hypertrophy, BDNF brain-derived neurotrophic factor, NTS nucleus tractus solitarius, RDN renal denervation, BP blood pressure, BAT baroreflex activation therapy, IL interleukin, RAS renin-angiotensin system, SIRT6 sirtuin 6, Runx2 runt-related transcription factor 2, CKD chronic kidney disease, SGLT2i sodium-glucose cotransporter-2 inhibitor, EV extracellular vesicle, FNDC5 fibronectin type-III domain-containing protein 5, miR microRNA, ACEI angiotensin-converting enzyme inhibitor, ACE2 angiotensin-converting enzyme-2, SHR spontaneously hypertensive rat, COVID-19 coronavirus disease 2019
Brain/autonomic nervous system
The brain receives various inputs from the peripheral system, organizes them, and determines central sympathetic outflow [1]. The sympathetic nervous system plays a major role in the control of diverse organs, including the heart, kidneys, and vasculature. Sympathetic overactivity is often accompanied by an imbalance of autonomic tone with decreased parasympathetic activity, which is a hallmark of the pathophysiology of hypertension and hypertensive organ damage.
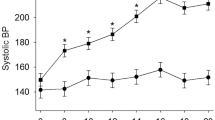
In clinical practice, autonomic neuromodulation for cardiovascular diseases, such as renal denervation, has attracted significant attention [2, 3]. Renal denervation and the involvement of renal sympathetic nerves in hypertension are discussed in another mini-review in this journal, so I describe recent studies investigating the other neural mechanisms that may modulate sympathetic activity. In particular, the role of afferent nerves carrying signals from peripheral organs to the central nervous system as well as efferent sympathetic nerves innervating the organs in cardiovascular regulation has recently been investigated. The stimulation of cardiac sympathetic afferent nerves increases sympathetic outflow and blood pressure. This sympathoexcitatory reflex is called the cardiac sympathetic afferent reflex (CSAR). CSAR is reported to be enhanced in experimental models of hypertension and chronic heart failure [4,5,6]. Transient receptor potential vanilloid 1 (TRPV1)-expressing cardiac afferent fibers are responsible for sensing and triggering CSAR activation [7]. Our recent study showed that cardiac TRPV1 expression and CSAR were increased in mice with pressure overload-induced cardiac hypertrophy and that TRPV1 knockout and selective denervation of TRPV1-expressing cardiac sympathetic afferent nerves similarly attenuated pressure overload-induced cardiac hypertrophy [8]. The activation of TRPV1-expressing cardiac afferent nerves was presumably associated with increased brain-derived neurotrophic factor in the brainstem nucleus tractus solitarius receiving the cardiac afferents, which could induce sympathoexcitation and cardiac hypertrophy. Afferent signals from white adipose tissue can also influence blood pressure through a sympathoexcitatory mechanism known as the adipose afferent reflex [9, 10]. Dalmasso et al. recently demonstrated that the stimulation of afferent sensory nerves from visceral white adipose tissue could increase blood pressure in normal mice [11]. They further showed that afferent signals from visceral white adipose tissue contributed to sympathetic activation and hypertension in male mice exposed to early life stress when fed an obesogenic diet [12]. This enhanced sympathetic outflow was most likely mediated by increased afferent signals from epididymal white adipose tissue projecting to brain areas with a pivotal role in developing neurogenic hypertension, such as the hypothalamic paraventricular nucleus (PVN) and rostral ventrolateral medulla (RVLM). In addition to these studies focusing on afferent signals from organs such as the heart and white adipose tissue, the study from Asirvatham-Jeyaraj et al. focused on the celiac ganglion as another potential target for antihypertensive neuromodulation. They showed that celiac ganglionectomy, a procedure that ablates efferent sympathetic nerves to and afferent sensory nerves from the splanchnic organs, decreased blood pressure as well as renal denervation in genetically hypertensive Schlager BPH/2 J mice [13]. The depressor response to the ganglionic-blocking agent hexamethonium, which indicates global neurogenic pressor activity, was significantly decreased by renal denervation, whereas this depressor response was not altered by celiac ganglionectomy. Therefore, both renal and splanchnic nerves contributed to hypertension in BPH/2 J mice, but likely through different mechanisms. As shown in this study, renal denervation and celiac ganglionectomy both attenuated hypertension to a similar degree in Dahl salt-sensitive rats [14]. In contrast, renal denervation had no effect in the angiotensin II-salt model and mild deoxycorticosterone acetate (DOCA)-salt model, but celiac ganglionectomy was effective in lowering blood pressure [15,16,17]. The effect of selective denervation of afferent nerves, including renal afferent nerves, on blood pressure also differs between hypertensive animal models [2, 18]. Taken together, the findings of these studies suggest that the efficacy of targeted denervation depends on the model studied and the organ systems and physiological processes affected. Finally, baroreflex activation as an antihypertensive neuromodulation other than denervation or ganglionectomy will now be described. Multiple clinical trials have shown that baroreflex activation therapy through carotid sinus stimulation is effective in decreasing blood pressure in treatment-resistant hypertensive patients [19,20,21]. However, there is currently no set of standardized electrical stimulation parameters, and the reported stimulus frequencies, amplitudes, and durations have varied widely among human trials. Domingos-Souza et al. showed that the ability of baroreflex activation to modulate hemodynamics and induce lasting vascular adaptation was critically dependent on the electrical parameters and duration of carotid sinus stimulation in spontaneously hypertensive rats (SHRs) [22]. This study provided an important rationale for improving baroreflex activation therapy in humans.
The brain receives inputs from circulating humoral factors, which affects sympathetic outflow. In particular, hypertension is associated with systemic inflammation, and the brain may play a key role in linking them. Cao et al. showed that intravenous injection, intracerebroventricular injection, or PVN microinjection of interleukin (IL)-17A similarly induced increases in blood pressure, heart rate, and renal sympathetic nerve activity [23]. Intravenous injection of IL-17A activated brain-resident glial cells and elevated the gene expression of inflammatory cytokines and chemokines through IL-17 receptor A within the PVN. The findings of this study suggest that IL-17A in the brain promotes neuroinflammation to enhance sympathetic activation and hypertension. As shown in this study, systemic administration of proinflammatory cytokines has been suggested to induce sympathetic activation and blood pressure elevation by acting on the brain [24,25,26], but it is not well understood how endogenous circulating proinflammatory cytokines are involved in the development of hypertension through the sympathetic nervous system because cytokines cannot cross the blood‒brain barrier. Brain perivascular macrophages are components of the blood‒brain barrier and are affected by circulating inflammatory cytokines. We recently showed that brain perivascular macrophages, which produce prostaglandin E2, thus activating neurons in response to circulating cytokines, contribute to the development of hypertension via sympathetic activation [27]. In addition, there is a link between obesity and hypertension. Gruber et al. showed in mice with high-fat, high-sugar diet-induced obesity, there was profound remodeling of the gliovascular interface in the hypothalamus, which includes preautonomic centers, resulting in arterial hypertension [28]. This process was driven by elevated leptin levels and an upregulation of the HIF1α-VEGF signaling axis in local astrocytes.
Kidneys
There is a close relationship between the kidneys and blood pressure. Kasacka et al. reported changes in the Wnt/β-catenin signaling pathway, which is a key pathway that regulates various cellular processes and tissue homeostasis and is also involved in damage and repair processes, in the kidneys of several hypertensive model rats [29]. They showed that the activity of the Wnt/β-catenin pathway was increased in SHRs and two-kidney, one-clip (2K1C) hypertensive rats, while it was inhibited in DOCA-salt rats, using kidney immunohistochemistry. The renin-angiotensin system (RAS) is increased in SHRs and 2K1C hypertensive rats; therefore, the findings of this study suggest an interaction between the RAS and Wnt/β-catenin signaling. The role of Wnt/β-catenin signaling in kidney injury and repair has been well reviewed in the recent literature [30, 31]. The intrarenal RAS is involved in BP regulation. In damaged kidneys with an impaired glomerular filtration barrier, liver-derived angiotensinogen filtered through damaged glomeruli regulates intrarenal RAS activity [32]. Matsuyama et al. further showed that the glomerular filtration of liver-derived angiotensinogen, depending on glomerular capillary pressure, causes a circadian rhythm of the intrarenal RAS with in vivo imaging using multiphoton microscopy [33]. Aldosterone-independent activation of renal mineralocorticoid receptors (MRs) has also been discussed and studied. Maeoka et al. used aldosterone synthase knockout mice and demonstrated that MRs were activated in the absence of aldosterone along distal convoluted tubule 2 and were partially activated in the cortical collecting duct, indicating that renal MRs are normally bound by hormones other than aldosterone [34]. This supports the use of MR antagonists in patients, even when aldosterone is not elevated.
Vascular system
Vascular system impairment is also associated with hypertension. Recently, an increasing number of studies have shown the vascular protective effect of sirtuin 6 (SIRT6), which is an NAD-dependent protein deacetylase and a member of the evolutionarily conserved sirtuin family. Grootaert et al. showed that SIRT6 protein expression was reduced in human and mouse plaque vascular smooth muscle cells (VSMCs) and was positively regulated by ubiquitin ligase C-terminus of HSC70-interacting protein (CHIP) [35]. SIRT6 regulated telomere maintenance and VSMC lifespan and inhibited atherogenesis, all of which were dependent on its deacetylase activity. Liu X et al. found that SIRT6 expression was downregulated in the aortae of aged rats and showed that SIRT6 knockdown enhanced Ang II-induced vascular adventitial aging by activating the NF-κB pathway in vitro [36]. Li et al. demonstrated that SIRT6 was markedly downregulated in peripheral blood mononuclear cells and in the radial artery tissue of patients with chronic kidney disease with vascular calcification [37]. They further showed that SIRT6 suppressed VSMC osteoblastic transdifferentiation and attenuated vascular calcification both in mice in vivo and in vitro. Mechanistically, SIRT6 deacetylated runt-related transcription factor 2 (Runx2) and promoted its ubiquitination and subsequent degradation through the ubiquitin‒proteasome system.
Skin vasoconstriction-mediated skin water conservation was newly indicated to contribute to hypertension. Ogura et al. showed that SHRs exhibited higher urine volume and lower urinary osmolality than normotensive Wistar-Kyoto rats (WKYs), without significant differences in water intake, urinary osmolyte excretion, or plasma osmolality between the groups [38]. SHRs showed higher blood pressure and skin sodium content and lower transepidermal water loss than WKYs. Skin vasodilation, induced by elevating body temperature, increased transepidermal water loss in both SHRs and WKYs; however, blood pressure was decreased in SHRs, but not in WKYs, by skin vasodilation. These findings suggest that skin water conservation, mediated by skin vasoconstriction and the accumulation of osmolytes such as sodium, may contribute to hypertension in SHRs. This concept has been indicated in other animal models [39, 40].
Potential treatments
There have been many potential treatments for hypertension and the associated organ damage. Here, the findings of several studies that may lead to potential drug repositioning will be described. Rivaroxaban, a direct factor Xa inhibitor, has been reported to have protective effects on the cardiovascular system. Daci et al. showed that pretreatment with rivaroxaban attenuated lipopolysaccharide (LPS)-induced acute vascular inflammation and contractile dysfunction in LPS-injected rats [41]. Nakanishi et al. demonstrated that rivaroxaban protected against cardiac dysfunction after myocardial infarction in mice [42]. Reductions in protease-activated receptor (PAR)-1, PAR-2, and proinflammatory cytokines in the infarcted area were associated with the cardioprotective effects of rivaroxaban. In addition, Narita et al. showed that rivaroxaban had a protective effect against cardiac hypertrophy and fibrosis by inhibiting PAR-2 signaling in renin-overexpressing hypertensive mice [43]. Sodium-glucose cotransporter-2 (SGLT2) inhibitors have been demonstrated to have cardio- and renoprotective effects in clinical studies. In addition, a blood pressure-lowering effect of SGLT2 inhibitors has been suggested in animal studies. Kravtsova et al. showed that dapagliflozin treatment blunted the development of hypertension with increases in glucose and Na+ excretion without secondary effects on the expression and function of other Na+ transporters and channels along the nephron and systemic and intrarenal RAS in Dahl salt-sensitive hypertensive rats [44]. Zhao et al. showed that canagliflozin attenuated the development of hypertension by directly alleviating vasoconstriction in Dahl salt-sensitive hypertensive rats [45]. Salt-induced vascular transient receptor potential channel 3 upregulation resulted in augmented vasoconstriction in salt-sensitive hypertensive rats by facilitating sodium-calcium exchanger 1-mediated vascular calcium uptake, which could be alleviated by canagliflozin. Furthermore, although SGLT2 inhibitors may have a sympathoinhibitory effect, which might be responsible for their protective effects on the cardiovascular system, their mechanism remains unclear.
Extracellular vesicles
Extracellular vesicles (EVs) have been studied in many aspects, including disease diagnosis, biological signaling, and novel therapeutics, in this decade. Ochiai-Homma et al. showed that pendrin in urinary EVs can be a useful biomarker for the diagnosis and treatment of primary aldosteronism, and this finding was supported by studies using a rat model of aldosterone excess [46]. Lugo-Gavidia et al. indicated that circulating platelet-derived EVs were positively associated with nocturnal blood pressure at baseline and therapy-induced blood pressure changes over a 12-week treatment period with ambulatory blood pressure monitoring [47]. Platelet-derived EVs may provide an integrated measure of blood pressure changes achieved with pharmacotherapy. In addition, Chi et al. demonstrated that fibronectin type-III domain-containing protein 5 (FNDC5)/irisin-enriched EVs contributed to exercise-induced protection against vascular aging by increasing SIRT6 stability [48]. The findings of this study indicate that the exerkine FNDC5/irisin may be a potential target for aging-related vascular comorbidities. EVs also play a role as mediators of cell communication [49]. Wang et al. revealed an inverse correlation between circulating microRNA-92a levels and pulse wave velocity in humans and mice [50]. The findings of their in vitro study indicated that endothelial cell microRNA‑92a may be transported to VSMCs via EVs to regulate phenotypic changes in VSMCs, leading to arterial stiffness.
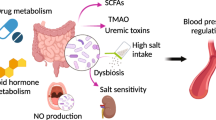
Gut microbiota
Studies on the role of gut microbiota in blood pressure regulation have also been accumulating. One of the topics in this field is the interaction between microbiota and antihypertensive drugs [51, 52]. Wu et al. showed that the abundances of several phyla and genera, including Proteobacteria, Cyanobacteria, Escherichia-Shigella, Eubacterium nodatum and Ruminococcus, were higher in DOCA-salt hypertensive rats than in control rats, while these changes were reversed by treatment with oral captopril, an angiotensin-converting enzyme inhibitor (ACEI) [53]. Of particular interest, the genera Bifidobacterium and Akkermansia, reported as beneficial bacteria in the gut, were abundant only in hypertensive rats treated with captopril. These results provide evidence that captopril has the potential to rebalance the dysbiosis of DOCA-salt rats, suggesting that the alteration of the gut flora by captopril may contribute to the hypotensive effect of this drug. Yang et al. showed that the blood pressure-lowering effect of quinapril, another ACEI, was more pronounced in SHRs treated with antibiotics than in SHRs not treated with antibiotics [54]. The depletion of gut microbiota in SHRs with antibiotics was associated with decreased gut microbial catabolism of quinapril as well as a significant reduction in the bacterial genus Coprococcus. C. comes, an anaerobic species of Coprococcus, harbored esterase activity and catabolized the ester quinapril in vitro. The coadministration of quinapril with C. comes reduced the antihypertensive effect of quinapril in SHRs. Importantly, C. comes selectively reduced the antihypertensive effects of the ester ramipril but not the nonester lisinopril. The findings of this study suggest that human commensal C. comes catabolizes the ester ACEI in the gut and lowers its antihypertensive effect.
The risk for coronavirus disease 2019 (COVID-19) in hypertensive patients and patients treated with RAS inhibitors has been discussed [55,56,57,58], and there are some animal studies investigating this issue from the aspect of gut microbiota. Li et al. showed that the expression of angiotensin-converting enzyme-2 (ACE2) and transmembrane protease serine-2 (TMPRSS2), key molecules in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, in the gut epithelium was increased in SHRs compared to normotensive rats [59]. Changes in the gut microbiome associated with short-chain fatty acids, particularly butyrate, were found in both hypertension and COVID-19 [60]. They further demonstrated that butyrate downregulates genes essential for SARS-CoV-2 infection, such as Ace2 and Tmprss2, but also upregulates toll-like receptors and other antiviral pathways [61]. These findings imply that the increased risk of COVID-19 in individuals with hypertension is partly due to the cumulative depletion of butyrate-producing bacteria in the gut.
Perspectives
In this mini-review, recent topics on basic research in hypertension were introduced. For many of these findings, the pathophysiology and underlying mechanism are not confined to a single organ. The brain receives neural and humoral inputs from the peripheral system and determines the central sympathetic outflow, which plays a major role in the control of diverse organs, including the heart, kidneys, and vasculature. Important mechanisms, such as Wnt/β-catenin signaling and SIRT6 activity, shown in this mini-review, are involved in homeostasis and pathogenesis across organs and diseases. EVs are also known to contribute to intracellular and interorgan crosstalk. The gut microbiome has become a key player in systemic diseases, including hypertension. The pathogenesis of hypertension is multifactorial and highly complex; therefore, it is reasonable that interorgan communication has recently become an important perspective in the study of hypertension and hypertensive organ damage. On the other hand, each researcher tends to focus only on his or her own area of expertise in trying to elucidate the pathogenesis of hypertension. To truly understand the pathogenesis of hypertension and to apply this understanding to treatments for conquering hypertension, interresearcher communication and collaboration should also be further facilitated (Fig. 2).
References
Hirooka Y. Sympathetic activation in hypertension: importance of the central nervous system. Am J Hypertens. 2020;33:914–26.
Katsurada K, Shinohara K, Aoki J, Nanto S, Kario K. Renal denervation: basic and clinical evidence. Hypertens Res. 2022;45:198–209.
Kario K, Hoshide S, Mogi M. A recent advance in Renal denervation to clinical practice. Hypertens Res. 2022 (e-pub ahead of print 20221005; https://doi.org/10.1038/s41440-022-01050-8).
Chen WW, Xiong XQ, Chen Q, Li YH, Kang YM, Zhu GQ. Cardiac sympathetic afferent reflex and its implications for sympathetic activation in chronic heart failure and hypertension. Acta Physiol (Oxf). 2015;213:778–94.
Zhu GQ, Xu Y, Zhou LM, Li YH, Fan LM, Wang W, et al. Enhanced cardiac sympathetic afferent reflex involved in sympathetic overactivity in renovascular hypertensive rats. Exp Physiol. 2009;94:785–94.
Wang HJ, Wang W, Cornish KG, Rozanski GJ, Zucker IH. Cardiac sympathetic afferent denervation attenuates cardiac remodeling and improves cardiovascular dysfunction in rats with heart failure. Hypertension. 2014;64:745–55.
Zahner MR, Li DP, Chen SR, Pan HL. Cardiac vanilloid receptor 1-expressing afferent nerves and their role in the cardiogenic sympathetic reflex in rats. J Physiol. 2003;551:515–23.
Shibata R, Shinohara K, Ikeda S, Iyonaga T, Matsuura T, Kashihara S, et al. Transient receptor potential vanilloid 1-expressing cardiac afferent nerves may contribute to cardiac hypertrophy in accompany with an increased expression of brain-derived neurotrophic factor within nucleus tractus solitarius in a pressure overload model. Clin Exp Hypertens. 2022;44:249–57.
Xiong XQ, Chen WW, Zhu GQ. Adipose afferent reflex: sympathetic activation and obesity hypertension. Acta Physiol (Oxf). 2014;210:468–78.
Cao W, Shi M, Wu L, Li J, Yang Z, Liu Y, et al. Adipocytes initiate an adipose-cerebral-peripheral sympathetic reflex to induce insulin resistance during high-fat feeding. Clin Sci (Lond). 2019;133:1883–99.
Dalmasso C, Leachman JR, Osborn JL, Loria AS. Sensory signals mediating high blood pressure via sympathetic activation: role of adipose afferent reflex. Am J Physiol Regul Integr Comp Physiol. 2020;318:R379–r389.
Dalmasso C, Leachman JR, Ghuneim S, Ahmed N, Schneider ER, Thibault O, et al. Epididymal fat-derived sympathoexcitatory signals exacerbate neurogenic hypertension in obese male mice exposed to early life stress. Hypertension. 2021;78:1434–49.
Asirvatham-Jeyaraj N, Gauthier MM, Banek CT, Ramesh A, Garver H, Fink GD, et al. Renal denervation and celiac ganglionectomy decrease mean arterial pressure similarly in genetically hypertensive schlager (BPH/2J) mice. Hypertension. 2021;77:519–28.
Foss JD, Fink GD, Osborn JW. Reversal of genetic salt-sensitive hypertension by targeted sympathetic ablation. Hypertension. 2013;61:806–11.
King AJ, Osborn JW, Fink GD. Splanchnic circulation is a critical neural target in angiotensin II salt hypertension in rats. Hypertension. 2007;50:547–56.
Kandlikar SS, Fink GD. Splanchnic sympathetic nerves in the development of mild DOCA-salt hypertension. Am J Physiol Heart Circ Physiol. 2011;301:H1965–73.
Osborn JW, Fink GD, Kuroki MT. Neural mechanisms of angiotensin II-salt hypertension: implications for therapies targeting neural control of the splanchnic circulation. Curr Hypertens Rep. 2011;13:221–8.
Randhawa PK, Jaggi AS. TRPV1 channels in cardiovascular system: a double edged sword? Int J Cardiol. 2017;228:103–13.
Bisognano JD, Bakris G, Nadim MK, Sanchez L, Kroon AA, Schafer J, et al. Baroreflex activation therapy lowers blood pressure in patients with resistant hypertension: results from the double-blind, randomized, placebo-controlled rheos pivotal trial. J Am Coll Cardiol. 2011;58:765–73.
Illig KA, Levy M, Sanchez L, Trachiotis GD, Shanley C, Irwin E, et al. An implantable carotid sinus stimulator for drug-resistant hypertension: surgical technique and short-term outcome from the multicenter phase II Rheos feasibility trial. J Vasc Surg. 2006;44:1213–8.
de Leeuw PW, Alnima T, Lovett E, Sica D, Bisognano J, Haller H, et al. Bilateral or unilateral stimulation for baroreflex activation therapy. Hypertension. 2015;65:187–92.
Domingos-Souza G, Santos-Almeida FM, Meschiari CA, Ferreira NS, Pereira CA, Pestana-Oliveira N, et al. The ability of baroreflex activation to improve blood pressure and resistance vessel function in spontaneously hypertensive rats is dependent on stimulation parameters. Hypertens Res. 2021;44:932–40.
Cao Y, Yu Y, Xue B, Wang Y, Chen X, Beltz TG, et al. IL (Interleukin)-17A acts in the brain to drive neuroinflammation, sympathetic activation, and hypertension. Hypertension. 2021;78:1450–62.
Wei SG, Zhang ZH, Beltz TG, Yu Y, Johnson AK, Felder RB. Subfornical organ mediates sympathetic and hemodynamic responses to blood-borne proinflammatory cytokines. Hypertension. 2013;62:118–25.
Wei SG, Yu Y, Felder RB. Blood-borne interleukin-1beta acts upon the subfornical organ to upregulate the sympathoexcitatory milieu of the hypothalamic paraventricular nucleus. Am J Physiol Regul Integr Comp Physiol. 2017. https://doi.org/10.1152/ajpregu.00211.2017.
Wei SG, Yu Y, Zhang ZH, Felder RB. Proinflammatory cytokines upregulate sympathoexcitatory mechanisms in the subfornical organ of the rat. Hypertension. 2015;65:1126–33.
Iyonaga T, Shinohara K, Mastuura T, Hirooka Y, Tsutsui H. Brain perivascular macrophages contribute to the development of hypertension in stroke-prone spontaneously hypertensive rats via sympathetic activation. Hypertens Res. 2020;43:99–110.
Gruber T, Pan C, Contreras RE, Wiedemann T, Morgan DA, Skowronski AA, et al. Obesity-associated hyperleptinemia alters the gliovascular interface of the hypothalamus to promote hypertension. Cell Metab. 2021;33:1155–.e1110.
Kasacka I, Piotrowska Z, Domian N, Acewicz M, Lewandowska A. Canonical Wnt signaling in the kidney in different hypertension models. Hypertens Res. 2021;44:1054–66.
Schunk SJ, Floege J, Fliser D, Speer T. WNT-β-catenin signalling - a versatile player in kidney injury and repair. Nat Rev Nephrol. 2021;17:172–84.
Nagasu H. Importance of wnt-catenin signaling in hypertensive kidney diseases. Hypertens Res. 2021;44:1546–7.
Matsusaka T, Niimura F, Shimizu A, Pastan I, Saito A, Kobori H, et al. Liver angiotensinogen is the primary source of renal angiotensin II. J Am Soc Nephrol. 2012;23:1181–9.
Matsuyama T, Ohashi N, Aoki T, Ishigaki S, Isobe S, Sato T, et al. Circadian rhythm of the intrarenal renin-angiotensin system is caused by glomerular filtration of liver-derived angiotensinogen depending on glomerular capillary pressure in adriamycin nephropathy rats. Hypertens Res. 2021;44:618–27.
Maeoka Y, Su XT, Wang WH, Duan XP, Sharma A, Li N, et al. Mineralocorticoid receptor antagonists cause natriuresis in the absence of aldosterone. Hypertension. 2022;79:1423–34.
Grootaert MOJ, Finigan A, Figg NL, Uryga AK, Bennett MR. SIRT6 protects smooth muscle cells from senescence and reduces atherosclerosis. Circ Res. 2021;128:474–91.
Liu X, Jiang D, Huang W, Teng P, Zhang H, Wei C, et al. Sirtuin 6 attenuates angiotensin II-induced vascular adventitial aging in rat aortae by suppressing the NF-kappaB pathway. Hypertens Res. 2021;44:770–80.
Li W, Feng W, Su X, Luo D, Li Z, Zhou Y, et al. SIRT6 protects vascular smooth muscle cells from osteogenic transdifferentiation via Runx2 in chronic kidney disease. J Clin Invest. 2022;132:e150051.
Ogura T, Kitada K, Morisawa N, Fujisawa Y, Kidoguchi S, Nakano D, et al. Contributions of renal water loss and skin water conservation to blood pressure elevation in spontaneously hypertensive rats. Hypertens Res. 2022; https://doi.org/10.1038/s41440-022-01044-6).
Kovarik JJ, Morisawa N, Wild J, Marton A, Takase-Minegishi K, Minegishi S, et al. Adaptive physiological water conservation explains hypertension and muscle catabolism in experimental chronic renal failure. Acta Physiol (Oxf). 2021;232:e13629.
Wild J, Jung R, Knopp T, Efentakis P, Benaki D, Grill A, et al. Aestivation motifs explain hypertension and muscle mass loss in mice with psoriatic skin barrier defect. Acta Physiol (Oxf). 2021;232:e13628.
Daci A, Da Dalt L, Alaj R, Shurdhiqi S, Neziri B, Ferizi R, et al. Rivaroxaban improves vascular response in LPS-induced acute inflammation in experimental models. PLoS One. 2020;15:e0240669.
Nakanishi N, Kaikita K, Ishii M, Oimatsu Y, Mitsuse T, Ito M, et al. Cardioprotective effects of rivaroxaban on cardiac remodeling after experimental myocardial infarction in mice. Circ Rep. 2020;2:158–66.
Narita M, Hanada K, Kawamura Y, Ichikawa H, Sakai S, Yokono Y, et al. Rivaroxaban attenuates cardiac hypertrophy by inhibiting protease-activated receptor-2 signaling in renin-overexpressing hypertensive mice. Hypertens Res. 2021;44:1261–73.
Kravtsova O, Bohovyk R, Levchenko V, Palygin O, Klemens CA, Rieg T, et al. SGLT2 inhibition effect on salt-induced hypertension, RAAS, and Na(+) transport in Dahl SS rats. Am J Physiol Ren Physiol. 2022;322:F692–f707.
Zhao Y, Li L, Lu Z, Hu Y, Zhang H, Sun F, et al. Sodium-glucose cotransporter 2 inhibitor canagliflozin antagonizes salt-sensitive hypertension through modifying transient receptor potential channels 3 mediated vascular calcium handling. J Am Heart Assoc. 2022;11:e025328.
Ochiai-Homma F, Kuribayashi-Okuma E, Tsurutani Y, Ishizawa K, Fujii W, Odajima K, et al. Characterization of pendrin in urinary extracellular vesicles in a rat model of aldosterone excess and in human primary aldosteronism. Hypertens Res. 2021;44:1557–67.
Lugo-Gavidia LM, Burger D, Nolde JM, Carnagarin R, Chan J, Bosio E, et al. Platelet-derived extracellular vesicles correlate with therapy-induced nocturnal blood pressure changes. J Hypertens. 2022;40:2210–8.
Chi C, Fu H, Li YH, Zhang GY, Zeng FY, Ji QX, et al. Exerkine fibronectin type-III domain-containing protein 5/irisin-enriched extracellular vesicles delay vascular ageing by increasing SIRT6 stability. Eur Heart J. 2022; https://doi.org/10.1093/eurheartj/ehac431).
Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21:9–17.
Wang C, Wu H, Xing Y, Ye Y, He F, Yin Q, et al. Endothelial-derived extracellular microRNA-92a promotes arterial stiffness by regulating phenotype changes of vascular smooth muscle cells. Sci Rep. 2022;12:344.
Mishima E, Abe T. Role of the microbiota in hypertension and antihypertensive drug metabolism. Hypertens Res. 2022;45:246–53.
Kyoung J, Atluri RR, Yang T. Resistance to antihypertensive drugs: is gut microbiota the missing link? Hypertension. 2022;79:2138–47.
Wu H, Lam TYC, Shum TF, Tsai TY, Chiou J. Hypotensive effect of captopril on deoxycorticosterone acetate-salt-induced hypertensive rat is associated with gut microbiota alteration. Hypertens Res. 2022;45:270–82.
Yang T, Mei X, Tackie-Yarboi E, Akere MT, Kyoung J, Mell B, et al. Identification of a gut commensal that compromises the blood pressure-lowering effect of Ester angiotensin-converting enzyme inhibitors. Hypertension . 2022;79:1591–601.
Kai H, Kai M, Niiyama H, Okina N, Sasaki M, Maeda T, et al. Overexpression of angiotensin-converting enzyme 2 by renin-angiotensin system inhibitors. Truth or myth? A systematic review of animal studies. Hypertens Res. 2021;44:955–68.
Shibata S, Arima H, Asayama K, Hoshide S, Ichihara A, Ishimitsu T, et al. Hypertension and related diseases in the era of COVID-19: a report from the Japanese Society of Hypertension Task Force on COVID-19. Hypertension Res. 2020;43:1028–46.
Ishida M. How to deal with hypertension in the COVID-19 era—the impact “ON” and “OF” hypertension. Hypertension Res. 2022;45:548–50.
Wojciechowska W, Terlecki M, Klocek M, Pac A, Olszanecka A, Stolarz-Skrzypek K, et al. Impact of arterial hypertension and use of antihypertensive pharmacotherapy on mortality in patients hospitalized due to COVID-19: the CRACoV-HHS study. Hypertension. 2022;79:2601–10.
Li J, Stevens BR, Richards EM, Raizada MK. SARS-CoV-2 receptor ACE2 (Angiotensin-Converting Enzyme 2) is upregulated in colonic organoids from hypertensive rats. Hypertension. 2020;76:e26–8.
Sharma RK, Stevens BR, Obukhov AG, Grant MB, Oudit GY, Li Q, et al. ACE2 (Angiotensin-Converting Enzyme 2) in cardiopulmonary diseases: ramifications for the control of SARS-CoV-2. Hypertension. 2020;76:651–61.
Li J, Richards EM, Handberg EM, Pepine CJ, Raizada MK. Butyrate regulates COVID-19-relevant genes in gut epithelial organoids from normotensive rats. Hypertension. 2021;77:e13–6.
Acknowledgements
I sincerely appreciate the editors of Hypertension Research for giving me the opportunity to write this review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The author declares no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shinohara, K. Emerging topics on basic research in hypertension: interorgan communication and the need for interresearcher collaboration. Hypertens Res 46, 638–645 (2023). https://doi.org/10.1038/s41440-023-01176-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-023-01176-3