Abstract
Purpose
Barriers to the implementation of pharmacogenomics in clinical practice have been thoroughly discussed over the past decade.
Methods
The objective of this scoping review was to characterize the peer-reviewed literature surrounding the experiences and actions of prescribers, pharmacists, or genetic counselors when using pharmacogenomic information in real-world or hypothetical research settings.
Results
A total of 33 studies were included in the scoping review. The majority of studies were conducted in the United States (70%), used quantitative or mixed methods (79%) with physician or pharmacist respondents (100%). The qualitative content analysis revealed five major methodological approaches: hypothetical clinical case scenarios, real-world studies evaluating prescriber response to recommendations or alerts, cross-sectional quantitative surveys, cross-sectional qualitative surveys/interviews, and a quasi-experimental real-world study.
Conclusion
The findings of this scoping review can guide further research on the factors needed to successfully integrate pharmacogenomics into clinical care.
Similar content being viewed by others
INTRODUCTION
Barriers to the expanded use of pharmacogenomics in clinical practice have been thoroughly discussed over the past decade.1,2,3,4 While many of these barriers have been addressed, obstacles persist that preclude the successful application of pharmacogenomics into routine clinical practice. These obstacles include an underdeveloped clinical decision support (CDS) infrastructure, lack of third-party payer coverage policies and reimbursement, and limited clinician and patient understanding.1,5,6,7,8,9 Several of these barriers were highlighted in recent work from the Implementing Genomics into Practice (IGNITE) consortium, which ranked 28 constructs for their importance to the future sustainability of genomic medicine.10 Interestingly, three of the top five ranked constructs (1, 2, and 4) focused on provider needs and included: (1) expanded genomic education, (2) making CDS tools available, and (3) integrating genomic information into clinical workflow.
Numerous descriptive or cross-sectional studies assessing the attitudes of providers toward pharmacogenomics have been published. Descriptive papers often come from the implementation initiatives established at academic hospitals.11,12,13,14 Cross-sectional studies focus on the attitudes, awareness, and concerns of physicians, community pharmacists, cardiologists, and psychiatrists. The findings of these studies demonstrate that the majority of physicians and community pharmacists, as well as specialists such as cardiologists and psychiatrists, have positive views of pharmacogenomics, yet feel unprepared to deliver it in their practices.6,15,16,17,18 While these papers clarify the nuances and considerations necessary to establish pharmacogenomics in practice, they typically do not include a measurement or assessment of the interventions’ impact on those delivering them to patients. Real-world assessments and intervention-based studies are crucial to provide actionable insights enabling clinical pharmacogenomics use for patient care.
With these considerations, the overall research goal of this scoping review is to assess how the prospective or retrospective experiences and actions of prescribers, pharmacists, or genetic counselors have been measured when using pharmacogenomic information in either real-world practice or a hypothetical research setting. This is an important gap in the literature to address as many studies to date have focused more on implementation strategies rather than collecting data on the decision making or experiences of the providers who will be responsible for acting on this information in clinical care.
MATERIALS AND METHODS
Study design
A scoping review is a synthesis of the existing literature on a topic in terms of the volume, nature, and characteristics of the primary research.19 A scoping review was selected to assess the extent, range, and nature of evidence to summarize heterogeneous methods or disciplines, without pursuing a quality assessment of the literature.20 The steps to conduct a scoping review are as follows: (1) clarify and link the review purpose and research question, (2) balance feasibility with breadth and comprehensiveness of the review process, (3) use an iterative team approach when selecting studies, (4) extract data, and (5) incorporate a quantitative summary and qualitative thematic analysis of results and state implications of study findings.21 To increase the methodological transparency and uptake of these findings, the recent checklist extension by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) published for scoping reviews (PRISMA-ScR) was used throughout this study.22
Eligibility criteria
To be included in the scoping review, studies had to include an outcome that measured the experiences or actions of a physician or advanced-practice provider, pharmacist, or genetic counselor when engaged in actual or hypothetical pharmacogenomic testing scenarios. At least 50% of the data must have come from responses or decisions made by physicians (MD/DO), advanced-practice providers (nurse practitioners or physician assistants), pharmacists (RPh/PharmD), or genetic counselors (CGC). The scoping review excluded studies that were purely descriptive, anecdotal, or opinion in nature. To achieve our stated research goal, these exclusions focused our findings to studies that collected and reported provider decision making or experience data when acting on pharmacogenomic information. This exclusion approach eliminated cross-sectional provider studies focused only on attitudes and awareness, not actual use of pharmacogenomics, as well as studies that only describe the strategies and learnings of clinical pharmacogenomic implementation. Studies not primarily focused on pharmacogenomics, published before the year 2000, or published in a language other than English were also excluded.
Information sources and search
Potentially relevant papers were searched using the MEDLINE® and Embase® bibliographic databases. The search was executed in December 2020 and included studies published through the end of June 2020. Search strategies were developed by the lead author and refined through discussion with other authors. Two individual searches were performed in MEDLINE® and Embase® combining various alternative search terms for “pharmacogenetics” and “health personnel,” “genetic counselors,” “pharmacists,” “physicians,” “education,” “surveys,” or “questionnaire.” “Nurse practitioner” and “physician assistant” were not included as explicit search terms, but appropriate studies that utilized these individuals as prescribers were included. The full search strategy is outlined in Supplement Table 1.
Selection of sources of evidence
Prior to beginning the selections, a screening form was developed and agreed upon by the authors. Two authors (N.J.K. and T.J.D.) independently and iteratively reviewed titles and abstracts, then full papers, making decisions to include or exclude at each stage. At the completion of each stage, the authors discussed their individual assessments and came to consensus on study inclusion.
Data charting and data elements
The data charting process used the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist to determine which variables to extract from the included studies. Final variables were extracted by two authors (N.J.K. and T.J.D.) and included author and publication year, study location, research aims, study design and methods, population and setting, outcome(s) of interest, and major findings.
Synthesis of results
Finally, two authors (N.J.K. and M.R.) performed an inductive content analysis of the study designs and methods extracted during data charting to structure the results. This inductive analysis was first performed independently, with these authors coming together to discuss initial findings and codes. These codes were then applied to each included study by the lead author. The authors met once more to ensure coding consistency before finalizing the results.
RESULTS
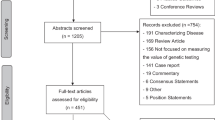
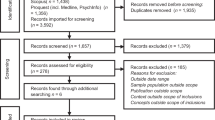
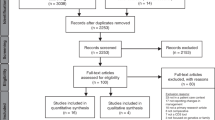
Fig. 1 provides an overview of the number of studies screened, determined eligible (with reasons for exclusion at each stage), and included in the review findings.
Characteristics of sources of evidence
A total of 592 unique studies were identified from MEDLINE®. The search of Embase® produced 527 unique studies that were not captured in the MEDLINE® search. A total of 33 studies underwent complete data extraction (Table 1). All studies, except for three, were conducted in the United States and Europe.23,24,25 Among the 33 studies, 24 contained only physician participants, 7 involved both physicians and pharmacists, and 2 only included pharmacists.26,27 None of the studies included respondents who identified as genetic counselors.
Results of individual sources of evidence
Table 2 outlines the extracted data for each included study. The qualitative content analysis revealed five major methodological approaches: hypothetical clinical case scenarios, real-world studies evaluating prescriber response to recommendations or alerts, cross-sectional quantitative surveys, cross-sectional qualitative surveys/interviews, and a quasi-experimental real-world study. The following sections define each methodological approach, identify specific study aims, and briefly describe major study findings.
Hypothetical clinical case scenarios
Studies in this section engaged with a provider in an exercise that mimics some form of real-world clinical decision making. Of the ten studies included in this section, three themes were identified: information seeking, prescribing tasks, and other. The first of these themes, information seeking, includes three studies directly measuring the time to complete certain tasks involving pharmacogenomic information use.28,29,30 The specific tasks were (1) modifying the appropriate dosage for a hypothetical patient based on pharmacogenomic alert messages triggered by the medication ordered and patient-specific laboratory, (2) searching online resources for pharmacogenomic information (UpToDate® and PharmGKB) to guide hypothetical clinical decisions, and (3) identifying correct dosages using algorithm-produced interpretations of genetic tests during organ transplantation immunosuppressive treatment. These studies cut across physician specialties, from internists to cardiologists to oncologists, and included multiple disease states and/or pharmacogenes. The sample size was small for these studies, ranging from 6 to 10 physicians. The variability between the findings of each of the included studies was high given the inherent differences among clinical decision support systems (CDSS) and study tasks. One study used two scenarios to gauge improvements task completion times.30 Comparisons across studies were difficult to establish due to variability in information-seeking tasks.
The second theme, prescribing tasks, included three studies where the prescribers were presented with hypothetical scenarios and evaluated on the actions they might take with pharmacogenomic information.31,32,33 All studies in this section used quantitative methods. The sample size was larger for this theme compared to information seeking, ranging from 15 to over 200 physicians. The measured variables included the percentages of physicians who would change a decision or initial orders based on new pharmacogenomic information, response to or dismissal of CDSS messages, as well as prescriber-reported evaluations of whether the alerts or information were helpful to decision making. Overall, these providers agreed that the alerts were helpful and there was high utilization of the CDS resources.
The third theme, other, included four studies among three unique approaches.34,35,36,37 The first two focused on assessments of PGx-CDS usability, using both quantitative and qualitative assessments.34,35 The third evaluated psychiatrists’ use of irrelevant genomic information (average symptom change associated with a genetic biomarker when pre- and post-symptom scores were already given).36 Finally, a survey examined the intercountry differences as drivers of oncologist test ordering. Similar to the previous section, providers involved in the studies cut across specialties and quantitative outcomes were reported. However, the sample in one study included both physicians and pharmacists.34 Attitudes toward the CDSS (measured as the usability, trustworthiness, usefulness, and workflow integration subscale totals) were moderately positive (mean score: 42.3 of 64), younger psychiatrists were significantly more likely to use irrelevant genomic information than their colleagues with more than 15 years’ experience, and US oncologists opted for testing more than their German counterparts.
Prospective or retrospective real-world studies of prescribing/testing decisions
This section includes ten studies, seven of which were prospectively designed, two that used a retrospective chart review method, and one that used a quantitative follow-up survey. These studies were further divided into three themes: evaluation of prescriber actions based on pharmacist or pharmacist-led surveillance service recommendation,38,39,40,41 prospective or retrospective evaluation of prescriber decision making based on a CDSS alert42,43,44,45 or communication of pharmacogenetic test results, the means of which was not explicitly stated,46 and finally, physician opinion of their patients’ clinical status following pharmacogenomic testing.24
Among the four studies in the first theme evaluating pharmacist-led services, the primary aim was to ascertain the frequency with which prescribers accepted these recommendations and for what types of patients (i.e., phenotype) did this occur. Study sample size ranged widely from actions taken on 18 to 514 patients. Prescriber response to the pharmacist recommendation was separated by the metabolizer status of the patient in two of the studies.39,41 Acceptance of pharmacist recommendations overall was high in three of the studies (73%, 89%, and 100%).38,39,40 However, lower acceptance rates were observed in the fourth study when the pharmacist recommended therapeutic modification, reporting a therapy change in 57.6% of poor metabolizers and only 33.2% of intermediate metabolizers.41 The authors point to previous studies that conclude that cardiologists remain uncertain about the clinical significance of a CYP2C19 intermediate metabolizer, as well as the increased out-of-pocket patient cost when switching patients to ticagrelor or prasugrel.47,48
In the second theme, evaluating prescriber decision making, the outcome of interest was adherence to an internal CDSS or interpreted results and guidance from the testing lab/company. Two of the five studies in this theme produced outcomes from a retrospective chart review to evaluate adherence to testing.43,44 Interestingly, both studies used a “pretest CDSS alert,” meaning that rather than guiding the provider on prescribing, it informed them that testing is indicated prior to any initial dosing. Pretest alerts resulted in about 20% of recommended tests being ordered in one study44 and 90% of tests being ordered in the other study.43
The final study in this section stood on its own in terms of methodological approach. Focused solely on mental health, this study captured psychiatrist opinions regarding their patient’s status following pharmacogenomic testing. No physicians reported a worse outcome for their patients and 23% indicated an improvement.24
Cross-sectional quantitative surveys
The cross-sectional quantitative survey section included six studies that employ unique methods to measure response from providers involved in pharmacogenomics.25,26,47,49,50,51 Given the uniqueness of each study, no themes were developed here. Five studies had between 18 and 159 physician respondents.25,47,49,50,51 In the other study, the reader is informed of the number of participating pharmacies (n = 5) and the number of patients engaged by these pharmacists (n = 69).26
One study was based on an actual implementation project across a series of community pharmacies.26 This study captured pharmacists’ perspectives and experiences with delivering pharmacogenomics and their perceptions of the patients’ experiences. The survey was offered after the communication of CYP2C19 and SLCO1B1 test results. In a second study, a discrete choice experiment was conducted along with a randomized controlled trial involving azathioprine use.50 This study examined the tradeoffs health-care providers were willing to make across numerous variables including predictive ability of the test, wait time for results, and information provision. A third study evaluated both psychiatrists’ and general practitioners’ attitudes toward pharmacogenomic testing for antidepressant and antipsychotic medications at the time of a patient follow-up visit, 6–8 weeks after test results were received.25 The smallest of the studies surveyed several physician specialties and evaluated the percent of time results were accessed, the number of medication changes, and the reasons for not accessing results or changing medications.51 The last three studies were conducted in academic pharmacogenomic implementation programs. The fifth study aimed to uncover cardiology and noncardiology providers’ perceptions of who should be notified of pharmacogenomic results and who should be primarily responsible for managing the patient.47 The sixth study assessed the positive or negative aspects of a pharmacogenomic alert.49
The results of the discrete choice experiment study revealed several interesting tradeoffs patients and health care providers were willing to make for higher levels of information and predictive ability. Both patients and physicians were willing to wait 2 days for a 1% improvement in test accuracy. Yet, patients were more willing to wait 19 days to obtain more information compared to 8 days for health-care providers.50 In the study of cardiology and noncardiology providers, there was a strong agreement that results should be returned to both the specialist treating (90% vs. 90% agreement) and the original prescriber (80% vs. 96%), as well as agreement that the specialist should be responsible for acting on the result (80% vs. 74%).47
Cross-sectional qualitative research methods
Five of the six studies included in this section utilized interviews.22,27,48,52,53 The remaining study utilized three focus groups with clinicians.54 The number of interviews ranged from 15 to 94 individuals. One focused on mental health providers and elicited specific reasons for and against using test results.23 A second study targeted primary care physicians to understand the value and utility of pharmacogenomics, as well as its use to guide clinical decision making.55 The third study included both primary care and cardiology providers and utilized a thematic approach to measure the knowledge and attitudes of clinicians participating in a large pharmacogenomics implementation program.48 The fourth study was one of only two included studies in this review to focus solely on pharmacists as the respondent.27 The last study was part of a prospective, observational, feasibility study that included pre- and post-study evaluations with primary care providers.53
Each interview-based study identified between three and five themes, with several similarities. The first study’s themes were test ordering and result receiving experiences, result utilization, and perceived advantages and disadvantages. Mental health–care service clinicians acknowledged the advantages of utilizing the test results but described difficulties when ordering and receiving tests.23 The second study’s themes were perceived value and utility of pharmacogenomic testing, challenges to implementation in practice, and provider and patient needs. Providers recognized the benefits of pharmacogenomic testing but also discussed challenges such as privacy concerns, costs, insurance coverage, and test interpretation.55 The third study identified three theme categories: preparation and knowledge, pharmacogenomics usage in practice, and future management of genomic variants. Providers expressed an inability to stay updated on new evidence and conveyed support for clinical decision support.48 Results of particular interest include the noted advantages of test results for decision confirmation and reassurance. However, perceived disadvantages included using genomic results at the expense of clinical judgment and worries of handoffs to providers outside established implementation programs.23,48 The prospective feasibility study found that once primary care physicians (PCPs) used pharmacogenomic information, they realized better relationships with patients and medication management skills. Reactions to pharmacogenomics producing a competitive edge were mixed, and respondents desired more testing guidance and electronic health record (EHR) support.53 The focus group study used a thematic approach to the analysis, but was singularly focused on capturing mental health clinician reaction to a CDS prototype, features left to be desired, and potential concerns or unintended consequences.54
Quasi-experimental
Only one study used a quasi-experimental approach, a methodology involving the manipulation of one or more variables, but without random participants assignment.52 Framing the study as a pilot, the authors used a two-armed intervention trial to assess provider utilization of pharmacist support in pharmacogenetic testing. The study arms consisted of six physicians within a pharmacist-in-house clinic and seven physicians within a pharmacist on-call clinic. This study found that physicians in the pharmacist in-house arm ordered significantly more tests (48 of 63 total tests ordered) and consulted pharmacists at nearly twice the rate compared to the pharmacist on-call arm.
DISCUSSION
This scoping review examined the characteristics of 33 peer-reviewed studies, illuminating the prevailing methods utilized to examine pharmacogenomic use in practice. The decision to identify methods used in implementing pharmacogenomics was a reaction to the saturation of the literature with cross-sectional health-care provider awareness and attitude studies. A common thread in these awareness and attitude studies is that health-care providers find pharmacogenomics useful to patient care, but lack the knowledge to deliver it effectively.8,15,16,56,57
The studies identified in this scoping review demonstrate a continued focus on decision making within the EHR. Most of these studies employed hypothetical case-based scenarios designed to mimic real-world practice. These types of studies fit well at the intersection of the three genomic sustainability constructs outlined at the beginning of this review: clinician education, CDS tools, and workflow integration.10 The importance of well-designed CDSS has been a central focus among leading pharmacogenomic implementation programs. The Clinical Pharmacogenetics Implementation Consortium’s (CPIC) Informatics Working Group provides best practice suggestions for integrating CDSS with pharmacogenomics for clinical delivery.58 Other leaders in the field point to the importance and challenge of developing standardized results and identifying who should receive a CDSS alert.59,60 The feasibility of the studies in this section is driven by the translation of pharmacogenomic information into a discrete data field that can be called upon when applicable. EHRs without this functionality make it challenging for prescribers to use pharmacogenomic information efficiently.61 Given the variability between studies, future research on prescriber interactions with a pharmacogenomic CDSS should pursue comparative and longitudinal study designs to elucidate the most effective way to deliver this information. Additional use of hypothetical clinical case scenarios methodologies would be useful for furthering our understanding of optimal CDS for pharmacogenomics. Digital pilot engagement meant to mimic the EHR may enhance provider competency ahead of the complex system integrations and improve adoption.
These review findings also indicate a concerted effort from several ongoing implementation programs to understand who should be acting on pharmacogenomic information and how well these providers perform. This was typically achieved in one of three ways: measuring adherence or compliance to a pharmacist or CDSS-based dosing and therapy recommendation, measuring engagement with the CDSS and subsequent medication changes, or measuring test ordering rates based on a pretest alert prior to dosing determination. Future studies should consider following providers over time to assess adherence behavior changes. Clinicians’ limited exposure to pharmacogenomics has been previously noted in the literature.7,15,56 This unfamiliarity may contribute to a hesitancy to adopt these suggestions immediately. Supplementing these studies with qualitative assessments of recommendation adherence would strengthen these studies, help identify areas in need of interventions, and better understand which providers are optimally positioned to deliver pharmacogenomic information across specialty.
There is a continued need to communicate the value and validity of pharmacist or CDSS-based recommendations more broadly. Pharmacist leadership and involvement with crafting the delivery of pharmacogenomics in clinical care has been strong and this leadership role should continue in new practice settings.5,12,13,62 The individual institution’s infrastructure will most likely guide whether a CDS- or pharmacist-guided recommendation is most appropriate for delivering clinical pharmacogenomics. Future studies focused on a patient-facing role for pharmacists in pharmacogenomics should implement prospective, observational studies and experimental designs. Findings from these study designs may be more impactful to the clinical community and will enable more rapid implementation in real-world settings.
The feasibility of delivering pharmacogenomics through community pharmacists is an ongoing area of research.17,45,63 Two studies identified in this scoping review identified some of the key considerations to operationalize this approach, such as the time needed for a pharmacogenomic consult, perceived patient understanding, and the pharmacist’s ability to interpret information correctly.26,52 Both studies were conducted by the same group of researchers and in the same state, thus the findings’ generalizability may be limited. However, these studies provide a model for future researchers to replicate and establish broader validity. Pharmacist engagement in pharmacogenomics at both the patient level and implementation level has clear support from leading professional organizations, including the American Society for Health-System Pharmacists and the American Pharmacists Association.64,65 The growth of physician–pharmacist collaborative models, such as formal collaborative practice agreements, is a sustainable path to implementing pharmacogenomics services.66 Furthermore, additional research surrounding the integration of genetic counselors into the physician–pharmacist collaboration would enhance patient experience and should be experimentally explored.
Our scoping review identified numerous study designs that reported both drug–gene agnostic and drug–gene specific findings across a variety of provider types and specialties. Clinical implementation of pharmacogenomics is progressing such that critical appraisals of the best implementation approach can be compared. Future research focusing on the implementation strategy for the same drugs, genes, clinical service, or testing approach across different institutions would be of particular value. Prospectively designing these studies to capture provider experience or decision making would further our understanding of the best implementation strategies. While most studies from this review included only physicians, future research should assess pharmacists’ real-world experiences using pharmacogenomic information, making clinical decisions or suggestions based on this information, and their role in developing best practice. This will help achieve one of the research directions of Volpi et al.: to study the pharmacist as the “clinical champion” for pharmacogenomics.5
This review contains some limitations. First, many of the studies included were published from mature pharmacogenomic implementation programs, likely due to the nature of our research question and inclusion requirements. As a result of this, the majority of included studies were conducted in academic medical centers. While this reflects a gap in previous literature and is not a limitation of our study, more insights would be gained from additional research exploring these same ideas in community pharmacies and hospitals. Second, the nature of a scoping review does not allow for the synthesis of results across studies and thus this was not our aim. However, this review identified key features of the pharmacogenomics implementation literature that can help guide future research priorities.
In summary, this scoping review compiles recent studies assessing the experiences or actions of health-care professionals engaged in pharmacogenomic information use. Our findings illustrate a range of study designs from the actual use of pharmacogenomic information to hypothetical scenarios designed to mimic the real world. Prospective and retrospective real-world data collection and quasi-experimental designs were common, and these studies likely best demonstrate how pharmacogenomic data can be efficiently and effectively implemented into routine clinical decision making. Given the diversity of settings implementing pharmacogenomics, less resource-intensive study designs, such as longitudinal surveys or interviews, still provide value and may be more feasible in resource-constrained environments. Depending on the health-care setting, resources available, and research question, any of these methods, when appropriately designed, may be useful to further understand the use of pharmacogenomic information.
References
Keeling, N. J. et al. Preemptive pharmacogenetic testing: exploring the knowledge and perspectives of US payers. Genet. Med. 21, 1224–1232 (2019).
Arwood, M., Chumnumwat, S., Cavallari, L., Nutescu, E. & Duarte, J. Implementing pharmacogenomics at your institution: establishment and overcoming implementation challenges. Clin. Transl. Sci. 9, 233–245 (2016).
Relling, M. V. & Evans, W. E. Pharmacogenomics in the clinic. Nature. 526, 343–350 (2015).
Ginsburg, G. S. & Phillips, K. A. Precision medicine: from science to value. Health Aff. (Millwood). 37, 694–701 (2018).
Volpi, S. et al. Research directions in the clinical implementation of pharmacogenomics: an overview of US programs and projects. Clin. Pharmacol. Ther. 103, 778–786 (2018).
Owusu Obeng, A. et al. Physician-reported benefits and barriers to clinical implementation of genomic medicine: a multi-site IGNITE-Network survey. J. Pers. Med. https://doi.org/10.3390/jpm8030024 (2018).
Giri, J., Curry, T. B., Formea, C. M., Nicholson, W. T. & Rohrer Vitek, C. R. Education and knowledge in pharmacogenomics: still a challenge? Clin. Pharmacol. Ther. 103, 752–755 (2018).
Amara, N., Blouin-Bougie, J., Bouthillier, D. & Simard, J. On the readiness of physicians for pharmacogenomics testing: an empirical assessment. Pharmacogenomics J. 18, 308–318 (2018).
O’Connor, S. K., Michaels, N. & Ferreri, S. Expansion of pharmacogenomics into the community pharmacy: billing considerations. Pharmacogenomics. 16, 175–180 (2015).
Levy, K. D. et al. Opportunities to implement a sustainable genomic medicine program: lessons learned from the IGNITE Network. Genet. Med. 21, 743–747 (2019).
Rosenman, M. B. et al. Lessons learned when introducing pharmacogenomic panel testing into clinical practice. Value Health. 20, 54–59 (2017).
Crews, K. R. et al. Development and implementation of a pharmacist-managed clinical pharmacogenetics service. Am. J. Health Syst. Pharm. 68, 143–150 (2011).
Johnson, J. A. et al. Institutional profile: University of Florida and Shands Hospital Personalized Medicine Program: clinical implementation of pharmacogenetics. Pharmacogenomics. 14, 723–726 (2013).
Cavallari, L. H. et al. Implementation of inpatient models of pharmacogenetics programs. Am. J. Health Syst. Pharm. 73, 1944–1954 (2016).
Stanek, E. J. et al. Adoption of pharmacogenomic testing by US physicians: results of a nationwide survey. Clin. Pharmacol. Ther. 91, 450–458 (2012).
Tuteja, S. et al. Community pharmacists’ attitudes towards clinical utility and ethical implications of pharmacogenetic testing. Pers. Med. 10, 793–800 (2013).
Alexander, K. M., Divine, H. S., Hanna, C. R., Gokun, Y. & Freeman, P. R. Implementation of personalized medicine services in community pharmacies: perceptions of independent community pharmacists. J. Am. Pharm. Assoc. 54, 510–517 (2014). 5 p following 517.
Johansen Taber, K. A. & Dickinson, B. D. Pharmacogenomic knowledge gaps and educational resource needs among physicians in selected specialties. Pharmacogenomics Pers. Med. 7, 145–162 (2014).
Arksey, H. & O’Malley, L. Scoping studies: towards a methodological framework. Int. J. Soc. Res. Methodol. 8, 19–32 (2005).
Tricco, A. C. et al. A scoping review on the conduct and reporting of scoping reviews. BMC Med. Res. Methodol. https://doi.org/10.1186/s12874-016-0116-4 (2016).
Levac, D., Colquhoun, H. & O’Brien, K. K. Scoping studies: advancing the methodology. Implement. Sci. 5, 69 (2010).
Tricco, A. C. et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): checklist and explanation. Ann. Intern. Med. 169, 467–473 (2018).
Dunbar, L. et al. Clinician experiences of employing the AmpliChip® CYP450 test in routine psychiatric practice. J. Psychopharmacol. (Oxf). 26, 390–397 (2012).
Walden, L. M. et al. Genetic testing for CYP2D6 and CYP2C19 suggests improved outcome for antidepressant and antipsychotic medication. Psychiatry Res. 279, 111–115 (2019).
Walden, L. M. et al. Physicians’ opinions following pharmacogenetic testing for psychotropic medication. Psychiatry Res. 229, 913–918 (2015).
Moaddeb, J., Mills, R. & Haga, S. B. Community pharmacists’ experience with pharmacogenetic testing. J. Am. Pharm. Assoc. 55, 587–594 (2015).
van der Wouden, C. H. et al. Assessing the implementation of pharmacogenomic panel-testing in primary care in the Netherlands utilizing a theoretical framework. J. Clin. Med. https://doi.org/10.3390/jcm9030814 (2020).
Devine, E. B. et al. Usability evaluation of pharmacogenomics clinical decision support aids and clinical knowledge resources in a computerized provider order entry system: a mixed methods approach. Int. J. Med. Inf. 83, 473–483 (2014).
Heale, B. S. E., Khalifa, A., Stone, B. L., Nelson, S. & Del Fiol, G. Physicians’ pharmacogenomics information needs and seeking behavior: a study with case vignettes. BMC Med. Inform. Decis. Mak. 17, 113 (2017).
Lærum, H., Bremer, S., Bergan, S. & Grünfeld, T. A taste of individualized medicine: physicians’ reactions to automated genetic interpretations. J. Am. Med. Inform. Assoc. 21, 143–146 (2014).
Nishimura, A. A. et al. Physician perspectives of CYP2C19 and clopidogrel drug–gene interaction active clinical decision support alerts. Int. J. Med. Inf. 86, 117–125 (2016).
Overby, C. L., Devine, E. B., Abernethy, N., McCune, J. S. & Tarczy-Hornoch, P. Making pharmacogenomic-based prescribing alerts more effective: a scenario-based pilot study with physicians. J. Biomed. Inform. 55, 249–259 (2015).
Peppercorn, J., Hamilton, E., Marcom, P. K., Beskow, L. & Lyman, G. H. Pharmacogenetic testing in the face of unclear clinical efficacy: lessons from cytochrome P450 2D6 for tamoxifen. Cancer. 119, 3703–3709 (2013).
Blagec, K., Romagnoli, K. M., Boyce, R. D. & Samwald, M. Examining perceptions of the usefulness and usability of a mobile-based system for pharmacogenomics clinical decision support: a mixed methods study. PeerJ. 4, e1671 (2016).
Wegwarth, O., Day, R. W. & Gigerenzer, G. Decisions on pharmacogenomic tests in the USA and Germany. J. Eval. Clin. Pract. 17, 228–235 (2011).
McMichael, A. J. et al. The influence of genotype information on psychiatrists’ treatment recommendations: more experienced cinicians know better what to ignore. Value Health. 20, 126–131 (2017).
Nguyen, K. A. et al. Utilizing a user-centered approach to develop and assess pharmacogenomic clinical decision support for thiopurine methyltransferase. BMC Med. Inform. Decis. Mak. 19, 194 (2019).
Bain, K. T., Schwartz, E. J., Knowlton, O. V., Knowlton, C. H. & Turgeon, J. Implementation of a pharmacist-led pharmacogenomics service for the Program of All-Inclusive Care for the Elderly (PHARM-GENOME-PACE). J. Am. Pharm. Assoc. 58, 281–289.e1 (2018).
Ferreri, S. P. et al. Implementation of a pharmacogenomics service in a community pharmacy. J. Am. Pharm. Assoc. 54, 172–180 (2014).
Nutescu, E. A. et al. Feasibility of implementing a comprehensive warfarin pharmacogenetics service. Pharmacotherapy. 33, 1156–1164 (2013).
Peterson, J. F. et al. Physician response to implementation of genotype-tailored antiplatelet therapy. Clin. Pharmacol. Ther. 100, 67–74 (2016).
van der Wouden, C. H., Bank, P. C. D., Özokcu, K., Swen, J. J. & Guchelaar, H.-J. Pharmacist-initiated pre-emptive pharmacogenetic panel testing with clinical decision support in primary care: record of PGx results and real-world impact. Genes. https://doi.org/10.3390/genes10060416 (2019).
Manzi, S. F. et al. Creating a scalable clinical pharmacogenomics service with automated interpretation and medical record result integration—experience from a pediatric tertiary care facility. J. Am. Med. Inform. Assoc. 24, 74–80 (2017).
Ubanyionwu, S. et al. Evaluation of prescriber responses to pharmacogenomics clinical decision support for thiopurine S-methyltransferase testing. Am. J. Health Syst. Pharm. 75, 191–198 (2018).
Bank, P. C. D. et al. A pilot study of the implementation of pharmacogenomic pharmacist initiated pre-emptive testing in primary care. Eur. J. Hum. Genet. 27, 1532–1541 (2019).
Ielmini, M. et al. The utility of pharmacogenetic testing to support the treatment of bipolar disorder. Pharmacogenomics Pers. Med. 11, 35–42 (2018).
Peterson, J. F. et al. Attitudes of clinicians following large-scale pharmacogenomics implementation. Pharmacogenomics J. 16, 393–398 (2016).
Unertl, K. M., Jaffa, H., Field, J. R., Price, L. & Peterson, J. F. Clinician perspectives on using pharmacogenomics in clinical practice. Pers. Med. 12, 339–347 (2015).
St Sauver, J. L. et al. Integrating pharmacogenomics into clinical practice: promise vs reality. Am. J. Med. 129, 1093–1099 (2016).
Payne, K. et al. Valuing pharmacogenetic testing services: a comparison of patients’ and health care professionals’ preferences. Value Health. 14, 121–134 (2011).
Borden, B. A. et al. Assessment of provider-perceived barriers to clinical use of pharmacogenomics during participation in an institutional implementation study. Pharmacogenet. Genomics. 29, 31–38 (2019).
Haga, S. B. et al. Primary care providers’ use of pharmacist support for delivery of pharmacogenetic testing. Pharmacogenomics. 18, 359–367 (2017).
Dressler, L. G., Bell, G. C., Abernathy, P. M., Ruch, K. & Denslow, S. Implementing pharmacogenetic testing in rural primary care practices: a pilot feasibility study. Pharmacogenomics. 20, 433–446 (2019).
Goodspeed, A. et al. Leveraging the utility of pharmacogenomics in psychiatry through clinical decision support: a focus group study. Ann. Gen. Psychiatry. https://doi.org/10.1186/s12991-019-0237-3 (2019).
Lemke, A. A. et al. Primary care physician experiences with integrated pharmacogenomic testing in a community health system. Pers. Med. 14, 389–400 (2017).
Haga, S. B., Burke, W., Ginsburg, G. S., Mills, R. & Agans, R. Primary care physicians’ knowledge of and experience with pharmacogenetic testing. Clin Genet. 82, 388–394 (2012).
McCullough, K. B. et al. Assessment of the pharmacogenomics educational needs of pharmacists. Am. J. Pharm. Educ. 75, 51 (2011).
Hoffman, J. M. et al. Developing knowledge resources to support precision medicine: principles from the Clinical Pharmacogenetics Implementation Consortium (CPIC). J. Am. Med. Inform. Assoc. 23, 796–801 (2016).
Caudle, K. E. et al. Standardization can accelerate the adoption of pharmacogenomics: current status and the path forward. Pharmacogenomics. 19, 847–860 (2018).
Freimuth, R. R. et al. Implementing genomic clinical decision support for drug-based precision medicine. CPT Pharmacomet Syst Pharmacol. 6, 153–155 (2017).
Hicks, J. K., Dunnenberger, H. M., Gumpper, K. F., Haidar, C. E. & Hoffman, J. M. Integrating pharmacogenomics into electronic health records with clinical decision support. Am. J. Health Syst. Pharm. 73, 1967–1976 (2016).
Owusu-Obeng, A. et al. Emerging roles for pharmacists in clinical implementation of pharmacogenomics. Pharmacotherapy. 34, 1102–1112 (2014).
Suppiah, V., Lim, C. X. & Hotham, E. Community pharmacists and their role in pharmacogenomics testing: an Australian perspective drawing on international evidence. Aust. J. Prim. Health. 24, 441–447 (2018).
ASHP statement on the pharmacist’s role in clinical pharmacogenomics. Am. J. Health Syst. Pharm. https://doi.org/10.2146/sp150003 (2015).
Wiisanen Weitzel, K. Pharmacists advancing role in pharmacogenomics. J. Am. Pharm. Assoc. 58, 593–595 (2015).
Center for Disease Control and Prevention. Advancing Team-Based Care Through Collaborative Practice Agreements: A Resource and Implementation Guide for Adding Pharmacists to the Care Team. (Atlanta, GA, Department of Health and Human Services, 2017).
Acknowledgements
J.M.H.’s work on this paper was funded by the National Institutes of Health (NIH) for CPIC (R24GM115264; U24HG010135) and the American Lebanese Syrian Associated Charities (ALSAC). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Keeling, N.J., Dunn, T.J., Bentley, J.P. et al. Approaches to assessing the provider experience with clinical pharmacogenomic information: a scoping review. Genet Med 23, 1589–1603 (2021). https://doi.org/10.1038/s41436-021-01186-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41436-021-01186-x