Abstract
Background
To assess the long-term visual outcomes in patients with posteriorly located choroidal melanoma treated with ruthenium plaque brachytherapy between January 2013 and December 2015.
Methods
A retrospective review was conducted on consecutive patients treated with ruthenium plaque brachytherapy for post-equatorial choroidal melanoma with available Snellen visual acuity before and after treatment, and the development and treatment of radiation complications.
Results
There were 219 patients with posterior choroidal melanoma treated with ruthenium plaque brachytherapy. Median follow up was 56.5 months, range 12–81 months. Final visual acuity was ≥6/12 in 97 (44.3%) patients, 6/12 to 6/60 in 57 (26.0%), <6/60 in 55 (25.1%) and 10 (4.6%) eyes were enucleated. Radiation maculopathy was the most common radiation complication encountered, occurring in 53 (24.2%) patients. Of these, final visual acuity was 6/12 in 10 patients (18.9%), 6/12 to 6/60 in 26 (49.1%), <6/60 in 16 (30.2%) and 1 eye (1.9%) was enucleated. Twenty-five (47%) with radiation maculopathy were treated with intravitreal anti-angiogenic therapy, 27 (51%) were monitored and one (2%) was treated with scatter photocoagulation. Eyes treated with intravitreal anti-angiogenic therapy had better final vision than those observed or treated with retinal laser (chi-square, p = 0.04). On multivariate analysis, close proximity to the optic nerve and fovea, and large or notched plaque type was associated with final vision worse than 6/12.
Conclusion
Most patients treated with ruthenium plaque brachytherapy for posterior choroidal melanoma retain 6/60 vision, with almost half retaining 6/12 vision at long term follow up.
Similar content being viewed by others
Introduction
In the management of medium-sized choroidal melanomas, studies have demonstrated no survival advantage of enucleation over plaque brachytherapy [1, 2]. The most commonly used radioisotopes for plaque brachytherapy are iodine-125 and ruthenium-106, which emit gamma and beta radiation, respectively. Ruthenium plaques, which have a more rapid isodose fall off than iodine, have been found to cause less radiation-related side effects while achieving similar rates of local control and patient survival in tumours that are approximately 6 mm or less thick [3,4,5,6,7].
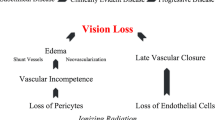
Vision preservation after plaque brachytherapy is mostly determined by certain tumour characteristics: thick or wide tumours that are close to the macula or optic nerve require high radiation doses to these structures and have worse vision outcomes [6, 8,9,10]. Anterior tumours, particularly those involving the iris and ciliary body, tend to have better vision outcomes and were excluded from this study. Patient factors, such as pre-treatment vision [6, 9, 11], older age [6] and diabetes [8], and other tumour characteristics, such as the presence of tumour-related exudative retinal detachment [8] and breach of Bruch’s membrane [8], have also been associated with worse visual outcomes. Vision is lost due to radiation effects on the tumour: i.e., toxic tumour syndrome, or on healthy ocular structures: i.e., cataract, radiation macular oedema, radiation optic neuropathy, retinal ischaemia, neovascular glaucoma and vitreous haemorrhage [12]. Over the past 15 years, significant advances have been made in the management of these complications. For example, intravitreal anti-angiogenic therapy is used routinely for radiation maculopathy and in some patients who develop radiation optic neuropathy [13, 14]. Similarly, toxic tumour syndrome is now recognised as a distinct clinical entity and it can respond to transpupillary thermotherapy or endoresection [15]. In addition, efforts are made to optimise the radiation prescription to minimise the development of radiation side-effects. Plaques can be positioned eccentrically, with a small posterior margin, for tumours close to the optic nerve or macula [16]. Similarly, administering radiation at a slower dose rate and lower apical dose may minimise the rate of radiation maculopathy and optic neuropathy [17,18,19].
The main aim of this study was to document the visual outcomes after ruthenium plaque brachytherapy for posterior choroidal melanomas in our centre during a time period when contemporary measures to minimize the development of, and effectively treat, radiation-related side effects have been in use.
Materials and methods
Patients and data collection
This was a retrospective cohort study of consecutive patients with posterior choroidal melanomas treated with ruthenium plaque brachytherapy at the London Ocular Oncology Service between January 2013 and December 2015. Patients were excluded if: (1) the tumour had previously been treated; (2) the tumour involved the iris or ciliary body; (3) the posterior edge of the tumour was anterior to the equator as determined by review of widefield colour photography (Optos California; Optos plc, Dunfermline, Scotland); and (4) the patient was lost to follow up in the first 12 months after treatment. The study adhered to the tenets of the Declaration of Helsinki and was conducted after obtaining approval from the Audit Committee of Moorfields Eye Hospital Clinical Audit Department (Number: 497). Patient medical records and imaging studies (B scan ultrasonography, optos widefield colour and fundus autofluorescence photography and optical coherence tomography (OCT) (Heidelberg Spectralis, Heidelberg Engineering, GmBH, Heidelberg, Germany)) were reviewed to determine baseline patient, tumour and treatment characteristics and clinical outcomes.
Ruthenium plaque protocol
A standard surgical technique for ruthenium plaque brachytherapy was employed, similar to that described by Damato et al. [16] In brief, circular 12, 15, or 20 mm or notched 20 mm ruthenium plaques were sutured to bare sclera overlying a choroidal melanoma whose location was identified by transpupillary or transscleral transillumination. The plaque size and location of insertion were selected so that the plaque overlapped the tumour margins by at least 2 mm, unless close to the optic nerve head or fovea, when the plaque positioning was offset with a smaller posterior margin of 1 mm. A dose of 80, 100, or 120 Gy was prescribed, with an over or under-prescription of up to 10% (usually 5% or less) allowed. Both insertion and removal were typically under general anaesthesia; however, surgery was performed with a regional anaesthesia and sedation as required due to medical comorbidities. The standard follow-up protocol for patients treated with ruthenium plaque brachytherapy was: 3–4 monthly in the first year, 6-monthly in the second year and annually after that unless tumour control or treatment complications dictated otherwise.
Outcome measures
Best-correct visual acuity (BCVA) was measured using a Snellen chart with pinhole correction if required. Baseline vision was defined as the BCVA at the clinic review immediately prior to plaque brachytherapy. Vision was then collected at yearly intervals, with the BCVA at the clinic review closest to desired timepoint used. If no clinic review occurred within 6 months of the desired timepoint, no vision outcome was recorded for that year. Final vision was the BCVA at the last recorded follow up appointment. Demographic data such as age, sex and medical history were collected from chart review, as were data pertaining to tumour features, radiotherapy data and local tumour control. A tumour was defined as juxtapapillary if its posterior margin was within 2 disc diameters from the optic nerve, macular if its posterior margins was within the superotemporal and inferotemporal retinal vascular arcades, superior if its posterior margin and the majority of the tumour was above the superonasal and superotemporal vascular arcades, nasal if its posterior margin and the majority of the tumour was within the superonasal and inferonasal retinal vascular arcades, inferior if its posterior margin and the majority of the tumour was below the inferonasal and inferotemporal retinal vascular arcades and temporal if its posterior margin and the majority of the tumour was outside the macula and within the inferotemporal and superotmeporal arcade. Treatment failure was defined as an eye which had progressive tumour growth after treatment or local tumour recurrence at any timepoint.
Data on radiation complications and their treatment were collected from chart and imaging review, and included radiation optic neuropathy (new optic nerve swelling or pallor), radiation macular oedema (new cystoid swelling clinically or on OCT macula), ischaemic retinopathy (the presence of retinal neovascularisation), macular atrophy (foveal retinal thinning in the absence of prior macular oedema), cataract, toxic tumour syndrome (exudate surrounding the margin of the tumour with or without macular oedema), vitreous haemorrhage and neovascular glaucoma (intraocular pressure >21 mmHg with rubeosis).
Techniques used to maximise vision outcomes
A number of techniques are used to maximise vision outcomes of patients at our centre. Ruthenium is the only radioisotope used as it has a more favourable side-effect profile than iodine or palladium, with similar rates of local tumour control for tumours up to approximately 6 mm in thickness [3,4,5, 20]. For tumours thicker than this, proton beam radiotherapy is used. For tumours close to the optic nerve or macula, plaques are placed eccentrically with a targeted posterior margin of 1 mm, to minimize the radiation dose to these structures [21]. Radiation dosing is adjusted based on thickness of the tumour and proximity to the optic nerve and macula: thin tumours, close to these structures are treated with 80 Gy to the apex, instead of our standard dosing of 100 Gy to the apex.
Patients are not treated prophylactically to prevent radiation complications, but rather these are treated when they develop. Intavitreal anti-vascular endothelial growth factor inhibitor (anti-VEGF) injections, using bevacizumab, is the treatment most commonly used for radiation macular oedema at our centre [14, 22, 23]. Our protocol is to conduct an analysis of the foveal avascular zone by fluorescein or OCT-angiography. Treatment is offered in the absence of widespread disruption of perifoveal vascular flow. A course of 3 injections is given and then an assessment based on visual acuity and OCT response, in a treat and extend manner. Treatment for radiation optic neuropathy, similarly with intravitreal bevacizumab, is offered if the optic nerve was swollen and the patient has a symptomatic loss of vision [13, 24]. Proliferative retinopathy, characterized by retinal neovascularisation or vitreous haemorrhage, is treated with scatter argon laser photocoagulation if the view allows, or a vitrectomy for non-clearing vitreous haemorrhage if there is no suspicion of treatment failure and good visual prognosis [25]. Toxic tumour syndrome is treated with intravitreal bevacizumab injections and/or TTT to the tumour surface [12]. Cataract surgery is offered for visually significant cataracts in patients with good visual prognosis once tumour regression has been demonstrated, typically at least one year after ruthenium plaque brachytherapy. Neovascular glaucoma is treated conservatively in most patients as, once established, visual prognosis tends to be poor.
Data analysis
The primary outcome measure was the proportion of eyes with BCVA 6/12 or better, between 6/12 and 6/60, worse than 6/60 and enucleated at 1, 2, 3, 4, 5 years and at the final visit. Descriptive statistics were performed on the baseline characteristics, vision outcomes, radiation treatment, radiation complications and local tumour control. Differences between the groups that achieved a final BCVA of 6/12 or better and those that did not were assessed by univariate and multivariate analyses. For multivariate analysis, a binary logistic regression model was applied. The dependent variable was achieving a final BCVA of 6/12 or not. The independent variables were the collected baseline patient, tumour and radiation treatment characteristics that had a p < 0.10 on univariate analyses. Data were presented as the mean ± standard deviation (SD) when normally distributed or as median [interquartile range, range] (IQR) if not. Normality was assessed using the Shapiro-Wilks test. Differences in continuous variables between two groups were compared using a Student’s t-test or the Mann-Whitney U test for normally and not normally distributed data, respectively. Differences in proportions between two and multiple groups were analysed using Fisher’s exact and Chi-square test, respectively. A P-value of <0.05 was considered statistically significant. All data were analysed using a commercially available software package (SPSS® 27; IBM Corporation, Armonk, NY, USA).
Results
Between January 2013 and December 2015, 351 patients were treated with ruthenium plaque brachytherapy. Of these, 219 were included in this study. Patients were excluded for the following reasons: iris or ciliary body involvement (N = 42), posterior border anterior to the equator (N = 11), prior treatment for their choroidal melanoma (N = 13), less than 12 months follow up after plaque brachytherapy (N = 15), treatment for conditions other than choroidal melanoma (N = 30) and insufficient data on our electronic medical record to allow analysis (N = 21).
Baseline characteristics
The median age at diagnosis was 64.0 years [IQR 54.0–72.0, range 21–91]. Ninety-six of 219 (43.8%) patients were female. Twenty-seven of 219 (12.2%) had diabetes, with 79 of 219 (36.1%) having hypercholesterolaemia and 47 of 219 (21.5%) smoking tobacco. The baseline tumour, vision, and radiation treatment characteristics are presented in Table 1. Eight patients had a tumour thickness of more than 6 mm, considered the upper limit suitable for ruthenium plaque brachytherapy [26].
Treatment outcomes
The median follow-up time was 56.5 months [IQR 49.0–66.3, range 12–81]. BCVA at the latest recorded visit was ≥ 6/12 in 97 (44.3%) patients, 6/12 to 6/60 in 57 (26.0%), < 6/60 in 55 (25.1%) and 10 (4.6%) eyes were enucleated. Of the enucleated eyes, 8 were for lack of tumour control, 1 was for each neovascular glaucoma and non-clearing vitreous haemorrhage that precluded adequate assessment of tumour response. Vision outcomes were available for 213, 193, 179, 164, and 132 patients at 1, 2-, 3-, 4- and 5-years post-treatment, respectively. Vision outcomes at various timepoints are presented in Table 2. Treatment failure occurred in 34 (15.5%) patients by the end of the study. Treatment failure occurred in 2 of 8 (25%) patients with a tumour thickness > 6 mm and 30 of 185 (16%) of those ≤ 6 mm. This difference was not significant, p = 0.62. Eleven treatment failures occurred in the first year after ruthenium plaque brachytherapy, 11 in the second year, 6 in the third year, 5 in the fourth year and 1 after the fourth year.
Radiation complications
Radiation complications, their treatment and final BCVA are presented in Table 3. The most common radiation complication was the development of radiation macular oedema, which occurred in 53 of 219 patients (24.2%). Of these, final BCVA was ≥6/12 in 10 patients (18.9%), 6/12 to 6/60 in 26 (49.1%), <6/60 in 16 (30.2%) and 1 eye (1.9%) was enucleated. Twenty-seven patients with radiation macular oedema were observed, 25 were treated with intravitreal anti-VEGF injections and one was treated with scatter retinal photocoagulation. Of eyes treated with intravitreal anti-VEGF injections, final BCVA was ≥6/12 in 4 patients (16%), 6/12 to 6/60 in 18 (72%) and <6/60 in 3 (12%). Eyes that were treated with intravitreal anti-VEGF injections had significantly better final BCVA than those observed or treated with scatter laser (chi-square, p = 0.04). Data regarding the number of anti-VEGF injections given was available for 22 or 25 patients. A median of 3 [IQR 3.0 to 6.0, range 1–12] intravitreal anti-VEGF injections were provided. All but one patient was treated with intravitreal bevacizumab who received intravitreal bevacizumab initially and then intravitreal aflibercept after a recurrence of oedema.
Patients who developed toxic tumour syndrome, radiation optic neuropathy, macular atrophy, vitreous haemorrhage and neovascular glaucoma had poor vision outcomes. In each of these groups, more than 50% of patients had final BCVA worse than 6/60. More than 70% of patients who developed macular atrophy and neovascular glaucoma had final BCVA worse than 6/60.
Baseline predictors of vision of 6/12 or better
On univariate analysis, juxtapapillary or macular tumour locations, greater tumour thickness, less distance to optic nerve or fovea, presence of lipofuscin on autofluorescence, exudative retinal detachment, worse baseline vision, slower delivery of radiation and use of a 20 mm circle or notched plaque were all significantly less likely to achieve a final BCVA of ≥6/12. (Table 4) Importantly, eyes that achieved a final BCVA of ≥6/12 had a median distance of 5.0 mm or more to the optic nerve and fovea. On multivariate analysis, correcting for interactions between the baseline characteristics, only tumour proximity to nerve and macula and plaque type used remained significant predictors of final BCVA worse than 6/12 (Table 5).
Discussion
Main findings
The main finding of this study was that most patients treated with ruthenium plaque brachytherapy for posterior choroidal melanoma maintained 6/60 vision. In our study, 44.3% of patients retained vision of 6/12 or better and 70.3% achieved a vision of 6/60 or better at a median of 56.5 months follow up. Distance to the optic nerve and fovea were the most important predictors of final vision, with a distance of 5.0 mm or more from these structures portending good visual outcomes. The rate of local tumour control achieved was comparable to other studies.
Included patients
The tumours included in this study were small and medium-sized choroidal melanomas according to the Collaborative Ocular Melanoma Study Group classification [27]. The median thickness was 3.0 mm and the tumours were posteriorly located, with a median distance of the posterior border to the optic nerve head of 3.9 mm and to the fovea of 3.0 mm. Only posteriorly located tumours were included in this study because their treatment generates the most controversy. Some centres opt to preferentially treat these tumours with proton beam or stereotactic radiosurgery because it requires less surgical precision than plaque brachytherapy and potentially higher rates of local tumour control [28, 29]. At our centre, proton beam radiotherapy is generally used for larger tumours and those in a juxtapapillary location where plaque brachytherapy cannot be performed due to tumour configuration, so we cannot directly compare the outcomes of our patients treated with protons and ruthenium plaques. There is limited comparative evidence to help clinicians choose between the different radiotherapy modalities. This series may serve as a useful, contemporary comparator for other groups who treat similar lesions with other forms of radiotherapy.
We included all primarily treated posterior uveal melanomas, with no exclusion based on thickness of the tumour. In certain circumstances, typically a strong patient preference, patients whose tumours are more than 6 mm thick will be treated with ruthenium plaque brachytherapy. The maximum thickness treated in the period of this study was 7.2 mm. The rate of treatment failure was not significantly different for tumours greater than 6 mm than those less than 6 mm. However, caution should be used in interpreting this as large tumour size is a well recognised cause for local treatment failure after ruthenium plaque brachytherapy [30].
Vision outcomes compared to other studies
Compared to previous studies, a higher proportion of our patients achieved mid-level vision (6/12 to 6/60) and less had poor vision (worse than 6/60), although comparing retrospective studies is fraught. At baseline, 77% of the patients had BCVA of 6/12 or better. This is similar to previous studies [6, 11, 31, 32]. Vision gradually declined with time from treatment. Despite this, at the latest visit, over 70% of patients had 6/60 vision or better. The most comparable large study to our is by Bergmann et al. [11] That study included 579 patients with choroidal melanoma treated in Sweden with ruthenium plaque brachytherapy between 1979 and 2003. The included tumours were similar in thickness and slightly more posterior than those in our study, distances of 2.0 mm and 3.0 mm to the fovea optic nerve head, respectively. At 5 years, 31% retained 6/12 vision and 49% 6/60 or better. This compares to 39% and 66% in our cohort at the same time points. The main difference was that more patients in our cohort retained a vision of 6/12 to 6/60.
Radiation complications
The radiation complications experienced by our patients were similar to other studies. Some complications had very high rates of visual loss: patients who developed toxic tumour syndrome, radiation optic neuropathy, macular atrophy, vitreous haemorrhage and neovascular glaucoma. In each of these groups, more than 50% of patients had final BCVA worse than 6/60.
The most commonly encountered radiation complication was radiation macular oedema, occurring in 24.2% of patients. The treatment of radiation macular oedema is rapidly evolving since described by Finger in 2011 [33]. Recent studies have shown that prophylactic intravitreal anti-VEGF after iodine brachytherapy and proton beam radiotherapy for uveal melanoma improves vision and reduces the incidence of macular oedema [34, 35]. At our centre, we treat when a patient becomes symptomatic rather than prophylactically, in part because the rates of radiation macular oedema are lower with ruthenium than iodine brachytherapy. Recently, the first prospective trials of intravitreal anti-VEGF therapy for radiation macular oedema after plaque brachytherapy have been published [22, 36, 37]. Our cohort received less intravitreal anti-VEGF injections than the prospective studies, with a median of 3 injections. The largest prospective study with 37 patients, by Schefler et al, achieved a BCVA of 6/12 or better in 30% and 6/60 or better in 83% at 1 year with the best outcome in the group receiving monthly injections. By comparison 16% and 88% of the patients treated with intravitreal anti-VEGF injections for radiation macular oedema in this present study achieved 6/12 or 6/60 or better vision, respectively. Achieving 6/60 or better therefore seems to be possible either with regular dosing or with a treat and extend regimen. The rate of achieving 6/12 or better vision in this study is lower than Finger’s long-term and large cohort study in which 65% achieved this vision level with a mean post-treatment follow up of 6.5 years [14]. In that series, patients who developed symptomatic radiation maculopathy after predominantly palladium plaque brachytherapy, were treated with regular intravitreal bevacizumab injections [14]. Our patients were likely treated less frequently and had more severe macular oedema than those in Finger’s cohort and this may account for the difference in outcomes.
Predicting vision outcomes from baseline characteristics
The strongest relationship between a baseline characteristic and vision outcomes was distance to the fovea; plaque type and distance to the optic nerve were also significant in the logistic regression model, which corrects for interactions between the baseline characteristics. Proximity to the optic nerve and macula are regularly found to predict visual outcomes in patients treated with ruthenium plaque brachytherapy [6, 11, 32]. In our cohort, better vision was found in patients treated with smaller plaques. This is similar to the recent findings of Jouhi et al who compared vision outcomes in patients with tumours less than 10 mm in LBD treated with 10 mm and 15 mm plaques [38]. Despite the tumours being located closer to the fovea, patients treated with 10 mm plaques had better vision outcomes and same rates of local tumour control those treated with 15 mm plaques in Jouhi et al. cohort of 164 patients treated between 1998 and 2014 in Finland [38]. Our findings corroborate theirs. Other baseline characteristics that are variably found to be linked to vision outcomes, such as radiation dose rate [19], tumour thickness [11, 31], and LBD [31] were not significant predictors in our cohort.
Local tumour control
The local failure rate found in this study of 15.5% at a mean follow up 56.5 months. This is comparable to other studies of ruthenium plaque brachytherapy for choroidal melanoma with this length of follow up [11, 30,31,32]. The rate of tumour control achieved by Damato et al in Liverpool, with 3% failure rate at 7 years, has not been replicated elsewhere and is the high-water mark reported in the literature [16]. Achieving a balance between tumour control and minimizing ocular side effects is complex, but continued research is necessary to improve outcomes. This study is useful as a benchmarking tool.
Clinical and research implications
This large cohort study from a tertiary referral centre provides real-world, contemporary treatment outcomes. It corroborates recent evidence that the use of smaller plaques likely results in better vision outcomes, and they should be chosen when doing so will not alter rates of local treatment failure.
Many research questions remain. In particular, the best treatment algorithm for radiation macular oedema remains unanswered. An ongoing prospective study of a treat and extend protocol for radiation maculopathy will hopefully provide vital data on this. The role of prophylactic anti-angiogenic therapy in patients treated with ruthenium plaque brachytherapy should also be explored.
There are a number of weaknesses to mention. This is a retrospective study with the associated biases. In particular, no standard algorithm for the grading or management of radiation macular oedema was employed, including in whom to initiate treatment with intravitreal anti-VEGF injections or the regimen followed once begun. However, the value of this real-world data is primarily for use in patient counselling and as a comparator for future studies.
Conclusions
Most patients treated with ruthenium plaque brachytherapy for posterior choroidal melanomas retain 6/60 or better vision with approximately half retaining 6/12 or better vision. Ongoing research is needed to identify effective methods of preventing the development of complications associated with very poor vision outcomes and to optimise outcomes in those with reversible complications.
Summary
What was known before
-
Ruthenium plaque brachytherapy for post-equatorial choroidal melanoma achieves good local tumour control, but loss of vision due to radiation side effects is common
-
Modern treatments, including intravitreal anti-VEGF injections, can improve vision outcomes in some patients
What this study adds
-
Good long-term vision can be achieved in the real-world setting at a busy tertiary ocular oncology centre - with 44% of patients having a final vision of 6/12 or better at a median follow up of 56 months
-
Patients treated with smaller plaques, independent of tumour size or location, achieved better vision and this should be considered if it will not impair local tumour control
References
Diener-West M, Earle JD, Fine SL, Hawkins BS, Moy CS, Reynolds SM, et al. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma, III: Initial mortality findings. COMS Report No. 18. Arch Ophthalmol Chic Ill 1960. 2001;119:969–82.
Collaborative Ocular Melanoma Study Group. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma: V. Twelve-year mortality rates and prognostic factors: COMS report No. 28. Arch Ophthalmol Chic Ill 1960. 2006;124:1684–93.
Takiar V, Voong KR, Gombos DS, Mourtada F, Rechner LA, Lawyer AA, et al. A choice of radionuclide: Comparative outcomes and toxicity of ruthenium-106 and iodine-125 in the definitive treatment of uveal melanoma. Pract Radiat Oncol. 2015;5:e169–176.
Wilkinson DA, Kolar M, Fleming PA, Singh AD. Dosimetric comparison of 106Ru and 125I plaques for treatment of shallow (<or=5 mm) choroidal melanoma lesions. Br J Radiol. 2008;81:784–9.
Filì M, Trocme E, Bergman L, See TRO, André H, Bartuma K, et al. Ruthenium-106 versus iodine-125 plaque brachytherapy of 571 choroidal melanomas with a thickness of ≥5.5 mm. Br. J. Ophthalmol. 2020;104:26–32.
Damato B, Patel I, Campbell IR, Mayles HM, Errington RD. Visual acuity after Ruthenium(106) brachytherapy of choroidal melanomas. Int J Radiat Oncol Biol Phys. 2005;63:392–400.
Browne AW, Dandapani SV, Jennelle R, Stevanovic M, Lee TC, Murphree AL, et al. Outcomes of medium choroidal melanomas treated with ruthenium brachytherapy guided by three-dimensional pretreatment modeling. Brachytherapy. 2015;14:718–25.
Melia BM, Abramson DH, Albert DM, Boldt HC, Earle JD, Hanson WF, et al. Collaborative ocular melanoma study (COMS) randomized trial of I-125 brachytherapy for medium choroidal melanoma. I. Visual acuity after 3 years COMS report no. 16. Ophthalmology. 2001;108:348–66.
Espensen CA, Appelt AL, Fog LS, Gothelf AB, Thariat J, Kiilgaard JF. Predicting visual acuity deterioration and radiation-induced toxicities after Brachytherapy for choroidal melanomas. Cancers (Basel). 2019;11:1124.
Sagoo MS, Shields CL, Emrich J, Mashayekhi A, Komarnicky L, Shields JA. Plaque radiotherapy for juxtapapillary choroidal melanoma: treatment complications and visual outcomes in 650 consecutive cases. JAMA Ophthalmol. 2014;132:697–702.
Bergman L, Nilsson B, Lundell G, Lundell M, Seregard S. Ruthenium brachytherapy for uveal melanoma, 1979-2003: survival and functional outcomes in the Swedish population. Ophthalmology. 2005;112:834–40.
Groenewald C, Konstantinidis L, Damato B. Effects of radiotherapy on uveal melanomas and adjacent tissues. Eye. 2013;27:163–71.
Finger PT, Chin KJ. Antivascular endothelial growth factor Bevacizumab for radiation optic neuropathy: Secondary to plaque radiotherapy. Int J Radiat Oncol. 2012;82:789–98.
Finger PT, Chin KJ, Semenova EA. Intravitreal Anti-VEGF therapy for macular radiation retinopathy: A 10-year study. Eur J Ophthalmol. 2016;26:60–6.
Damato B. Progress in the management of patients with uveal melanoma. The 2012 Ashton Lecture. Eye Lond Engl. 2012;26:1157–72.
Damato B, Patel I, Campbell IR, Mayles HM, Errington RD. Local tumor control after 106Ru brachytherapy of choroidal melanoma. Int J Radiat Oncol Biol Phys. 2005;63:385–91.
Naseripour M, Jaberi R, Sedaghat A, Azma Z, Nojomi M, Falavarjani KG, et al. Ruthenium-106 brachytherapy for thick uveal melanoma: Reappraisal of apex and base dose radiation and dose rate. J Contemp Brachyther. 2016;8:66–73.
Mossböck G, Rauscher T, Winkler P, Kapp KS, Langmann G. Impact of dose rate on clinical course in uveal melanoma after brachytherapy with ruthenium-106. Strahlenther Onkol Organ Dtsch Rontgengesellschaft Al. 2007;183:571–5.
Jones R, Gore E, Mieler W, Murray K, Gillin M, Albano K, et al. Posttreatment visual acuity in patients treated with episcleral plaque therapy for choroidal melanomas: Dose and dose rate effects. Int J Radiat Oncol Biol Phys. 2002;52:989–95.
Danish H, Ferris MJ, Balagamwala E, Switchenko JM, Patel KR, Choudhary M, et al. Comparative outcomes and toxicities for ruthenium-106 versus palladium-103 in the treatment of choroidal melanoma. Melanoma Res. 2018;28:120–5.
Russo A, Laguardia M, Damato B. Eccentric ruthenium plaque radiotherapy of posterior choroidal melanoma. Graefes Arch Clin Exp Ophthalmol Albrecht Von Graefes Arch Klin Exp Ophthalmol. 2012;250:1533–40.
Fallico M, Reibaldi M, Avitabile T, Longo A, Bonfiglio V, Chronopoulos A, et al.Intravitreal aflibercept for the treatment of radiation-induced macular edema after ruthenium 106 plaque radiotherapy for choroidal melanoma.Graefes Arch Clin Exp Ophthmol Albrecht Von Graefes Arch Klin Exp Ophthalmol. 2019;257:1547–54.
Gupta A, Muecke JS. Treatment of radiation maculopathy with intravitreal injection of bevacizumab (Avastin). Retin Phila Pa. 2008;28:964–8.
Roelofs K, Larocque MP, Murtha A, Weis E. The Use of Intravitreal Anti-VEGF and Triamcinolone in the Treatment of Radiation Papillopathy. Ocul Oncol Pathol. 2018;4:395–400.
Bianciotto C, Shields CL, Pirondini C, Mashayekhi A, Furuta M, Shields JA. Proliferative radiation retinopathy after plaque radiotherapy for uveal melanoma. Ophthalmology. 2010;117:1005–12.
American Brachytherapy Society - Ophthalmic Oncology Task Force. Electronic address: paulfinger@eyecancer.com, ABS – OOTF Committee. The American Brachytherapy Society consensus guidelines for plaque brachytherapy of uveal melanoma and retinoblastoma. Brachytherapy. 2014;13:1–14.
Anon. Factors predictive of growth and treatment of small choroidal melanoma: COMS Report No. 5. The Collaborative Ocular Melanoma Study Group. Arch Ophthalmol Chic Ill 1960. 1997;115:1537–44.
Damato B, Kacperek A, Errington D, Heimann H. Proton beam radiotherapy of uveal melanoma. J Saudi Ophthalmol Soc. 2013;27:151–7.
Barker CA, Francis JH, Cohen GN, Marr BP, Wolden SL, McCormick B, et al. Ru plaque brachytherapy for uveal melanoma: Factors associated with local tumor recurrence.Brachytherapy. 2014;13:584–90.
Karimi S, Arabi A, Siavashpour Z, Shahraki T, Ansari I. Efficacy and complications of ruthenium-106 brachytherapy for uveal melanoma: A systematic review and meta-analysis. J Contemp Brachytherapy. 2021;13:358–64.
Isager P, Ehlers N, Urbak SF, Overgaard J. Visual outcome, local tumour control, and eye preservation after 106Ru/Rh brachytherapy for choroidal melanoma. Acta Oncol Stockh Swed. 2006;45:285–93.
Tarmann L, Wackernagel W, Avian A, Mayer C, Schneider M, Winkler P, et al. Ruthenium-106 plaque brachytherapy for uveal melanoma. Br J Ophthalmol. 2015;99:1644–9.
Finger PT, Mukkamala SK. Intravitreal anti-VEGF bevacizumab (Avastin) for external beam related radiation retinopathy. Eur J Ophthalmol. 2011;21:446–51.
Shields CL, Dalvin LA, Chang M, Mazloumi M, Fortin P, McGarrey M, et al. Visual outcome at 4 years following plaque radiotherapy and prophylactic intravitreal bevacizumab (Every 4 Months for 2 Years) for Uveal Melanoma: Comparison with nonrandomized historical control individuals. JAMA Ophthalmol. 2020;138:136–46.
Kim IK, Lane AM, Jain P, Awh C, Gragoudas ES. Ranibizumab for the prevention of radiation complications in patients treated with proton beam irradiation for choroidal melanoma. Trans Am Ophthalmol Soc. 2016;114:T2.
Schefler AC, Fuller D, Anand R, Fuller T, Moore C, Munoz J, et al. Randomized trial of monthly versus as-needed intravitreal ranibizumab for radiation retinopathy-related macular Edema: 1-year outcomes. Am J Ophthalmol. 2020;216:165–73.
Cennamo G, Montorio D, Bernardo R, Farella A, Liuzzi R, Breve MA, et al. Retinal vascular changes in radiation maculopathy after intravitreal ranibizumab by optical coherence tomography angiography. J Clin Med. 2020;9:E1618.
Jouhi S, Heikkonen J, Reijonen V, Raivio V, Täll M, Kivelä TT. Brachytherapy of Choroidal Melanomas Less Than 10 mm in Largest Basal Diameter: Comparison of 10-mm and 15-mm ruthenium plaques. Ophthalmology. 2021;128:140–51.
Author information
Authors and Affiliations
Contributions
Conceptualisation - VC, RO, KR, and GN. Data curation – RO, KR, GN, and IS. Data analysis and paper writing – RO. Editing, feedback on the paper, and project management - VC, MSS, BD, AKA, and GH. All authors accepted the final version of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
O’Day, R.F.J., Roelofs, K.A., Negretti, G.S. et al. Long-term visual outcomes after ruthenium plaque brachytherapy for posterior choroidal melanoma. Eye 37, 959–965 (2023). https://doi.org/10.1038/s41433-022-01944-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-022-01944-4
This article is cited by
-
Ocular oncology demystified
Eye (2023)
-
Ruthenium
Reactions Weekly (2023)