Abstract
Hemoglobin (Hb) Vancleave (NM_000518.5:c.431 A > T; dbSNP: rs33918338) is an extremely rare structural hemoglobin variant worldwide, and studies are limited. This report describes the case of a 16-year-old male patient who presented with secondary erythrocytosis. The diagnosis of Hb Vancleave, in combination with codon 41/42 (-TTCT) (NM_000518.5:c.126_129del; dbSNP: rs80356821), was confirmed by direct sequencing. This report highlights the importance of sequencing in the differential diagnosis of beta-thalassemia syndrome in Malaysia.
Similar content being viewed by others
Hemoglobin abnormalities are caused by molecular changes involving two primary globin gene clusters (alpha and/or beta) located on chromosomes 11 (beta; 11p15.4; OMIM# 141900)1 and 16 (alpha; 16p13.3; OMIM# 141800 and 141850)2. The clinical consequences are associated with qualitative (hemoglobinopathies) and/or quantitative (thalassemia) defects in globin chain expression3. Coinheritance of both structural hemoglobinopathies and thalassemia in a patient results in a continuum of complex phenotypes. To date, more than 100 high-oxygen-affinity hemoglobinopathies have been described worldwide, and their presence usually leads to erythrocytosis. Although patients with erythrocytosis are generally asymptomatic, identifying patients with high oxygen affinity is important in young patients or those with high hemoglobin concentrations and a family history of polycythemia4. A correct diagnosis is important for the clinical management and long-term outcome of patients4,5,6.
Hemoglobin Vancleave (HGVS: NM_000518.5:c.431A > T; dbSNP: rs33918338) is an extremely rare beta-globin chain variant, and its prevalence is unknown. Two entries for Hb Vancleave were found in public databases (HbVar ID: 1234 and IthaID: 1298), but no clinical case reports were found in the literature. The molecular basis of Hb Vancleave is based on a single-nucleotide substitution at codon 143 (CAC > CTC) of the HBB gene, which changes the coding amino acid from histidine (His) to leucine (Leu). Theoretically, the side chain difference between leucine and histidine will affect the biochemical characteristics of Hb Vancleave relative to normal hemoglobin. In the normal hemoglobin tetramer, histidine has a positively charged imidazole side chain, which is one of the vital components of the binding site for 2,3-diphosphoglycerate (2,3-DPG), which is essential for regulating the affinity of hemoglobin for oxygen. In contrast, in Hb Vancleave, histidine is substituted with leucine, which contains an isobutyl side chain that converts the 2,3-DPG binding site to a nonpolar form. This subsequently modifies the oxygen affinity of hemoglobin. The same effects of high oxygen affinity were also observed for Hb Abruzzo7, Hb Syracuse8, and Hb Little Rock9, which are similarly altered at codon 143 of the HBB gene.
Malaysia is a tropical country in Southeast Asia where multiethnic populations and mixed marriages are common. Thus, complex genetic interactions from different ethnic backgrounds cause a wide clinical spectrum of hemoglobin disorders in Malaysia. Most of the patients registered in the Malaysian Thalassemia Registry were Malays (63.95%), Chinese (11.75%), and Kadazan-Dusuns (11.36%). However, hemoglobin disorders have rarely been reported among Malaysian Indians10,11. Several clinically important mutations in the HBB gene have been reported in major ethnic groups in Malaysia. Codon 26 (GAG > AAG) Hb E, codon 41/42 (–TTCT), IVS 1–1 (G > T), and IVS 1–5 (G > C) are the most common disease-associated HBB gene variations in Malays, while codon 41/42 (–TTCT) and IVS 2–654 (C > T) were predominantly detected among the Malaysian Chinese population with beta-thalassemia12. Here, we report the first case of Hb Vancleave combined with a severe beta-thalassemia mutation associated with secondary erythrocytosis in a Malaysian Indian teenager.
A 16-year-old student (RS873/23) of Indian Sikh descent initially volunteered to participate in the National Thalassemia Screening Program for secondary school students in Malaysia. His parents provided informed consent for the screening program at his school. Peripheral blood samples and data were collected following the Declaration of Helsinki and the Malaysian National Institute of Health (NIH) guidelines for the conduct of research at Ministry of Health (MOH) institutions and facilities.
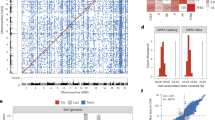
The patient was a smoker and currently vapes. At presentation, he was asymptomatic, with a Hb level of 18.3 g/dL. No history of transfusion was reported. The peripheral blood film (PBF) showed packed red cells with hypochromic microcytic erythrocytes for his age. He has two siblings, including his younger sister, who is also asymptomatic. His parents were not consanguineous. His father is a Malaysian Sikh who died at the age of 51 from heart disease and dyslipidemia. No paternal history of thalassemia was reported. His mother is a 42-year-old Indian woman from Uttar Pradesh, India, and she has three siblings who were suspected of having thalassemia, but the diagnosis was never confirmed. In 2014, it was suspected that she had thalassemia during pregnancy, yet no confirmatory test was performed. The family tree of patient RS873/23 is shown in Fig. 1A.
A Three-generation family tree of case RS873/23 shows both affected and asymptomatic family members. The arrow symbol marked the index case (III-1; RS873/23). Squares indicate male, circles indicate female, upward diagonal stripes indicate affected/ suspected proband, black-filled symbols indicate affected proband with compound heterozygous states of βCodon41/42/βVancleave genotype, and white symbols indicate unaffected proband. B High-performance liquid chromatography shows mildly increased HbA2, high HbF, and unknown variants. C Capillary electrophoresis shows a peak for HbA, HbA2, and HbF/Hb-variant. The blue arrow indicates the presence of the Hb variant. D, E show sequencing analysis of the HBB gene revealed double heterozygotes for Hb Vancleave (Codon 143 [CAC>CTC]) and Codon 41/42 (-TTCT), respectively. The top bar panel with reference sequence; yellow indicates the HBB transcript region, green indicates the HBB gene untranslated region, the second bar panel with consensus HBB gene sequence from case III-1, and the third- and fourth-panel sequencing chromatograms from the reverse and forward primers of the HBB gene, respectively. The red arrow indicates the mutant sequence.
The peripheral blood of patient RS873/23 was collected for full blood count (FBC) and hemoglobin analysis (Hb analysis). Both FBC and Hb analyses were performed in primary health care facilities. His FBC showed hypochromic microcytic erythrocyte indices, with a red blood cell (RBC) count, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and red cell width (RDW) of 8.94 × 106/µL, 64.2 fL, 20.5 pg and 20.4%, respectively. The peripheral blood film (PBF) showed packed erythrocytes with a hypochromic microcytic morphology. Hemoglobin analysis by capillary electrophoresis (CE; Sebia, France) revealed elevated HbA2 (4.2%) and normal HbA (82.3%) levels (Fig. 1B). An abnormal HbF peak was detected by CE. High-performance liquid chromatography (HPLC; Bio-Rad, USA) revealed that 76.1% of the hemoglobin variants were abnormal, with a retention time of 1.84 min. The HbA2 level was borderline high (3.7%), and the HbF level was elevated (16.8%) (Fig. 1C). The results of hemoglobin analysis suggested hereditary persistence of fetal hemoglobin (HPFH) or delta-beta-thalassemia.
Molecular investigations to detect common and unknown beta-globin gene cluster deletions that are associated with HPFH and delta-beta-thalassemia phenotypes were carried out by using multiplex gap polymerase chain reaction (MGap-PCR) and multiplex ligation probe-dependent amplification (MLPA). However, no deletions were detected. Therefore, we suspected that this patient might have compound or homozygous HBB gene mutations that can cause both elevated HbF (16.8%) and an abnormal peak (76.1%) at a retention time of 1.84 min on HPLC. Direct sequencing was carried out to detect disease-associated variations in the beta-globin gene (HBB; RefSeq ID: NM_000518.5), and the results confirmed the presence of compound heterozygous variants of codon 41/42 (-TTCT) [HGVS: (NM_000518.5:c.126_129del)] (Fig. 1D) and codon 143 (CAC > CTC) Hb Vancleave (Fig. 1E). A summary of the hematology and molecular test results in this study is provided in Table 1.
Our case is an incidental finding from the National Thalassemia Screening Program in Malaysia. This is the first case report in the literature and the first identification of Hb Vancleave in the Malaysian population. The majority of hemoglobin disorders are inherited as autosomal recessive. However, in some cases, disease-associated genetic variations in globin gene clusters can be caused by de novo or dominant genetic variations. In this study, we identified a case of a Malaysian patient with compound heterozygous variants of Hb Vancleave and codon 41/42 (-TTCT), which are rare among Indian Sikh in Malaysia. The inheritance pattern of these variations cannot be determined because parental samples were not available. Nevertheless, we speculate that his father might be homozygous for Hb Vancleave, which could cause polycythemia and hyperviscosity syndrome; this condition might have led to his heart problems and subsequent death at the age of 51 years, which is considered young for heart disease and complications of cardiomyopathy. The clinical phenotypes were comparable to those of another patient with high oxygen affinity—a 4-year-old Malay girl with homozygous Hb Tak who presented with symptomatic polycythemia and congestive heart failure13. Hb Tak is mildly unstable; thus, patients in a homozygous state are more symptomatic and present with symptoms early in life.
A common disease-associated frameshift variation at codon 41/42 (-TTCT) compounded with Hb Vancleave was detected in our index patient. The four-nucleotide deletion between codons 41 and 42 of the HBB gene results in a stop codon at codon 59, which subsequently terminates translation and reduces the rate of beta-globin chain synthesis. The genetic alteration of codon 41/42 (-TTCT) has been reported in many populations, including Chinese, Malaysian, Singaporean, Taiwanese, Thai, Punjabi, Pakistani, Japanese, Korean, English, and Indonesian populations14. Particularly in Malaysia, the codon 41/42 (-TTCT) variation is commonly found in Malaysian Chinese and Malays but has never been reported in Malaysian Indians12. We noted that the patient’s mother was originally from Uttar Pradesh, India, which has a high prevalence of beta-thalassemia with codon 41/42 (-TTCT) being one of the most common beta-thalassemia alleles15. Hence, based on the family history of the index patient, we postulate that codon 41/42 (-TTCT) and Hb Vancleave could have been inherited from the maternal and paternal sides, respectively. In addition, although both the Hb Vancleave and codon 41/42 (-TTCT) alleles were functionally altered, the Hb value was still high, likely due to physiological compensation from smoking and vaping habits.
Differential diagnosis of high oxygen affinity may be laborious and complex due to its plethora of clinical presentations and its unknown prevalence in the population5,16. Particularly in Malaysia, where hemoglobin disorders are highly prevalent, patients with erythrocytosis should be prioritized for hemoglobin anomaly investigations via direct DNA sequencing. This is an important step to ensure that clinical management is successful and that effective investigations can be performed for patients with erythrocytosis5,17. Previous studies have also shown that coinheritance of high-affinity hemoglobin with delta-beta thalassemia or beta-thalassemia leads to marked polycythemia; otherwise, patients are clinically healthy, but the risk of thrombosis increases later in life4. In addition, Percy and colleagues recommended that if the P50 value is not available, HPLC and Sanger sequencing can be used as alternative methods for the differential diagnosis of high-affinity hemoglobin. Erythrocytosis with concomitant iron deficiency can normalize hemoglobin levels and therefore make it difficult to detect high-affinity hemoglobin by performing only routine laboratory tests18. Some of the silent variants may be overlooked in the presumptive diagnosis. Hence, when encountering these atypical cases, molecular DNA analysis is recommended17,19. In summary, this is the first reported case of Hb Vancleave, a rare variant worldwide, in Malaysia. The present study highlights the importance of hemoglobin disorder awareness and the advantage of DNA analysis for patients with erythrocytosis, which can be challenging due to the clinical and genetic heterogeneity of thalassemia syndrome in Malaysia.
HGV Database
The relevant data from this Data Report are hosted at the Human Genome Variation Database at https://doi.org/10.6084/m9.figshare.hgv.3391. https://doi.org/10.6084/m9.figshare.hgv.3394.
References
Entry - *141900 - HEMOGLOBIN-BETA LOCUS; HBB - OMIM. Accessed October 13, 2023. https://www.omim.org/entry/141900?search=141900&highlight=141900
Entry Search - 141800 - OMIM. Accessed October 13, 2023. https://www.omim.org/search?index=entry&start=1&limit=10&sort=score+desc%2C+prefix_sort+desc&search=141800
Old J. Hemoglobinopathies and Thalassemias. In: Reference Module in Biomedical Research. 2014. https://doi.org/10.1016/B978-0-12-801238-3.05543-4
Bain B. J. Haemoglobinophaty diagnosis. Published online 2006:1-330.
NM, H & MN, S. Rare cases of haemoglobin variant presented with isolated erythrocytosis. Malays J. Med. Health Sci. 16, 114–116 (2020).
Yudin, J., Verhovsek, M., Joseph’, S. & Hamilton, H. How we diagnose and manage altered oxygen affinity hemoglobin variants. Am. J. Hematol. 94, 597–603 (2019).
Bonaventura, C. et al. Hemoglobin Abruzzo (beta143 (H21) His replaced by Arg). Consequences of altering the 2,3-diphosphoglycerate binding site. J. Biol. Chem. 250, 6273–6277 (1975).
Jensen, M., Oski, F. A., Nathan, D. G. & Bunn, H. F. Hemoglobin Syracuse (alpha2beta2-143(H21)His leads to Pro), a new high-affinity variant detected by special electrophoretic methods. Observations on the auto-oxidation of normal and variant hemoglobins. J. Clin. Invest 55, 469–477 (1975).
Bare, G. H. et al. Hemoglobin Little Rock (beta143 (H21) His leads to Gln). Effects of an amino acid substitution at the 2,3-diphosphoglycerate binding site. J. Biol. Chem. 249, 773–779 (1974).
Mohd Ibrahim, H. et al. Observational study on the current status of thalassaemia in Malaysia: a report from the Malaysian Thalassaemia registry. BMJ Open 10, e037974 (2020).
Tan JAMA, et al. Molecular defects in the β-globin gene identified in different ethnic groups/populations during prenatal diagnosis for β-thalassemia: A Malaysian experience. Clin. Exp. Med. Published online 2004. https://doi.org/10.1007/s10238-004-0048-x
Hassan, S., Ahmad, R., Zakaria, Z., Zulkafli, Z. & Abdullah, W. Z. Detection of β-globin gene mutations among β-thalassaemia carriers and patients in Malaysia: Application of multiplex amplification refractory mutation system-polymerase chain reaction. Malays. J. Med. Sci. 20, 13–20 (2013).
Amran, H. S. et al. Secondary polycythaemia in a Malay girl with homozygous Hb Tak. Malays. J. Pathol. 39, 321–326 (2017).
HbVar ID 849. Accessed October 21, 2023. https://globin.bx.psu.edu/cgi-bin/hbvar/query_vars3?mode=output&display_format=page&i=849
Agarwal, S., Pradhan, M., Gupta, U. R., Samai, S. & Agarwal, S. S. Geographic and ethnic distribution of β-thalassemia mutations in Uttar Pradesh, India. Hemoglobin 24, 89–97 (2000).
Perroud, C., Porret, N. & Rovó, A. “The Long Journey of Unexplained Erythrocytosis”: Erythrocytosis due to high-oxygen affinity hemoglobinopathy – hemoglobin variant little rock (HBB: c.432C>A) – A Report of a Swiss family and review of the literature. Acta Haematol. 146, 326–330 (2023).
Prakobkaew, N., Singsanan, S., Fucharoen, G., Surapot, S. & Fucharoen, S. Secondary erythrocytosis caused by hemoglobin Tak/(δβ)- thalassemia syndrome. Acta Haematol. 124, 115–119 (2010).
Percy, M. J. et al. Identification of high oxygen affinity hemoglobin variants in the investigation of patients with erythrocytosis. Haematologica 94, 1321–1322,, (2009).
Aziz A. et al. Rare cause of transfusion-dependent hemolytic anemia: A case report of HbE/Hb Nottingham and literature review. Pediatr. Blood Cancer. Published online March 6, 2022:e29629. https://doi.org/10.1002/PBC.29629
Acknowledgements
This research and publication fees were fully funded by the Institute for Medical Research (IMR), National Institute of Health (NIH), and Ministry of Health (MOH), Malaysia. We wish to thank the Director-General of Health, Malaysia, for permission to publish this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aziz, N.A., Musa, N.H., Mathews, M. et al. Rare coinheritance of hemoglobin vancleave with severe beta-thalassemia mutation in a patient with secondary erythrocytosis. Hum Genome Var 11, 19 (2024). https://doi.org/10.1038/s41439-024-00275-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41439-024-00275-y