Abstract
Objective
To report outcomes of tacrolimus immunosuppression after penetrating keratoplasty (PK) in very young children.
Methods
Retrospective, consecutive, cohort study of children undergoing PK at a tertiary children’s hospital between 2005 and 2016. Oral tacrolimus immunosuppression was given for 2 years, followed by topical tacrolimus.
Results
Fourteen children (20 eyes) had 24 PKs; nineteen eyes had primary PKs, five eyes had repeat PKs. Mean age at primary graft was 95 days (3.1 months) for anterior segment dysgenesis (ASD), 430 days (14.3 months) for non-ASD children. Eleven children (15 eyes) had ASD. Three children (five eyes) had non-ASD: two children (three eyes) had glaucoma-related corneal opacity and one child (two eyes) had congenital hereditary endothelial dystrophy (CHED).
One-year rejection-free survival rates following primary PK was 80% for ASD (n = 15) and 100% for non-ASD (n = 4). At final review, 5/15 of primary grafts for ASD were clear. 10/15 failed after a mean of 19 months, specifically attributable to infection (n = 2), rejection (n = 2) and glaucoma (n = 2). 4/4 primary non-ASD grafts are clear at final review (mean follow-up = 77 months). All repeat grafts (n = 5), failed after a mean of 38.25 months. Considering all grafts, 15/24 (62.5%) failed: 5/15 due to infection, 2/15 due to rejection, 8/15 due to glaucoma, phthisis, perforation or vascularised with no rejection. At last review (mean = 58.1 months, range 28–84), overall cohort survival is 37.5%. Final visual acuities range between 0.86 and 2.4 LogMAR.
Conclusion
We compare our results to published literature: 1-year graft survival was higher than previously reported, with lower failure due to rejection. Overall infection rates did not increase, however, proportionally, severe infections were higher. Overall graft survival is at least comparable to reported literature.
Similar content being viewed by others
Introduction
Anterior segment dysgenesis (ASD) is the commonest indication for penetrating keratoplasty (PK) in infancy. ASD is an umbrella term for a group of heterogeneous congenital anomalies which can be described by their constellation of features. Increasingly, underlying genetic mutations are understood, enabling classifications based on genotype rather than phenotype [1]. Associated co-morbidities such as cataracts and glaucoma, and the high risk of allogenic rejection result in a higher failure rate than most series in adults [2,3,4].
Younger children have a higher risk of corneal graft failure, probably due to more robust immune responses causing rejection [2, 5]. Brisker healing is evidenced by earlier structural integrity of the graft–host junction in children; at 4 weeks post graft, sutures can be removed in infants. Consequently graft survival rates are worse in younger children: overall graft survival is 52% in children under one year old, 61% in 1 to 3 year olds and 77% in children over five years old [2]. Graft rejection in particular was commoner in children under 5 years (52%), compared to children over five (22%) [2].
Corneal vascularisation, present in a significant proportion of ASD, compromises the immune privilege of the cornea. The extent of vascularisation proportionally increases the likelihood of rejection, and increases the length of time needed to successfully treat a rejection episode [6]. Blood vessels and associated lymph vessels at the graft–host junction allow the presentation of donor antigens to host T cells within host cornea, and within host-lymphatic tissue [6, 7]. Chemokines, such as IL-2, modulate the immune cell movement towards donor cornea and orchestrate immune mediated damage.
Calcineurin inhibitors such as cyclosporin and tacrolimus reduce production of IL-2 and reduce T-cell proliferation. Systemic cyclosporin has multiple side effects: 81% of adult patients experienced an adverse reaction [8]. Tacrolimus has a better systemic side effect profile, with comparable results to cyclosporin for reducing corneal allogeneic rejection in adults [9]. Tacrolimus is a FK506 binding protein that inhibits calcineurin’s ability to dephosphorylate nuclear factor in T cells. It has a history of successful use in solid organ transplant, ocular graft versus host disease, atopic keratoconjunctivitis and posterior uveitis [10], particularly in children.
Oral immunosuppression has improved survival rates of high-risk grafts in adults [11,12,13]. Meta-analysis of adult data did not favour a particular immunosuppressant due to low quality data with a risk of bias [14]. Meta-analysis concluded that mycophenolate, cyclosporin and tacrolimus improve rejection rates, but have no effect on graft survival [14, 15]. However, since publication of the meta-analysis, individual studies have shown that, in high-risk adult PKs, oral tacrolimus improves graft survival from 33 to 100% over a mean of 24 months [11] and topical tacrolimus reduces graft failure from 44 to 19% when added to topical prednisolone 1% [16, 17]. Low dose oral tacrolimus can reverse rejection episodes [18]. Topical cyclosporin for paediatric PKs increased rejection-free survival from 38.5% in control eyes, to 88.9% at 3 years [19].
Oral tacrolimus immunosuppression in infants and young children undergoing PK has not, so far been reported; we report our experience.
Methods
This is a retrospective case note review of consecutive children undergoing PK with oral tacrolimus immunosuppression over an 11-year period (2005–2016) at a tertiary children’s hospital. Informed consent was obtained for surgery and tacrolimus immunosuppression. Tacrolimus immunosuppression was given at the surgeon’s discretion. In the time period studied, four children had PK without immunosuppression. Indications for surgery, surgical details and detailed post-operative course and outcomes were recorded.
All procedures were performed by a single surgeon (SS). Donor tissue was chosen with a maximum acceptable age of 30 years, to optimise endothelial cell counts. The donor corneal button was oversized by 0.5–1 mm, and sutured with interrupted 10–0 nylon. Subconjunctival cefuroxime and triamcinolone acetonide (Kenalog 40 mg/mL, Bristol-Myers Squibb, Princeton, NJ USA) were administered to provide immunosuppression until oral tacrolimus cover became effective.
All of the children had suture removal between 4 and 8 weeks post-operatively, usually at two planned general anaesthetics. This was feasible because of the young age of the cohort. Early suture removal minimises suture-related risk of infection and reduces the risk of rejection episodes related to loosening of the sutures. If loose sutures were identified early, an unplanned procedure was organised for expedited suture removal.
Post-operative topical therapy included preservative free dexamethasone (0.1%, Minims, Bausch & Lomb, Surrey, UK) 2 hourly and chloramphenicol (0.5%, Minims, Bausch & Lomb, Surrey, UK) 4 times daily for 8 weeks. Topical steroids were tapered over the subsequent 3 months to 4 times a day, gradually reducing to twice daily after 1 year.
Tacrolimus was commenced within 1 week of surgery, at 0.1 mg/kg/day and titrated to maintain trough blood levels at 2–4 ng/ml. Three consecutive trough levels <1.8 ng/ml were defined as sub-therapeutic. Frequency of blood monitoring was reduced to 3 monthly once trough levels were stable.
A liver and small bowel transplant team coordinated dosing, blood monitoring, and adverse event reporting, due to their experience with immunosuppression. Baseline blood tests were taken for renal and hepatic function, with monitoring frequency reducing to 3 monthly in stable patients. Live attenuated vaccinations were avoided whilst immunosuppressed, but non-live (inactivated, recombinant or toxoid) vaccines were permitted.
After 2 years, oral tacrolimus was replaced with tacrolimus ointment twice daily (Protopic 0.03%, Astellas Pharma US, Inc., Northbrook, IL). Tacrolimus was withheld at the discretion of the hepatology and ophthalmology teams; permanently if the graft failed, or temporarily if systemic or ocular infection occurred.
The primary outcome measure was a clear corneal graft at last follow-up. This was defined as a compact and clear cornea, allowing a clear view of iris details. Secondary outcome measures were: number of rejection episodes, rejection-free survival, visual acuity and tacrolimus-related adverse outcomes. Graft rejection was defined as loss of graft clarity accompanied by intraocular inflammation or epithelial or endothelial rejection lines. Graft rejections were treated promptly with high dose steroids in a combination of topical, subconjunctival, sub-tenon or intravenous routes. Graft failure was defined as irreversible loss of central graft clarity that was no longer compatible with good visual function, from any cause. Visual acuity recorded as fix and follow, perception of light (PL) and no PL (NPL) were substituted with 2.40, 2.70 and 3.00 LogMAR [20].
Given the heterogeneity of pathology, and the small numbers of cases in our series, we divided the analysis of our cohort into those children who had PK due to ASD, of any type, and those who had corneal failure secondary to other causes. Further subdivision of phenotype with a view to risk stratification would not be statistically valid in a small series.
Results
Demographics
Fourteen children (twenty eyes) had 24 PKs; nineteen eyes had primary PKs, five eyes had repeat PKs (Table 1). Eleven children (15 eyes) had ASD: Peter’s anomaly phenotype (n = 7), ASD of varying phenotype (n = 3), sclerocornea (n = 3) and corneal perforation at birth (n = 2). Primary grafts occurred for ASD at a mean age of 95 days (6–375). One child underwent bilateral surgery at 6 days old. At the time of PK, additional procedures were based on clinical need including: lensectomy or division of dense iridolenticular adhesions (n = 6) and combined stem cell graft (n = 4). None of these eyes had undergone prior surgical intervention.
Three children (five eyes) had PKs for non-ASD causes. One child (two eyes) had congenital hereditary endothelial dystrophy (CHED), two children (three eyes) had glaucoma associated with non-acquired ocular causes. Two children had bilateral sequential primary PKs at a mean age of 430 days (range 181–607). A third child had a repeat graft at 1500 days old, she did not have tacrolimus immunosuppression for her primary PK (Table 2, case 16). Each child had undergone prior surgical procedures to control glaucoma (mean number: 5.75, range 5–9); but no additional interventions were undertaken at the time of PK.
All children remain under our care, one child died due to an underlying systemic condition. At final review, children had been followed up for a mean of 76.6 months (range 28–151).
Graft survival
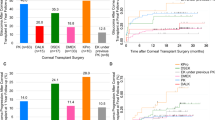
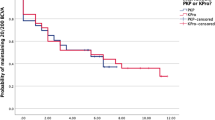
50% of all grafts in this series were functioning at 41 months. Graft survival for the entire cohort is shown in Fig. 1.
At final review, 5/15 primary PKs for ASD are surviving; mean survival duration is 43 (28–84) months. One-year survival was 11/15 (73%), 2-year survival was 10/15 (67%). One graft survives to date, beyond 5 years. One child had clear grafts at the time of his death, 28 and 31 months after his bilateral PKs. All four primary grafts for glaucoma continue to survive after a mean of 77 (74–83) months (Tables 1, 2).
Five eyes with ASD developed progressive thinning in the host cornea and peri-limbal sclera, requiring tectonic scleral grafts; of these only 1/5 grafts is surviving. Four eyes with ASD required glaucoma procedures (one Baerveldt tube insertion, three cyclophotocoagulation); 2/4 grafts continue to function.
Four eyes with ASD and one eye with glaucoma underwent repeat PK. All five grafts failed after a mean of 38 (7–94) months. One-year survival was 3/5 (60%), 2-year survival was 2/5 (40%) and 5-year survival was 1/5 (20%).
Graft failure
Considering all primary and secondary grafts 15/24 (62.5%) grafts failed. 5/15 (33%) failed due to infection, of which three had infective keratitis resulting in evisceration (Table 2). 3/15 (20%) failed with vascularisation and no rejection, 2/15 (13%) failed due to glaucoma. Two eyes (13%) developed phthisis and one eye (7%) perforated. The remaining two grafts rejected (13%).
Rejection whilst on oral tacrolimus occurred six times in four eyes of four children. 2/6 episodes resulted in graft failure. The remaining 4/6 episodes were successfully treated with combinations of topical, subconjunctival, orbital floor or systemic steroids (Table 3). At the time of rejection, tacrolimus trough levels were therapeutic in 5/6 cases.
Of 15 primary grafts surviving at 1 year, rejection-free survival rates were 9/11 (82%) for ASD and 4/4 (100%) for non-ASD. 3/5 repeat PKs were surviving at 1 year, 2/3 (60%) had no episodes of rejection. Of 14 primary grafts surviving at 2 years, rejection-free survival rates were 6/10 (60%) for ASD and 4/4 (100%) for non-ASD. 15/19 eyes (79%) never experienced rejection whilst on oral tacrolimus, and four eyes (21%) experienced one or more episodes of rejection (Table 3).
Seven grafts became vascularised: 3/7 post rejection episodes, 2/7 failed with vascularisation and no rejection, 2/7 vascularised after infection, which led to failure. If vascularisation was noted prior to graft failure, it was treated with fine needle diathermy and bevacizumab.
Systemic adverse effects of oral tacrolimus
We did not encounter any previously reported adverse effects including: nephropathy, neurotoxicity, hepatotoxicity, arterial hypertension, gum hyperplasia or hirsutism. None of the children suffered an immunosuppression-related illness and no-one was disadvantaged due to delay in receiving live vaccinations. One child had tooth decay and tacrolimus was stopped at the parent’s request, although no association was proven. Blood testing throughout the treatment period, supervised by a hepatology team, did not find any irreversible derangement of haematological or biochemical variables.
Visual outcome
All children received refractions on a minimum of an annual basis and glasses were prescribed if the child were to benefit from the prescription. Of those children with ASD, who had uniocular surgery, two had a normal fellow eye and received patching to optimise vision in their operated eye. The remaining three either opted for surgery in only one eye, or had pathology that was too severe to consider surgical intervention in the fellow eye. The operated eye was therefore their dominant eye. Last recorded uniocular visual acuity achieved while the graft was functioning is shown in Fig. 2. Uniocular recorded visual acuity ranged between 0.86 and 2.4 LogMAR (mean 1.64). At final review, all children with clear grafts have measurable vision.
Discussion
This is currently the only published series of children who have received oral tacrolimus for PK. Our cohort includes very young children, with congenital or infant-onset disease and therefore the risk of graft failure is high when compared to series describing children with later-onset, traumatic or progressive pathologies [1].
PK in infants and young children presents a unique set of challenges including the need to balance the risk of early surgery against the benefit of preventing intractable visual-deprivation amblyopia. Graft health is directly linked to prompt presentation and recognition when problems occur, which can be a challenge in young children with several ocular and systemic co-morbidities [21].
In published literature, rejection is the dominant reason for graft failure in infants and young children, followed by infection. We aimed to reduce the risk of graft failure, due to rejection, with the addition of oral immunosuppression. Multiple risk factors increase the likelihood of rejection, including: young age at surgery, heightened immune responses, previous anterior segment surgery, multiple concurrent procedures at the time of PK, and the need for large grafts, which allow donor antigens to contact host vessels [6, 22]. Previously published failure rates at 1-year range between 29 and 83% [5, 23,24,25,26] and 65% at 2 years [27]. In this cohort, the proportion of grafts that failed at 1 year was 27% and 33% at 2 years. 16/24 of PKs in our cohort were for children under 1 year old, with a 1-year failure rate of 25%.
Eyes with ASD are structurally abnormal, associated with limbal stem cell deficiency, loss of limbal architecture, corneal vascularisation and corneo-lenticular adhesions. Subsequent reduction of corneal immune privilege, increases the likelihood of rejection. Our survival rate of primary PK in ASD suggests that the drive for immunogenic rejection can be modulated with oral tacrolimus. Simultaneous lensectomy is a significant risk factor for graft failure, reducing overall survival to 19% [5]. Our experience was similar. Only 1/5 ASD eyes (20%) that underwent lensectomy was clear at final follow-up, compared to 5 of the remaining 10 cases (50%).
Glaucoma at the time of PK is an independent risk factor for failure [27]. Four eyes with childhood glaucoma or CHED had surviving grafts at 5 years. The improved graft survival in glaucoma, compared to ASD, is likely due to increased age at surgery, and no concurrent procedures at the time of PK.
Subsequent development of glaucoma risks graft failure, due to the need for further surgical interventions, with a hazard ratio of 4.09 [27]. Of the four ASD eyes that developed glaucoma, two grafts were functioning at final follow-up.
We found scleral thinning to be a serious adverse prognostic factor, occurring in four eyes (Nos. 1, 2, 11, 13). None of the eyes had raised intraocular pressure. Despite scleral patch grafting, three grafts failed (perforation, endophthalmitis leading to evisceration, and infective keratitis leading to corneal decompensation). Three eyes (1, 2, 13) had evidence of scleral thinning at presentation, which progressed post PK. In eye 13, thinning progressed for years, even when tacrolimus was discontinued. Eye 11 developed scleral thinning within 5 months of PK. We do not understand the mechanism for progressive scleral thinning, particularly as it was not associated with any thinning or stretching of the graft–host junction. We presume that it is a manifestation of abnormal tissues, and is unrelated to tacrolimus immunosuppression.
Severe infection was the dominant cause of failure, compared to rejection in our series, contrasting with previously published studies [2, 5, 28]. Suture abscesses were not a contributory factor in our series, due to the infections occurring after suture removal. Our infection rates compare to Al-Ghamdi’s series of childhood PKs where oral immunosuppression was not used (20.8% vs. 26%) [5]. Studies where infection rates were lower, involved cohorts of children who had surgery at an older age [2, 5, 29]. It is possible that infection is more likely to progress unnoticed in a young child who is unable to communicate symptoms.
Infection is an inherent risk factor in this difficult group of patients. As 3/5 of our cases resulted in endophthalmitis, it is not possible to ignore that infection in tacrolimus-immunosuppressed children may be more aggressive and more likely to progress to endophthalmitis, a devastating outcome. Delays in detection of infection, and presentation and complex ocular and social circumstances, contribute to delay in treatment. We ensure that oral immunosuppression is halted after 2 years in order to minimise the risk of serious infection.
Repeat PKs are at higher risk of rejection, as the immune system is primed to recognise donor corneal antigens. Rejection usually occurs earlier and in a more fulminant pattern in subsequent grafts [30]. Yang reported a second or subsequent PK in a patient with Peter’s anomaly has a <10% survival rate at 3 years [31]. In our cohort, all five repeat grafts failed after a mean of 38 (7–94) months. Maintaining the clarity of a repeat PK remains a challenge. Engagement from families and a close relationship with medical staff helps to ensure timely presentation when there are concerns regarding graft health [32].
The ability to provide functional vision in the early years is crucial for development of sight, mobility and social development. Only one child developed loss of corneal clarity in both eyes by 1 year old. The remaining children with ASD had a minimum of PL vision binocularly. Visual acuity was a minimum of 1.6LogMAR in the remaining eye of those who had an evisceration. This level of vision, as reported by the children’s families, is adequate for independent mobility, social interactions and in some cases, limited reading on tablets or computers.
Sub-therapeutic levels of oral tacrolimus were found in several cases at various times during the study period, without consequence on graft health. Five of the six rejection cases had therapeutic levels at the time of rejection (of which two grafts failed). This suggests that while tacrolimus lowers the rate of immunogenic rejection, it does not eliminate the risk. None of our patients suffered any toxicity, or adverse events as a result of immunosuppression.
Study limitations
There are inherent limitations to a report of this nature. A control group would improve scientific validity. We considered comparing outcomes with cases predating the use of tacrolimus but confounding variables invalidated this analysis. Due to small numbers of patients, it is not possible to further subdivide ocular phenotype and stratify transplantation risk.
Upon consideration of commencing systemic immunosuppression, as a multidisciplinary team, we discussed: the need for oral suppression of a systemic rejection process, target trough levels, duration of treatment, and step-down post oral therapy. Experience from solid organ transplantation, and paediatric PK rejection episodes informed decisions. At the time of writing the protocol, published major studies highlighted that the highest failure rate from rejection was seen in the first year after surgery, and stabilised after 2 years [5, 23, 24, 31, 32].
Systemic side effects of tacrolimus relatively contraindicated oral treatment beyond 2 years. Thereafter we used topical tacrolimus (0.03%) due to our experience of its use in sight-threatening paediatric ocular surface disease. We concede that our chosen protocol is not founded on comparative paediatric studies; and of itself merits further study. However, it is comparable with adult series [11], and we continue to use this regimen.
Conclusion
We report a small series of young children with varied complex ocular co-morbidities who had PK with oral tacrolimus immunosuppression. Infection, particularly endophthalmitis, remains a cause of poor outcome, though rejection-free survival, overall graft survival (including grafts lost from infection), is encouraging in the context of published series. Further study, involving a control group, is required to define the role of systemic tacrolimus in young children undergoing PK.
Moving forward, the challenge in paediatric graft survival will be to overcome the hurdles of infection and corneal graft vascularisation and aim to improve the survival of repeat grafts, as even a successful primary graft is unlikely to last the lifetime of the child.
We suggest that oral tacrolimus is a safe and well tolerated adjunct for post-operative immunosuppression and appears to benefit graft survival at 1 year.
Summary
What was known before
-
Paediatric corneal grafts have a high incidence of failure due to rejection and infection.
-
The likelihood of graft failure increases with the following risk factors: younger age at surgery, concomitant intraocular surgery, ASD and previous glaucoma.
What this study adds
-
Oral tacrolimus is a safe and well tolerated adjunct for post-operative immunosuppression. It appears to improve survival of paediatric corneal grafts at 1 year, with a reduction in graft failure due to rejection.
-
Oral tacrolimus does not increase the rate of failure due to intraocular infection, when compared to published literature.
-
Corneal grafts for infants and young children can enable vision adequate for independent mobility.
References
Ma AS, Grigg JR, Jamieson RV. Phenotype-genotype correlations and emerging pathways in ocular anterior segment dysgenesis. Hum Genet. 2019;138:899–915.
Aasuri MK, Garg G, Gokhle N, Gupta S. Penetrating keratoplasty in children. Cornea. 2000;19:140–4.
Kim YW, Choi HJ, Kim MK, Wee WR, You YS, Oh JY. Clinical outcome of penetrating keratoplasty in patients 5 years or younger: Peter’s anomaly versus sclerocornea. Cornea. 2013;32:1432–6.
Majander A, Kivel TT, Krootila K. Indications and outcomes of keratoplasties in children during a 40-year period. Ophthalmol. 2016;94:618–24.
Al-Ghamdi A, Al-Rajhi A, Wagoner MD. Primary pediatric keratoplasty: indications, graft survival, and visual outcome. J AAPOS. 2007;11:41–7.
Dua HS, Azuara-Blanco AA. Corneal allograft rejection: risk factors, diagnosis, prevention and treatment. Ind J Ophthalmol. 1999;47:3–9.
Qazi Y, Hamrah P. Corneal allograft rejection: immunopathogenesis to therapeutics. J Clin Cell Immunol. 2013;S9:1–21.
Lee JJ, Kim MK, Wee WR. Adverse effects of low-dose systemic cyclosporine therapy in high-risk penetrating keratoplasty. Graefes Arch Clin Exp Ophthalmol. 2015;253:1111–9.
Wei X, Chen XM, Wang L, Song JP, Deng YP. Effects of immunosuppressants after penetrating keratoplasty: meta-analysis of randomized controlled trials. Int J Ophthalmol. 2011;4:529–36.
Abud TB, Di Zazzo A, Kheirkhah A, Dana R. Systemic immunomodulatory strategies in high-risk corneal transplantation. J Ophthalmic Vis Res. 2017;12:81–92.
Sloper CML, Powell R, Dua H. Tacrolimus (FK506) in the management of high-risk corneal and limbal grafts. Ophthalmology. 2001;108:1838–44.
Chow SP, Cook SD, Tole DM. Long-term outcomes of high-risk keratoplasty in patients receiving systemic immunosuppression. Cornea. 2015;34:1395–9.
Dhaliwal JS, Mason BF, Kaufman SC. Long-term use of topical Tacrolimus (FK506) in high-risk penetrating keratoplasty. Cornea. 2008;27:488–93.
Abudou M, Wu T, Evans JR, Chen X. Immunosuppressants for the prophylaxis of corneal graft rejection after penetrating keratoplasty. Cochrane Database Syst Rev. 2015;8:CD007603.
Birnbaum F, Bohringer D, Sokolovska Y, Sundmacher R, Reinhard T. Immunosuppression with cyclosporine A and mycophenolate mofetil after penetrating high-risk keratoplasty: a retrospective study. Transplantation. 2005;79:964–8.
Magalhaes OA, Marinho DR, Kwitko S. Topical 0.03% tacrolimus preventing rejection in high-risk corneal transplantation: a cohort study. Br J Ophthalmol. 2013;97:1395–8.
Mills RA, Jones DB, Winkler CR, Wallace GW, Wilelmus KR. Topical FK-506 prevents experimental corneal allograft rejection. Cornea. 1995;14:157–60.
Joseph A, Raj D, Shanmuganathan V, Powell RJ, Dua HS. Tacrolimus immunosuppression in high-risk corneal grafts. Br J Ophthalmol. 2007;91:51–5.
Cosar CB, Laibson PR, Cohen EJ, Rapuano CJ. Topical cyclosporine in pediatric keratoplasty. Eye Contact Lens. 2003;29:103–7.
Day AC, Donachie PHJ, Sparrow JM, Johnston RL. The Royal College of Ophthalmologists’ National Ophthalmology Database study of cataract surgery: report 1, visual outcomes and complications. Eye. 2015;29:552–60.
Kusumesh R, Vanathi M. Graft rejection in pediatric penetration keratoplasty: Clinical features and outcomes. Oman J Ophthalmol. 2015;8:33–7.
Maguire MG, Stark WJ, Gottsch JD, Stulting RD, Sugar A, Fink NE, et al. Risk factors for corneal graft failure and rejection in the Collaborative Corneal Transplantation Studies. Ophthalmology. 1994;101:1536–47.
Karadag K, Chan TCY, Azari AA, Nagra PK, Hammersmith KM, Rapuano CJ. Survival of primary penetrating keratoplasty in children. Am J Ophthalmol. 2016;171:95–100.
Buzzonetti L, Ardia R, Petroni S, Petrocelli G, Valente P, Parrilla R, et al. Four years of corneal keratoplasty in Italian paediatric patients: indications and clinical outcomes. Graefes Arch Clin Exp Ophthalmol. 2016;254:2239–45.
Patel HY, Ormonde S, Brookes NH, Moffatt LS, McGhee CNJ. The indications and outcome of paediatric corneal transplantation in New Zealand: 1991-2003. Br J Ophthalmol. 2005;89:404–8.
Hovlykke M, Hjortdal J, Ehlers N, Nielson K. Clinical results of 40 years of paediatric keratoplasty in a single university eye clinic. Acta Ophthalmol. 2014;92:370–7.
Lowe MT, Keane MC, Coster DJ, Williams KA. The outcome of corneal transplantation in infants, children, and adolescents. Ophthalmology. 2011;118:492–7.
Trief D, Marquezan MC, Rapuano CJ, Prescott CR. Pediatric corneal transplants. Curr Opin Ophthalmol. 2017;28:477–84.
Dana MR, Moyes AL, Gomes JA, Rosheim KM, Schaumberg DA, Laibson PR, et al. The indications for and outcome in pediatric keratoplasty. A multicenter study. Ophthalmology. 1995;102:1129–38.
Bersudsky V, Blum-Hareuveni T, Rehany U, Rumelt S. The profile of repeated corneal transplantation. Ophthalmology. 2001;108:461–9.
Yang LL, Lambert SR, Lynn MJ, Stulting RD. Long-term results of corneal graft survival in infants and children with Peter’s anomaly. Ophthalmology. 1999;106:833–48.
Ganekal S, Gangangouda C, Dorairaj S, Jhanji V. Early outcomes of primary paediatric keratoplasty in patients with acquired, atraumatic corneal pathology. J AAPOS. 2011;15:353–5.
Acknowledgements
This work has been presented as a poster at Oxford Ophthalmological Congress, July 2018.
Author information
Authors and Affiliations
Contributions
Data collection: SP/MR. Data analysis: SP/MP/JA. Paper writing and review: SP/MP/GG/SS/AB.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Painter, S.L., Rana, M., Barua, A. et al. Outcomes following tacrolimus systemic immunosuppression for penetrating keratoplasty in infants and young children. Eye 36, 2286–2293 (2022). https://doi.org/10.1038/s41433-021-01855-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-021-01855-w
This article is cited by
-
Immunosuppressants
Reactions Weekly (2023)
-
Immunosuppressive Therapy for High-Risk Corneal Transplant
Current Ophthalmology Reports (2022)