Abstract
Ionogels are crosslinked networks—typically polymeric networks—swollen with ionic liquids. The unique properties of ionogels, such as nonvolatility, ionic conductivity, nonflammability, and high thermal and electrochemical stability, make them promising for a variety of applications. Examples include sensors, adhesives, energy storage devices, and ionotronics. While many ionogels require complex syntheses and suffer from poor mechanical properties, simpler strategies are emerging to produce tough ionogels, thereby improving the durability, enabling 3D printing, and broadening the application space of ionogels. This perspective highlights promising applications and future opportunities of ionogels.
Similar content being viewed by others
Introduction
Polymeric gels are solvent-swollen networks that are crosslinked physically (i.e., with noncovalent bonds such as hydrogen or ionic bonds) or chemically (i.e., with covalent bonds)1,2,3. Gels can be swollen with a variety of solvents, such as water (hydrogels), organics (organogels), or ionic liquids (ionogels). Ionic liquids (ILs) are low melting salts composed of cations and anions. The low melting point (in many cases, less than room temperature) arises because at least one of the ions in the IL has bulky side groups that make crystallization more difficult than it is for more conventional salts such as NaCl2.
ILs are nonvolatile, ionically conductive, and nonflammable, and they have high thermal and electrochemical stabilities3,4,5. Since there are approximately 1018 possible ILs, they have a diverse range of physicochemical properties2,3, such as solvent quality (i.e., ability to dissolve substances), viscosity, biocompatibility, hydrophobicity/hydrophilicity, density, freezing point, and stability. Section “Comparison of ionogels and hydrogels” indicates why these excellent properties make ionogels attractive for use as energy storage devices (i.e., supercapacitors and batteries), adhesives, sensors, and ionotronics/electronics4,6. While many gels have poor mechanical properties, a number of strategies are emerging to improve the mechanical properties and enable more applications2.
This perspective highlights the applications of ionogels. We first compare and contrast hydrogels and ionogels to highlight the unique properties of ionogels that motivate and enable potential applications. While there are no known commercial applications of ionogels to date, we discuss applications demonstrated in the literature, such as wearable sensors, energy harvesters, and batteries that utilize the special properties of ionogels. We highlight applications in which improved mechanical properties would be enabling. We also emphasize new applications, such as three-dimensional (3D) printing, made possible by emerging, one-step synthetic methods to create ionogels. Finally, we discuss future opportunities and untapped applications of ionogels.
Comparison of ionogels and hydrogels
We start by comparing ionogels and hydrogels because they are both polymeric networks swollen with solvents. Hydrogels, which can have soft and wet properties similar to biological tissues, were first discussed in the literature in the 1960s. In contrast, ionogels are still in their infancy, having been first reported in 19932,3. Hydrogels are encountered in daily life (e.g., jelly, contact lenses, tofu), whereas ionogels are typically only found in research environments.
Ionogels are superior to hydrogels in many regards due to the excellent properties of the ILs (Fig. 1). Since there are many ILs with a range of physicochemical properties, we can only generalize the differences between hydrogels and ionogels. First, consider the volatility of the solvent (Fig. 1). Water evaporates readily, so the masses and dimensions of hydrogels are unstable, especially in hot and dry environments. While the addition of salts or other deliquescent materials can retain some of the water in hydrogels, ionogels are inherently nonvolatile because ILs have approximately zero vapor pressure; that is, they do not evaporate or “dry out” over time2. Nonvolatility means that ILs can be used in vacuum conditions (including those used for spectroscopy, electron microscopy, and physical vapor deposition). In addition, nonvolatile ionogels can be handled without concern for breathing the IL. Many ILs are hygroscopic, which causes some ionogels to add mass in the presence of water, similar to hydrogels.
The water in hydrogels can evaporate, whereas the ILs in ionogels cannot. Ionogels can operate over a much broader range of temperatures than hydrogels. The water in hydrogels can undergo electrolysis, whereas ionogels are electrochemically stable over a much wider potential range. Water is a poor conductor relative to many ILs. Finally, there are 1018 different ILs that may be used for ionogels, but water is the only solvent for hydrogels.
Second, ionogels are also more thermally stable than hydrogels (Fig. 1). The narrow liquidus range of water (i.e., 0 °C for freezing and 100 °C for boiling) narrows the application ranges of hydrogels. In contrast, ionogels can be used safely over wide temperature windows (often −70 to 350 °C) without concern for phase changes1.
Third, ionogels have excellent electrochemical stability relative to hydrogels (Fig. 1). Water undergoes electrolysis to produce hydrogen and oxygen when the voltage is above ≈1.3 V7. In contrast, ILs remain electrochemically stable up to ≈4 V, which extends their applications to circuits and batteries that are often subjected to several volts1,8.
Fourth, ILs conduct ions better than pure water (Fig. 1). Ionically conductive solvents find use in electrochemical systems such as batteries and electrical double layer capacitors. In particular, ILs that use lithium ions (Li+) as cations have excellent ionic conductivities due to the high mobility of Li+, which is attractive in preparing solid-state electrolytes for batteries4,6.
Finally, ionogels are chemically and physiochemically diverse due to the range of available ILs. There are approximately 1018 species of ILs that can be formed by combining different cations and anions. This incredible range of solvents offers a broad library to choose from in producing a variety of ionogels, thus distinguishing ionogels from hydrogels, in which water is the only solvent.
Although we focus here on comparing ionogels with hydrogels due to their ubiquity, we briefly note that other types of gels are possible. For example, organogels are swollen with organic solvents. Organogels have poor inherent conductivities, although in some cases salts can be dissolved in the solvent to increase the conductivity. Due to the diversity of organic solvents, it is possible to tune the physicochemical properties of organogels, including the thermal and electrochemical stabilities, viscosities, volatilities, and densities.
Applications of ionogels
This section discusses several typical applications of ionogels based on the excellent features described in section “Comparison of ionogels and hydrogels”.
Adhesives
The cations and anions in ILs enable ionogels to form noncovalent bonds (e.g., hydrogen bonds, ion–dipole and dipole–dipole interactions, electrostatic bonds) at the interface between the ionogel and substrates, thereby endowing ionogels with adhesive properties (Fig. 2a)9,10,11. Note that adhesion depends not only on interfacial interactions between an adhesive and substrate but also on the viscoelasticity of the adhesive that distributes and dissipates stress12. Thus, not all ionogels are adhesive, but nevertheless, the unique bonds available for ILs facilitate adhesion to many substrates. For example, ionogels can adhere to glass and even polytetrafluorethylene (PTFE) in air (Fig. 2b)9. Hydrophobic ionogels can also adhere to multiple surfaces underwater without swelling (Fig. 2c)10. Ionogels are interesting adhesives because of the additional properties they can provide, such as optical transparency, thermal stability, and ionic conductivity.
a Ionogels have multiple adhesive bonding mechanisms9. b Such bonds enable certain ionogels to exhibit strong adhesion to diverse substrates9. Copyright 2020 Wiley-VCH. c Hydrophobic ionogels show good adhesion to various surfaces in both air and water10. d, e Hydrophobic ionogel-based wearable sensors can adhere to the skin to monitor d underwater human motions and e gestures in air10. Copyright 2021 RSC.
Sensors, ionotronics, and optics
Ionically conductive materials such as ionogels are useful for new types of ionotronic devices. The term “ionotronics” is an adaptation of the word “electronics” and is used to emphasize the importance of ions in such devices. In contrast to metals, which are opaque, ionotronic devices can be transparent. Ionotronics are intriguing for interfacing electrodes with biology, which naturally use ions13.
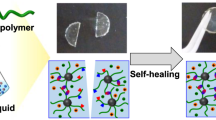
Ionogels can detect deformation and strain with the changes in resistance arising from changes in geometry. Using the adhesive principles from section “Adhesives”, ionogels can adhere to the body and detect human motion (e.g., breathing, crawling, gestures) (Fig. 2d, e)10. Sensitive strain sensors can be 3D printed with unique geometries14, while strain sensors with noncovalent bonds in the gel network can self-heal to improve their stability and lifetime10,15. In addition to strain, ionogels can also sense humidity by using hygroscopic ILs that absorb moisture from the environment and thereby change their resistance. In principle, ionogels could also sense changes in temperature via changes in the resistance or the electrical double layer capacitance at an electrode.
Since ionogels transmit light in addition to their inherent ability to transmit ionic signals, they can be used in more than one mode (e.g., dual mode) for sensing and information transmission. They can also be used for optical components, such as lenses or optical cables. Ionogels can also be loaded with additives to enable sensing or other functionality. For instance, SiO2 nanoparticles loaded in ionogels impart mechanochromic properties due to changes in the distances between particles upon deformation16,17. These ionogels, which exhibit different colors under deformation, can monitor mechanical deformations optically and electrically17. The dual-responsive materials can be used for optical camouflage and ionic communication and can even be used underwater if the ionogels are hydrophobic16. Other properties of ionogels—such as nonvolatility and high thermal stability—enable their use for sensing in harsh environments, including high vacuum and high temperature.
Energy harvesters and energy storage devices
Ionogels may serve as transparent and stretchable electrodes for energy harvesters. For example, ionogels can capacitively collect charges from tribocharged surfaces, such as polydimethylsiloxane (PDMS), as shown in Fig. 3a, b18. Due to the high thermal stabilities of the ionogels, the output current (~4 µA, area: 9 cm2) does not vary significantly between −20 and 60 °C (Fig. 3c)18. Additionally, when cut, ionogels can self-heal without performance degradation (Fig. 3d). Devices that harness mechanical energy may undergo involve significant deformation or physical contact and would benefit from tough mechanical properties, which is an opportunity for improvement.
a, b The schematics of a the structure (left) and photograph (right) and b the working mechanism of the ionogel-based triboelectric energy harvester18. c, d The performance of the c original and d healed energy harvesters at various temperatures18. Copyright 2022 Springer Nature. e Stable performance of Li+-batteries using SiO2-based ionogel electrolytes to suppress dendrite formation under extreme conditions (rate: 5 C, 80 °C)6. Copyright 2017 RSC.
Ionogels are also attractive as solid-state electrolytes in energy storage devices such as batteries and supercapacitors due to their electrochemical and thermal stabilities and ionic conductivities4,6,8. Ionogels with a high moduli (e.g., 60 MPa) can mitigate safety issues (e.g., short circuits) in Li+ batteries by preventing the growth of Li dendrites and strengthening devices against external impact2,4. SiO2 nanoparticles can be used to improve the moduli of ionogels by modifying them with functional groups (e.g., amine, alkyl) to enhance the compatibility between the SiO2 matrix and the Li+-based IL6. These modifications enable the battery to exhibit excellent performance even under extreme conditions (i.e., 5 C rate and 80 °C) by effectively suppressing Li dendrites that can form under such conditions (Fig. 3e)6. A capacity of 112 mA h g−1 can be maintained over 3000 cycles with a Coulombic efficiency >99.8%6. Since the SiO2 matrix is brittle, there is a safety concern in that it may fail under external impact6. One of the solutions is to introduce a soft and stretchable network into the rigid and brittle SiO2 network to form a double-network. During deformation, the SiO2 network has a large modulus but ultimately breaks, while the stretchable network can undergo large deformations and keep the gel intact, resulting in good ductility2,4. There are opportunities to improve the mechanical properties of the ionogels further for use in batteries.
Shape memory
Ionogels can be synthesized that contain glassy domains with glass transition temperatures above room temperature; thus, it is possible for certain ionogels to have shape-memory behavior5.
The shape memory occurs by heating the ionogel above the glass transition temperature (Tg), deforming it into a temporary shape, and then cooling it. The ionogel will retain the deformed shape until it is heated again, at which point it relaxes back to the original shape. In general, shape-memory polymers have been used for shrink wrapping, self-folding origami, and self-closing sutures. Usually, shape-memory polymers are thermoplastics; thus, ionogels may broaden the application space by introducing ionic conductivity and other unique properties to the field of shape-memory polymers.
Three-dimensional (3D) printing (e.g., actuators and sensors)
3D printing could broaden the applications and types of structures accessible with ionogels. To date, 3D printing has been used to pattern ionogel sensors and lattice structures with unique mechanical properties. Popular 3D printing methods, such as stereolithography (SLA), two-photon lithography, and digital light processing (DLP), use photopolymerization to convert liquid precursors to solid polymers with light. Many ionogels can be photopolymerized by free-radical polymerization to enable DLP 3D printing of arbitrary 3D structures5, such as a hollow, three-arm gripper that can be actuated pneumatically to grasp objects (Fig. 4a).
a Photos of 3D-printed ionogels with varied structures and a pneumatic three-arm gripper5. The precursor was dyed yellow with tartrazine. x is the molar fraction of acrylamide (AAm) in the total monomer (AAm + acrylic acid (AA))5. Copyright 2022 Springer Nature. b Photographs of 3D-printed PACMO ionogels with micron-scale pyramids and lattice structures14. c Pressure sensitivity comparison of the 3D-printed device (pyramid patter) and the control sensor (flat surface)14. Copyright 2022 RSC. d Photos of 3D-printed ionogel-based strain sensors19. e The intensity of the strain sensor signal increases with strain19. Copyright 2019 Wiley-VCH.
3D printing can be used to create structures for resistive sensing. For example, micron-scale pyramids and lattice structures detect touch by changes in resistance arising when the pyramids make better contact with the electrode surface (Fig. 4b)14. Compared to the sensitivity (~0.13 kPa−1) of a control device without pyramids, the device with pyramid patterns exhibited a higher sensitivity (~0.72 kPa−1), as shown in Fig. 4c14. 3D printing can also be used to create auxetic structures for sensing (Fig. 4d)19. Unlike SLA and DLP 3D printing, which use low viscosity liquids, this printing relies on a shear-thinning ionogel ink that can be extruded to provide a self-supporting structure that is cured under UV light. Auxetic structures have low densities (22% in area relative to a film) and high mechanical compliance. As shown in Fig. 4e, the sensor responds well to deformation, and the response increases with stretching19. Unfortunately, the mechanical properties of the ionogels described in these examples are poor (e.g., fracture strength: ~0.3 MPa, stretchability: <200% strain)14,19. Thus, there are opportunities for improvement.
Simplifying routes to toughen ionogels and broaden their applications
This perspective has highlighted several applications of ionogels, all of which would benefit from ionogels with improved mechanical properties. For example, many ionogels have low fracture strengths (<0.1 MPa); improving this value could enhance the ability of ionogels to resist external impacts. Many ionogels also have low moduli (e.g., ~0.1 MPa). Increasing the modulus to those of commonly used silicone elastomers (modulus ~1–10 MPa) might allow ionogels to be used as “drop in replacements” for a wide range of applications that currently use silicones5,9,10. In addition, for many applications, toughness (that is, the area under the stress‒strain curve) is important because it means that gels can dissipate energy in response to deformations and are less prone to failure.
Gels can be toughened by introducing mechanisms to distribute and dissipate the energy within the network during deformation. Examples include double polymer networks, phase-separated domains, sacrificial bonds (e.g., hydrogen and ionic bonds) between polymer chains, entanglements, and fillers. Synthetic strategies used to implement these mechanisms were summarized recently2. Many of these approaches are laborious. Thus, there is motivation to create simple strategies to impart toughness.
We briefly discuss one approach because of its simplicity: Phase separation—that is, ionogel compositions with limited solubility in the IL—can toughen gels. While solvated portions of the ionogel tend to be soft and stretchable, the portions of the gel that separate from the IL can have polymer-polymer interactions2,5. The noncovalent bonds between the polymer chains can break and reform multiple times during deformation to dissipate energy, thereby toughening the gel2.
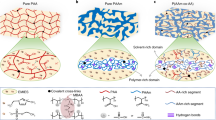
Recently, we demonstrated phase-separated ionogels fabricated by one-step free-radical polymerization; thus, they were compatible with 3D printing (cf. Fig. 4a)5. A pure poly(acrylic acid) (PAA) ionogel was transparent, and the pure polyacrylamide (PAAm) ionogel was opaque (Fig. 5a, top)5. When randomly copolymerized, two phases formed in the poly(acrylic acid-co-acrylamide) (P(AAm-co-AA)) copolymer ionogel: a solvent-rich domain and a polymer-rich domain. The phase behavior and glass transitions varied with composition, which can be used to tune the mechanical properties over a wide range, including soft/stiff, inextensible/stretchy, weak/strong, and brittle/tough (Fig. 5a)5. When optimized, the mechanical properties of these copolymer ionogels (fracture strength: ~13 MPa, Young’s modulus: ~50 MPa, stretchability: ~600% strain) far exceeded those of the pure PAA and PAAm ionogel constituents, as well as those of most tough hydrogels and ionogels, natural rubber, and cartilage (Fig. 5b–d)5. While these copolymer ionogels have excellent mechanical properties, their electrical properties have not been explored; doing so should extend their application space. The copolymer ionogels can also heal and exhibit shape memory since the polymer-rich domains reversibly soften upon heating above the Tg (composition dependent) and reform upon cooling (e.g., room temperature)5.
a Gels formed by polymerizing a 6 M solution of monomers (AA and AAm) in IL. The optical and mechanical properties changed significantly based on the molar fraction of AAm in the total monomer (AAm+AA)5. b The tensile curves of pure PAA (soft, blue data), pure PAAm (brittle, red data), and copolymer (tough, green data) ionogels5. c, d A comparison between the mechanical properties of tough ionogels and cartilage, natural rubber and various gels in terms of c fracture energy and d Young’s modulus in relation to fracture strength5. Copyright 2022 Springer Nature.
Conclusions and prospects
Ionogels—polymer networks swollen with ILs—are emerging gels that are in their infancy2. Ionogels have unique properties, including negligible vapor pressures, good ionic conductivities, and excellent thermal and electrochemical stabilities3,20. This perspective highlights applications that take advantage of the unique properties of ionogels while also motivating the need for ionogels with improved mechanical properties and facile synthetic methods to broaden their utility. Although we know of no existing commercial uses of ionogels, section “Applications of ionogels” highlights several applications of ionogels that have been demonstrated in the literature.
The beauty of ILs is that the cations and anions in ILs can be independently modified to enable a wide range of material possibilities. There are approximately 1018 ILs, so it is possible to find ILs that are biocompatible, toxic, acidic, basic, hydrophobic, or hydrophilic depending on their composition. Hence, ILs have a wide range of physicochemical features, resulting in a variety of ionogels.
Ionogels can conduct ionic charge in a way that is useful for energy devices (harvesters, capacitors, batteries) and ionotronics (sensors, diodes, and other devices that use ions as part of their operation). Most work on ionotronics (e.g., artificial muscles, e-skins, touchpads) has been focused on hydrogels13. Thus, there are many opportunities for ionogels, especially considering their diversity. Like hydrogels, ionogels are usually transparent, which makes it possible to form transparent devices that can transmit both light (optical devices) and ionic signals (electric devices). However, unlike hydrogels, ionogels are nonvolatile; thus, they can form devices without encapsulation and can also be used in high vacuum environments or in outer space. Moreover, the excellent thermal and electrochemical stabilities broaden the operating environments in which ionogels can safely operate relative to those of other gels.
Other properties, such as recovery, fatigue resistance, nonflammability, self-healing, shape-memory, and conductivity, of ionogels need to be further explored and exploited. It is also conceivable to introduce additives/nanoparticles into ionogels to make them optically active (e.g., electrochromic particles such as WO3), magnetically responsive (e.g., Fe3O4), or tougher (e.g., silica filler). Such composites could further expand the application range of ionogels.
Finally, since ionogels constitute a relatively new field, their promise in many key areas may be underappreciated or understudied. For instance, ionogels have seen few applications in soft robots and stretchable electronics. A better understanding of the biocompatibilities of ionogels could broaden their applications in biomedical fields (e.g., drug delivery, tissue engineering, and neural interfaces). In addition, the tunable polymer backbone and diverse ILs make ionogels useful in environmental fields such as extraction/harvesting heavy metal ions from wastewater and water retention in arid environments. For such applications, swelling of the ionogels is a consideration that needs more investigation.
Currently, many ILs are expensive, but with increased applications, the costs should decrease. The costs of ILs may also be reduced by simplifying the syntheses of ILs and using low-cost raw materials, economies of scale, and improved reuse of ILs by forming reversible/degradable polymer networks. In addition, recycling of ILs could mitigate the environmental impact of toxic ILs and enhance the sustainability of ionogels.
Challenges and opportunities
Despite their advantages, ionogels still face several challenges. First, most existing ionogels have poor mechanical properties, such as low fracture strengths (<1 MPa), small moduli (~0.1 MPa), and low fracture energies (<1000 J m−2)2,5. As a point of reference, these metrics are similar to those of gel-based contact lenses, which are soft and tear easily. The poor mechanical properties limit the application of ionogels. For instance, the solid-state electrolytes in Li+ batteries require moduli as high as ~60 MPa to suppress Li dendrite growth, which is a safety issue leading to short circuits4,5.
Fortunately, inspired by efforts to toughen hydrogels, a number of toughening mechanisms are emerging to produce ionogels with significantly enhanced mechanical properties (e.g., fracture strengths >10 MPa, moduli >40 MPa and fracture energies >10,000 J m−2)5,6,21. Section “Simplifying routes to toughen ionogels and broaden their applications” briefly discussed the toughening mechanisms, and a recent review highlighted synthetic strategies with which to produce tough gels2.
Second, the syntheses of tough ionogels are usually complex and laborious. One underlying issue is the difficulty in selecting appropriate starting materials to synthesize ionogels due to the vast number of ILs, the physicochemical properties of which are not fully understood. Consequently, the synthesis often involves solvent exchange (i.e., forming/dissolving the polymer in an organic solvent and then exchanging the solvent for the IL)2. Simplified synthetic routes would make it easier for researchers to produce ionogels and thereby use them for applications. Section “Simplifying routes to toughen ionogels and broaden their applications” highlights one example of a simple, one-step route used to create tough ionogels via phase separation5. Notably, these excellent mechanical properties are produced in a facile, single-step process, enabling 3D printing. To date, ionogels have mostly been neglected by the 3D printing community.
We hope this perspective will draw attention to ionogels by highlighting their promising properties and potential applications. There are many opportunities to create new materials (especially in simple ways) by combining the power of synthetic chemistry and the diversity of ILs. New ionogels, particularly those with tough mechanical properties, should open up many new possibilities.
References
Suen, J. W. et al. The role of interfaces in ionic liquid‐based hybrid materials (ionogels) for sensing and energy applications. Adv. Mater. Interfaces 9, 2201405 (2022).
Wang, M., Hu, J. & Dickey, M. D. Tough ionogels: synthesis, toughening mechanisms, and mechanical properties—a perspective. JACS Au. 2, 2645–2657 (2022).
Marr, P. C. & Marr, A. C. Ionic liquid gel materials: applications in green and sustainable chemistry. Green Chem. 18, 105–128 (2016).
Chen, N., Zhang, H., Li, L., Chen, R. & Guo, S. Ionogel electrolytes for high‐performance lithium batteries: a review. Adv. Energy Mater. 8, 1702675 (2018).
Wang, M. et al. Tough and stretchable ionogels by in situ phase separation. Nat. Mater. 21, 359–365 (2022).
Chen, N. et al. Biomimetic ant-nest ionogel electrolyte boosts the performance of dendrite-free lithium batteries. Energy Environ. Sci. 10, 1660–1667 (2017).
Li, H. et al. Ultrastretchable and superior healable supercapacitors based on a double cross-linked hydrogel electrolyte. Nat. Commun. 10, 1–8 (2019).
Qian, W., Texter, J. & Yan, F. Frontiers in poly(ionic liquid)s: syntheses and applications. Chem. Soc. Rev. 46, 1124–1159 (2017).
Zhu, J., Lu, X., Zhang, W. & Liu, X. Substrate‐independent, reversible, and easy‐release ionogel adhesives with high bonding strength. Macromol. Rapid Commun. 41, 2000098 (2020).
Wei, J., Zheng, Y. & Chen, T. A fully hydrophobic ionogel enables highly efficient wearable underwater sensors and communicators. Mater. Horiz. 8, 2761–2770 (2021).
Liu, L. et al. A superstrong and reversible ionic crystal‐based adhesive inspired by ice adhesion. Angew. Chem. 133, 9030–9041 (2021).
Abbott, S. Sticking Together: The Science of Adhesion (Royal Society of Chemistry, 2020).
Yang, C. & Suo, Z. Hydrogel ionotronics. Nat. Rev. Mater. 3, 125–142 (2018).
Zhang, M. et al. Self-healing, mechanically robust, 3D printable ionogel for highly sensitive and long-term reliable ionotronics. J. Mater. Chem. A 10, 12005–12015 (2022).
Yu, Z. & Wu, P. Underwater communication and optical camouflage ionogels. Adv. Mater. 33, 2008479 (2021).
Lyu, Q. et al. Bioinspired photonic ionogels as interactively visual ionic skin with optical and electrical synergy. Small 17, 2103271 (2021).
Peng, L., Hou, L. & Wu, P. Synergetic lithium and hydrogen bonds endow liquid‐free photonic ionic elastomer with mechanical robustness and electrical/optical dual‐output. Adv. Mater. 35, e2211342 (2023).
Liao, W. et al. Transparent, stretchable, temperature-stable and self-healing ionogel-based triboelectric nanogenerator for biomechanical energy collection. Nano Res. 15, 2060–2068 (2022).
Wong, J. et al. 3D printing ionogel auxetic frameworks for stretchable sensors. Adv. Mater. Technol. 4, 1900452 (2019).
Sun, Y. et al. Biomimetic chromotropic photonic‐ionic skin with robust resilience, adhesion, and stability. Adv. Funct. Mater. 32, 2204467 (2022).
Xiao, X. et al. Lead-adsorbing ionogel-based encapsulation for impact-resistant, stable, and lead-safe perovskite modules. Sci. Adv. 7, eabi8249 (2021).
Acknowledgements
M.D.D. acknowledges support from the Coastal Studies Institute. J.H. acknowledges the support of the National Natural Science Foundation of China (12272286).
Author information
Authors and Affiliations
Contributions
M.W.: conceptualization, data curation, writing—original draft, writing—review and editing. M.D.D.: conceptualization, writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, M., Hu, J. & Dickey, M.D. Emerging applications of tough ionogels. NPG Asia Mater 15, 66 (2023). https://doi.org/10.1038/s41427-023-00514-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41427-023-00514-8