Abstract
Metabolic competition between tumour cells and immune cells for limited nutrients is an important feature of the tumour microenvironment (TME) and is closely related to the outcome of tumour immune escape. A large number of studies have proven that tumour cells need metabolic reprogramming to cope with acidification and hypoxia in the TME while increasing energy uptake to support their survival. Among them, synthesis, oxidation and uptake of fatty acids (FAs) in the TME are important manifestations of lipid metabolic adaptation. Although different immune cell subsets often show different metabolic characteristics, various immune cell functions are closely related to fatty acids, including providing energy, providing synthetic materials and transmitting signals. In the face of the current situation of poor therapeutic effects of tumour immunotherapy, combined application of targeted immune cell fatty acid metabolism seems to have good therapeutic potential, which is blocked at immune checkpoints. Combined application of adoptive cell therapy and cancer vaccines is reflected. Therefore, it is of great interest to explore the role of fatty acid metabolism in immune cells to discover new strategies for tumour immunotherapy and improve anti-tumour immunity.
Similar content being viewed by others
Facts
-
1.
The reprogramming of fatty acid metabolism of immune cells can directly affect the activation and differentiation of immune cells to affect the host’s anti-tumor immunity;
-
2.
The disorder of lipid metabolism in cancer is an important factor affecting tumor progression and changing TME intercellular communication.
-
3.
Different types of fatty acid metabolism in different immune cell subsets will affect the function of immune cells.
-
4.
The study on the relationship between fatty acid metabolism and tumor immunotherapy has shown significant efficacy in many types of tumor therapy.
OPEN QUESTIONS
-
1.
How does disruption of fatty acid metabolism affect cell communication in the tumor microenvironment?
-
2.
Is the fatty acid metabolism of targeted immune cells targeted at a single stage?
-
3.
Does fatty acid metabolism have good therapeutic potential in tumor immunotherapy?
Introduction
After decades of research on cancer, the factors that affect cancer progression are referred to as characteristics of cancer [1]. In the background of the widespread occurrence of tumours, the serious effects of metabolic changes and immunosuppression of tumour cells have been broadly studied. Indeed, researchers consider the metabolic reprogramming of immune cells, which can directly affect activation and differentiation of immune cells or indirectly change the function of immune cells by affecting transcription, as a new marker for cancer [2]. A growing body of research has provided ample evidence that tumour immune escape is associated with metabolic reprogramming of the TME, in which cancer cells need to respond to hypoxia, acidification, and limited nutritional support while supporting their growth and metastasis [3]. Lipid metabolism, especially fatty acid metabolism, has been gradually recognized as a crucial part of metabolic processes, playing an important role in the survival and proliferation of tumour cells and supporting the differentiation and migration of immune cells in the TME [4]. Therefore, the TME can reprogramme the fatty acid metabolism of immune cells to influence the host’s anti-tumour immunity [5]. The changes in lipid metabolism in tumour cells and immune cells in the TME reshape the lipid composition and further link the related metabolic characteristics of tumours and other cells [6]. As an important component of triglycerides (TGs), phospholipids and glycolipids, fatty acids provide energy or cellular signal support for almost all cells to maintain normal (or abnormal) physiological and pathological functions. Therefore, the combination of metabolic targets and immunotherapy has great potential and prospects.
The immune cells in tumours are regulated by the cancer cells to protect them from being killed by the immune system, a situation that has aroused great interest, and the resulting immunotherapy has shown an unusual effect in the fight against cancer. At present, immunotherapy mainly includes immune checkpoint blockade (ICB), adoptive cell therapy (ACT), and tumour vaccines, among others [7]. Despite great progress as a therapeutic strategy to promote the immune response to tumour antigens, its clinical application is limited because it is only effective for a small number of patients due to immunosuppression, tumour heterogeneity, drug resistance and other problems. In this review, we mainly discuss the fatty acid metabolism of immune cells in the TME and explore the goal of providing more therapeutic strategies.
Fatty acid metabolism in the tumour microenvironment
The survival, proliferation and metastasis of cancer cells in hypoxia, acidification and other harsh environments depend on metabolic reprogramming. Various changes in cellular metabolism contribute to tumour progression, invasion, and metastasis and even regulate the resistance of cancer cells to treatment [8, 9]. However, tumour cells can adapt to changes in the environment and use different metabolic patterns to ensure their growth in harsh environments to cope with oxidative stress and drug exposure. Researchers regard this interesting metabolic adaptation as a self-protection measure for cancer cells against treatment before mutation and regeneration. Overall, targeting the metabolism of tumour cells should be an effective way to improve anticancer treatment or to achieve re-sensitization cancer to drugs, though it remains unclear whether the metabolic changes are related to drug resistance.
The Warburg effect, whereby cancer cells show stronger glucose uptake than normal tissues, was proposed as early as the 1920s; it was further found that through glycolysis, cancer cells are more likely to convert glucose to lactic acid, regardless of oxygen content [10]. As the earliest discovered adaptive metabolism, glucose metabolism through the glycolysis pathway provides a carbon source for energy and key biochemical precursor synthesis in cancer, and some tumours obtain carbon and amino nitrogen by increasing glutamine consumption [11]. Thus far, it is generally agreed that the ability of tumour lipid synthesis is enhanced and that it is closely related to glucose metabolism. The disorder of lipid metabolism in cancer is an important factor affecting cancer progression and changing intercellular communication in the TME [12,13,14,15,16]. Indeed, the increase in exogenous FA synthesis and uptake and synthesis of endogenous FA in highly proliferative cancer cells and the TME are important manifestations of lipid metabolic adaptation [17,18,19]. Fatty acids and other lipids (triglycerides, phospholipids, sterols, etc.) in cancer cells provide energy, membrane components and signal molecular composition and are involved in the regulation of energy supply, proliferation, metastasis, environmental adaptation and therapeutic responses [17, 20, 21].
Fatty acid metabolism includes de novo synthesis of fatty acids, fatty acid decomposition (mainly β-oxidation) and the transport of metabolites. Under the background of cancer biology, a large number of studies have shown that de novo synthesis of fatty acids plays a critical role in activation of carcinogenesis and is the main way for cancer cells to obtain fatty acids [22,23,24]. However, recent research suggests that some tumour cells and tissues are simultaneously able to utilize lipid synthesis and breakdown (including lipolysis and lipophagy) pathways to obtain fatty acids, further supporting their survival and proliferation [25,26,27].
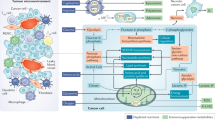
Glycolysis, the tricarboxylic acid (TCA) cycle, and the pentose phosphate pathway (PPP) provide the major substrate for intracytoplasmic fatty acid synthesis, namely, (FAS)-acetyl-CoA [28]. Acetyl-CoA carboxylases (ACCs) are rate-limiting enzymes in de novo synthesis of fatty acids, regulating the first step of conversion of acetyl-CoA carboxylate to malonyl-CoA at a 7:1 ratio, which is catalysed by fatty acid synthase (FASN), to produce saturated palmitate (FA16:0) [17]. Saturated palmitate is then elongated or desaturated to other saturated or unsaturated long-chain fatty acids (LCFAs). In addition, fatty acids can be condensed with glycolysis-derived glycerols to produce various combinations of triacylglycerols and phospholipids as key components of many cellular structures [29]. β-Oxidation in mitochondria is the main oxidation mode of fatty acid decomposition, providing much energy. Among them, short-chain fatty acids (SCFAs) and medium-chain fatty acids (MCFAs) can directly enter mitochondria, whereas long-chain fatty acids (LCFAs) depend on the carnitine shuttle system and are first converted to fatty acyl-CoA with the assistance of fatty acyl-CoA synthetase. Fatty acyl coenzyme A is converted to fatty acyl carnitine under the catalysis of the rate-limiting enzyme carnitine palmitoyltransferase 1 (CPT1) [30] and enters mitochondria with the help of carnitine/acylcarnitine translocase (CACT). The process from acyl carnitine to acyl coenzyme A is completed under the catalysis of carnitine palmitoyltransferase 2 (CPT2), followed by decomposition to acetyl coenzyme A in mitochondria via multiple steps. Acetyl-CoA then enters the tricarboxylic acid cycle (TCA) to produce ATP [31]. Cellular fatty acid uptake depends on specific transporters expressed on the plasma membrane, including FA transporter (FAT), the FA transporter family (FATPs) and plasma membrane FBP-binding protein (FABP) [32] (Fig. 1). Recent studies have also found that tumour cells can absorb fatty acids through exosome transport, which is not described in this review [33].
Fatty acid metabolism of immune cells
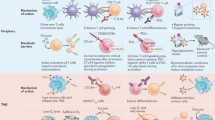
There is evidence that immune system function is related to fatty acids, energy supply and nutritional support at the cellular level [34, 35]. The combination of nutritional availability and different metabolic states generally determines the executable energy-consuming activities of different immune cells. Metabolic plasticity may be the key factor for the immune system to adapt to changes in the microenvironment [36]. Researchers believe that immune cells have different metabolic characteristics; specifically, different immune cell subsets possess different metabolic characteristics [37,38,39]. Metabolic processes, including glycolysis, the citric acid (TCA) cycle, the pentose phosphate pathway (PPP), fatty acid synthesis and oxidation, amino acid metabolism and oxidative phosphorylation, may be changed to meet the energy requirements of different immune cell subsets for functional growth and proliferation [40]. This means that fatty acids affect immune cell function, regardless of their type; extracellular fatty acids can then be recognized and absorbed into the cells through the above-mentioned process, becoming the substrate for β-oxidation and complex lipid synthesis as well as nuclear receptor signals [41, 42]. In this review, we focus on the role of fatty acid metabolism in different immune cell subsets and put forward new insights into the possible role of fatty acids in immune metabolism (Table 1).
CD8+ T cells and fatty acid metabolism
T cells with immune activity are important factors involved in anti-tumour immunity. Fatty acid metabolism is of great significance to T cells. De novo synthesis of fatty acids provides raw materials for their proliferation, and fatty acid oxidation provides energy for them and plays an important role in the differentiation of memory function of CD8+ T cells and regulation of subset differentiation of CD4+ T cells [43]. As the most important lymphocyte subset in the TME, CD8+ T cells, also known as CTLs, mainly destroy cancer cells by releasing granzymes, perforins and other effectors. Maintenance of their effector function generally requires the assistance of aerobic glycolysis. However, due to the hypoxia or low glucose microenvironment in cancer, CD8+ T cells increase fatty acid uptake and catabolism and initiate fatty acid oxidation [44].
Researchers have focused on regulation of fatty acid anabolism in T cells [45, 46], and interestingly, different studies have reported opposite results. Lipid metabolism in CD8+ T cells may play an immunosuppressive role. In breast cancer, leptin/STAT3 signalling is driven by obesity, which aggravates fatty acid oxidation (FAO) and reduces glycolysis in CD8+ T cells through the PD-L1 channel, which leads to inhibition of effector function and tumour growth [47]. In another study, PD-L1-mediated blockade of T-cell differentiation into effector subsets was mediated by inhibiting glycolysis and promoting fatty acid oxidation [48]. Earlier experiments have suggested that the activity and function of CD8+ T cells decreases significantly after treatment with tumour-derived free fatty acids [49, 50]. However, in gastric cancer, the lack of fatty acids may cause the memory T cells residing in tissues to lose their functions because they mainly rely on fatty acid oxidation for energy supply [51]. IL15 can up-regulate expression of carnitine palmitoyltransferase-1A (CPT1A), the key rate-limiting enzyme of fatty acid oxidation, and increase the spare respiration capacity (SRC) of CD8+ T memory cells to cope with additional stress [52]. In another study, expression of CPT1A was up-regulated through the CD28 costimulatory signalling pathway, which can enhance fatty acid oxidation and mitochondrial respiratory function [53].
By examining the immunophenotype of the TME in a mouse breast cancer model, it was shown that the new selective inhibitor of phosphatidylinositol 3-kinase α (PI3Kα) CYH33 can weaken the inhibition of CD8+ T cells mediated by M2-like macrophages by reprogramming macrophages and promote the metabolism of FA in tumour tissue to enhance infiltration and activity of CD8+ T cells, exerting an anti-tumour effect [54]. The regulation of immune activity by short-chain fatty acids (SCFAs) may be achieved by inducing metabolic changes. Studies have revealed that valerate and butyrate significantly enhance the anti-tumour activity of cytotoxic T lymphocytes (CTLs) and chimeric antigen receptor (CAR) T cells by increasing the production of effector cytokines (CD25, IFN-γ and TNF-α) in CD8+ T cells. At the same time, the function of mTOR as a metabolic sensor of central cells was increased [55]. Due to the shift of mammalian rapamycin complex target 1 (mTORC1) and mTORC2 signal inhibition of the immune response from effector to memory [56, 57], the importance of T-cell activation and enhancement of mTOR signalling may also be caused by positive feedback through fatty acid metabolism, suggesting that targeted fatty acid metabolism may play a role in adoptive T-cell therapy.
Fatty acid catabolism supports the survival of effector CD8+ T cells under harsh TME conditions. Oxisome proliferation-activated receptor α (PPAR- α) is responsible for regulation of fatty acid oxidation and can restore part of the effector function of CD8+ TILs in low-glucose and hypoxic TMEs [44]. Prostaglandins are a group of unsaturated fatty acids produced by arachidonic acid enzymes. Studies have shown that metastatic mouse renal carcinoma (RECA) cells produce excessive prostaglandin eosin 2 (PGE_2), which inhibits initiation of tumour-specific CTLs; this is achieved by blocking the IFNγ signal between intercellular cell adhesion molecule-1 (ICAM-1) and lymphocyte receptor lymphocyte function-associated antigen (LFA-1) [58]. Bezafibrate is a complex agonist of proliferation-activated receptor γ (PPAR γ) co-activator 1-α (PGC-1α). By promoting expression of CPT1A, long-chain acyl-CoA dehydrogenase (LCAD) and fatty acid oxidation-related genes in CD8+ TILs, it maintains the number of CTLs and inhibits tumour progression in a lung cancer transplanted tumour model [59], which is consistent with the research of Saibil et al. [60]. However, due to simultaneous upregulation of glycolysis, further exploration is needed to attribute the results to fatty acid oxidation.
Researchers have long found that depletion of CD8+ T cells in the TME is one of the important factors restricting the effectiveness of tumour treatment. In depleted CD8+ T cells, inhibitory receptors such as cytotoxic T lymphocyte antigen-4 (CTLA-4), programmed death-1 (PD-1), T-cell immunoglobulin-3 (TIM-3) and lymphocyte activation gene 3 (LAG-3) are significantly up-regulated and affect the FA metabolism of T cells in the TME [61, 62]. PD-1 promotes fatty acid oxidation of T cells and increases expression of CPT1A [48]. In a study of non-small cell lung cancer, PD-1hiCD8+ TILs had a higher lipid content and uptake capacity than PD-1lowCD8+ T cells [63].
Moreover, all kinds of lipids present in the TME have been proven to have certain immunomodulatory effects. For example, CD36, an FA transport receptor and lipid oxidation scavenging receptor, increases expression of CD36 in melanoma and colon cancer cell lines, induces lipid peroxidation and increased fatty acid uptake, and causes functional inhibition and iron death in CD8+ CTL cells in a CD36-dependent manner (Fig. 2) [64, 65]. We will not talk too much about lipid peroxidation here, but it may be our next research direction. Fatty acid metabolism changes have a variety of effects on CD8+ T cells, and the above studies have shown that fatty acid metabolism plays an important role in the activation, differentiation and effector function of CD8+ T cells. These possible promotive or inhibitory effects depend on the type, source, and transport mode of fatty acids and interaction with CD8+ T cells. Therefore, more evidence in T-cell ab initio fatty acid synthesis, catabolism and uptake and other related experiments are needed with regard to the regulatory targets of T-cell function.
Treg cells and fatty acid metabolism
Th1, Th2, Th17 and Treg cells are all subgroups that differentiate from immature CD4+ T cells under antigen stimulation and have different metabolic characteristics. After T cells first interact with antigen-presenting cells, TCR recognizes the ligands presented by MHC and activates with the help of the costimulatory signal CD28, relying on aerobic glycolysis to provide rapid production of ATP. At the same time, nucleotide production, lipid synthesis and a certain amount of Warburg effect are also necessary [66]. Mammalian target of rapamycin (MTOR) is the main target of the above metabolic mechanism, and phosphatidylinositol 3-kinase-protein kinase B (PI3K-AKT) signalling and c-Myc are the main pathways that induce its downstream regulation [67].
FAs have been shown to be involved in activating differentiation of CD4+ T-cell subsets [45, 68]. As a widely studied cancer-promoting factor, Twist-1 has the characteristics of increased FA oxidation and decreased glycolysis in heavily stimulated Th1 subsets. The purpose may be to protect lymphocytes from oxidative stress by promoting fatty acid oxidation [69]. Soraphen A, for example, can specifically inhibit acetyl-CoA carboxylase 1 (ACC1) to block Th17 differentiation, possibly by inhibiting de novo fatty acid synthesis of T cells [68], and differentiation and activation of naive CD4+ T cells into Th17 subsets depend on FA synthesis [70]. However, in the two groups of experiments, the Treg subgroup had different characteristics, suggesting that the Th17/Treg subgroup may carry out the opposite metabolic programme, and this subgroup seems to be able to regulate the metabolic target.
Such a change in fatty acid metabolism may also be an important factor affecting the uniqueness of Treg cells in the TME. Treg cells increase their glycolysis rate and lipid biosynthesis, and fatty acid oxidation helps to amplify their metabolic signals [71, 72]. Abnormally increased or decreased fatty acid synthesis and storage seem to be harmful to Treg cells, such as abnormal changes in triglycerides and inhibition or loss of lysosomal acid lipase. Increased storage of triglycerides leads to impaired Treg inhibition [73]. In prostate cancer, N-cadherin knockout decreased expression of genes related to fatty acid synthesis and the content of free fatty acids in eTreg cells, though the survival time of Treg cells with sufficient free fatty acids was significantly prolonged [74]. In gastric cancer, large amounts of free fatty acids also seem to be a metabolic advantage of Treg cells because these cells consume fatty acids more preferentially and efficiently than CD8+ T cells [75]. Tregs can also increase De novo synthesis of fatty acids and promote maturation and function by up-regulating the activity of steroid regulatory element-binding proteins (SREBPs). However, at the same time, SREBPs also up-regulate expression of PD-1. It was found that down-regulation of SREBP expression by targeted SREBP cleavage activating protein (SCAP) increased apoptosis in Treg cells and could be used in combination with PD-1 blockers to activate an anti-tumour immune response [76].
Compared with CD8+ T cells, the increase in special metabolic pathways, such as fatty acid oxidation, may be one of the main reasons why Treg cells have the advantage of being able to proliferation in hypoxia, low glucose and other harsh environments, which also explains why Tregs induce peripheral immune tolerance and lead to tumour cell immune escape [77, 78]. The energy provided by fatty acid oxidation maintains the survival and differentiation of Treg cells and accelerates fatty acid catabolism through phosphorylation and activation of the AKT/mTor pathway [79]. Studies have found that inhibition of mTor may indeed induce T cells to differentiate into Treg cells and enhance their fatty acid oxidation function [80, 81]. The role of the PPAR signalling pathway was also found in the metabolic adaptation of Tregs. Treg cells highly express CD36 in tumours from cancer patients or mouse models and promote mitochondrial metabolic adaptation by enhancing fatty acid uptake and activating the PPAR-β pathway while reducing metabolic stress caused by an acidified TME [82].
All the above studies suggest that Treg cells in the TME play an important role in tumour immune escape, which requires the coordination of metabolic adaptation. Therefore, regulating the number or function of Treg cells by targeting fatty acid metabolism seems to be an effective means to induce and enhance the antitumour immune response and improve the immunotherapy effect.
Macrophages and fatty acid metabolism
As an important part of the second line of defence, macrophages can respond quickly to a variety of invading material and physiological challenges. Therefore, macrophages, as innate immune cells, need to maintain stable and flexible functions in the body [83, 84]. In some cancers, macrophages account for the majority of immune cells and show plasticity under different conditions [85]. Macrophages are generally divided into M1 (pro-inflammatory type) and M2 (anti-inflammatory type) according to their function, surface markers and secreted cytokines. Different subsets also have different metabolic characteristics.
Glycolysis and the pentose phosphate pathway (PPP) up-regulation and increased glutamine decomposition and lipogenesis all support the metabolic needs of M1 macrophages [36]. This metabolic regulation assists M1 macrophages in exerting bactericidal and anti-tumour effects. This killing effect on cancer cells is mediated by cytotoxicity or antibody-dependent cytotoxicity (ADCC), but it may cause damage to host tissue [86].
On the other hand, M2 macrophages mainly rely on enhancement of oxidative phosphorylation and β-oxidation of fatty acids to provide ATP, and the biomaterials proline and polyamines depend on metabolism by arginase [87]. Because the TME is rich in fatty acids, M2 TAMs easily increase the degree of fatty acid oxidation [88, 89]. Studies have shown that fatty acid oxidation and lipid accumulation are necessary for the maintenance of the immunosuppressive TAM phenotype [90,91,92]. Related studies have also described the role of M2 macrophages in tissue remodelling and tumour growth. We believe that M2 macrophages assist tumour proliferation and metastasis and achieve immune escape by antagonizing inhibition of tumour immunity and promotion of tumour angiogenesis [86]. Anti-inflammatory signals such as IL-4, IL-10 and IL-13 are involved in inducing the above processes and bolster fatty acid oxidation and oxidative phosphorylation [93,94,95]. Fatty acid oxidation induces differentiation of anti-inflammatory macrophages [39]; in the absence of fatty acid transporters, macrophages are more likely to differentiate into pro-inflammatory macrophages [96]. Although researchers are still exploring the significance of fatty acid oxidation leading to M2 polarization of macrophages, it has also been suggested that the use of fatty acids can reduce M1 polarization of macrophages.
Prostaglandin E3 (PGE3) can produce indirect anti-tumour activity by inhibiting the M1 polarization induced by LPS and interferon γ and promoting interleukin-4-mediated M2 polarization. PGE3 selectively promote M2 polarization and inhibit M1 and TAM polarization, thus playing an anti-inflammatory and anti-tumour role in prostate cancer [97]. In head and neck cancer and squamous cell carcinoma, macrophages are polarized by fatty acids in the TME [98]. In prostate cancer, the fatty acid omega-3 inhibits tumour invasion by inhibiting angiogenesis and the T-cell inhibition induced by M2 macrophages, which further highlights the therapeutic potential of host G protein-coupled receptor 120 (GPR120)-dependent omega-3 fatty acids in inhibiting M2 macrophages in prostate cancer [99].
Researchers have confirmed the role of tumour microenvironmental factors in promoting different phenotypes of cells, proved this role of regulating glycolysis-related enzymes in promoting the TAM phenotype, and verified the existence of an inhibitory phenotype by observing high expression of arginase-1 and CXCR1 in the TAM population [100]. It has also been reported that accumulation of unsaturated fatty acids-oleic acid and saturated fatty acids-nonstearic acid and polarization of macrophages lead to an immunosuppressive phenotype. During co-culture of polarized macrophages and CD4+ T cells, the decrease in the proliferation of CD4+ T cells confirmed the effect of oleic acid on the immunosuppressive properties of macrophages, and up-regulation of nitric oxide synthase and arginase-1 was the reason why oleic acid was able to regulate macrophage polarization, playing an immunosuppressive role; arginase-1 simultaneously regulates inhibition of T cells [92, 101]. It has been found that PPAR-γ participates in M2 macrophage polarization [102]; PPARδ inhibits systemic inflammation by improving fatty acid metabolism and insulin sensitivity [103]. High concentrations of linoleic acid and arachidonic acid, agonists of PPARδ, promote M2 polarization of TAMs in the TME [104, 105]. In hepatocellular carcinoma, down-regulation of receptor-interacting protein kinase 3 (RIPK3) promotes fatty acid metabolism by reducing the PPAY cleavage mediated by reactive oxygen species, induces increased M2 polarization of TAMs and accelerates tumour growth [106]. In a mouse breast cancer model, caspase-1 interacts with medium-chain acyl CoA dehydrogenase (MCAD) to cleave PPAR γ at Asp64, inhibit fatty acid oxidation, increase LD accumulation, and promote macrophage differentiation and cancer progression [104]. Fatty acids are often stored in lipid droplets, which can provide PPARδ ligands to up-regulate the target gene of PPAR δ in ovarian cancer to induce TAM polarization [107]. Arachidonic acid is also often stored in lipid droplets of white blood cells. It is the raw material for ab initio synthesis of eicosanoic acid, an inflammatory mediator, and mediates activation, proliferation and inflammation after release [108,109,110]. As an organelle, lipid droplets store large amounts of fat, play an important role in vesicle transport, protein folding and storage, and act as a signal platform in autophagy [27, 111, 112]. There are not a few studies on LD-mediated fatty acid metabolism, suggesting that targeting lipid droplets as potential cancer therapy is a feasible research direction.
As an efficient drug delivery mode, nano-drug loading has a unique characteristic in affecting fatty acid metabolism in macrophages. We believe that the metabolites of macrophages in different polarization states are important drivers of their signal transduction. It was found that synthesized TLR7/8 agonists and fatty acid oxidation inhibitors are loaded on metabolic supramolecular nano-particles (MSNPs) and delivered to macrophages. In vitro, the phenotype of macrophages changes from M2 to M1 after MSNP treatment, with enhanced phagocytosis. In vivo experiments of 4T1 cell line mice showed that MSNP injection delays tumour growth and enhances anti-tumour immunity [113]. In another group of experiments, siRNA loaded onto a nano-platform with reductive response characteristics was used to silence monoacylglycerol lipase (MGLL) and the key receptor regulating macrophage phenotype (endogenous cannabinoid receptor-2 CBMQ 2), inhibit production of free fatty acids in pancreatic cancer cells and induce TAM reprogramming to M1, increasing secretion of tumour-killing factors (TNF-α, IL-12), both of which exert anti-tumour effects [114]. Although studies have found that direct elimination of macrophages does not have a direct impact on tumour growth [115, 116], specific targeted regulation of TAMs is an effective treatment choice for many diseases. The above experiments reveal the importance of regulating fatty acid metabolism in macrophages or TAMs and provide us with the possibility of targeting fatty acid metabolism in macrophages for cancer immunotherapy.
Dendritic cells and fatty acid metabolism
Dendritic cells (DC) play an important role in the initiation and persistence of immune response. Dendritic cells are activated by signals transmitted by pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs). Dendritic cells present treated antigens to T cells to complete the initiation stage of the immune response. Conventional DCs (cDCs) and plasma cell-like DCs (pDCs) are common phenotypes of DC cells. CDCs mainly promote the anti-tumour T-cell response, whereas pDCs are often related to tumour immunosuppression and immune tolerance [117].
In general, cDCs are activated by TLR agonists and activate the PI3K-AKT and TbK1-IKKε signalling pathways, which promote glycolysis to enhance anabolism and assist in its function [118, 119]. Interestingly, after activation of DCs stimulated by the TLR9 agonist CPGA, glycolysis did not increase but increased FAO and oxidative phosphorylation (OXPHOS), which was induced by DC autocrine type 1 interferon (IFN) and regulated by PPARα [120]. In another study, fatty acid-binding protein 5 (FABP5) was found to regulate TLR signal transduction through the IRF7 and NFκB pathways, and its overexpression promoted Treg production. In contrast, deletion of FABP5 reduced production of pDC cytokines, which decreased Treg production and delayed tumour progression [121]. Fatty acids can also be transmitted by tumour-derived exosomes (TDEs). In many cancer cell lines, fatty acids delivered by TDEs can be induced by PPARα to lead to excessive lipid droplet production, enhance fatty acid oxidation and drive DC immune dysfunction by shifting the metabolic model to mitochondrial oxidative phosphorylation [33]. This is consistent with what we mentioned earlier, indicating that PPAR α may be an effective target for immunotherapy.
The function of DCs can be regulated by fatty acid metabolism reprogramming. High expression of the cDC scavenger receptor MSR1 greatly increases fatty acid uptake, and abnormal accumulation of fatty acids impair the antigen-presenting ability of cDCs; use of acetyl-CoA carboxylase and 5-(tetradecycloxy)-2-furoic acid (TOFA) blocks fatty acid synthesis and restores the T-cell anti-tumour response [122]. Accumulation of oxidized lipids, including fatty acids, in DC cells blocks cross-presentation of antigens by reducing the expression of surface MHCI complexes [123]. In another study, the hyperlipidaemic environment of liver DCs correlated positively with immunogenicity [124]. It is suggested that the effect of lipid content on the function of DCs may be twofold. For example, in an ovarian cancer model, inhibition of FASN reduces fat synthesis and recovers part of the function of DC cells, thus enhancing the anti-tumour immune response [125]. Researchers co-cultured DCs with secreted alpha-fetoprotein (AFP) in hepatocellular carcinoma and found that expression of SREBP-1 and PGC1-α decreased and down-regulated fatty acid synthesis, mitochondrial metabolism and the basal oxygen consumption rate (OCR), which led to DC dysfunction and immunosuppression [126], emphasizing the potential of AFP as a target for immunotherapy of hepatocellular carcinoma.
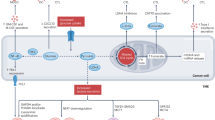
In addition to acting as metabolic substrates, fatty acids have a role as signalling molecules. Prostaglandin E2 (PGE2) is an unsaturated fatty acid produced by tumours that inhibits secretion of the chemokines CCL5 and XCL1 by NK cells, resulting in recruitment of cDCs and a decrease in chemokine receptors in cDCs. Such a decrease in cDC abundance in the TME leads to tumour immune escape (Fig. 3) [127]. The combination of PGE2 and Toll-like receptor agonists (TLRs) can be used as a new standard for DCs with the ability to secrete and migrate cytokines, namely, TLR-P DCs, which successfully induce the response of antigen-specific CD8 T cells to tumour-associated antigens and damage cross-presentation of protein antigens in human DCs and CD8 T cells in a dose-dependent manner [128]. Therefore, blocking the PGE2 signalling pathway can reverse tumour immune escape, which may be a promising target for tumour immunotherapy. The above findings show that enhancement of fatty acid synthesis and accumulation of fatty acids in the TME hinder the function of tumour-related DCs, resulting in a decrease in the ability of DCs to activate T cells to induce an anti-tumour immune response. Therefore, fatty acid metabolism targeting cancer DCs may have a positive therapeutic effect on improving the immunosuppressive tumour microenvironment.
Fatty acid metabolism and tumour immunotherapy
In recent years, researchers have regarded changes in fatty acid metabolism and related signals as markers of cancer and have revealed their importance for cancer progression through experimentation. Particularly in the TME, various fatty acids may play an important role in activating the anti-tumour immune response by regulating the function and differentiation of immune cells. Considering the role of fatty acid metabolism in the pathogenesis and progression of tumours, changes in fatty acid metabolism in immune cells are often regulated by various signals in tumour cells and further induce immune escape. Therefore, it is very important to explore how tumours affect the fatty acid metabolism of immune cells in the TME and to study the reprogramming of fatty acid metabolism as a target. In this section, we review studies on the relationship between fatty acid metabolism and tumour immunotherapy and explore the possibility of further development of immunotherapy (Fig. 4).
Immune checkpoint blockade (ICB) therapy is often referred to as an innovation in the field of cancer treatment and shows significant efficacy in many types of tumour treatment, but only approximately 1/4 of cancer patients can benefit from ICB because of immunosuppression and immune tolerance [129,130,131]. Encouragingly, one study found that fatty acid metabolism of targeted immune cells improves the efficacy of ICB. It was found that fenofibrate, a PPAR-α agonist, enhances the anti-tumour function of CD8+ TILs in mouse colon cancer and synergizes with a PD-1 blocker [132]. Similarly, PPAR-γ co-activator 1α (pGC-1α) has a synergistic effect with PD-1 blockers and may be achieved by up-regulating CPT1, which is necessary for the oxidation of CTL fatty acids, and forming a complex with Bcl2 to prevent CTL apoptosis [133]. In a recent study of a mouse Lewis lung cancer cell model, the pGC-1 α activator bezafibrate maintained the cell survival and function of CTLs by increasing expression of fatty acid oxidation-related genes (PGC-1α, CPT1A) in CTLs, and synergistic therapy with an anti-PD-L1 antibody enhanced its tumour-killing ability [134]. These experimental results show that pGC-1α agonists combined with PD-1/PD-L1 antibodies enhance the tumour-killing ability of CD8+ TILs and support the great potential of PPAR as a fatty acid metabolism target, as mentioned above.
Using metabolic drugs to modulate the immunosuppressive TME has been shown to be an effective therapeutic approach. Studies have shown that M2 TAMs and Treg cells enhance the immunosuppression of the tumour microenvironment and promote immune escape. Experiments have shown that Treg cells enhance the immunosuppressive TME by inhibiting the secretion of IFN-γ by CD8+ T cells, which is the basis of the TAM-M2 polarization induced by SREBP1 mediated by fatty acid synthesis [135]. In a tumour-bearing mouse model, an SREBP1 inhibitor led to a large lack of free fatty acids and resulted in an increase in CD8+ T cells and a decrease in M2 TAMs under combined treatment with anti-PD-1 therapy, enhanced antitumour immunity and inhibited tumour growth [135]. At the same time, Treg cells in the TME express the SREBP gene to mediate the antitumour immune response. FASN-mediated fatty acid synthesis and SREBP cleavage-activating protein (SCAP) cooperate to regulate PD-1 gene expression and maintain Treg cell inhibition [76]. It has been suggested that the SCAP/SREBP signalling pathway has a synergistic inhibitory effect with anti-PD-1 therapy in M2-TAM and Treg cells and enhances anti-tumour immunity.
In another study, detecting the concentration of ultra-long-chain fatty acids (VLCFAs) in serum was able to assess the therapeutic response of urinary cancer patients treated with immune checkpoint inhibitors (nivolumab or atezolizumab), which may be related to the fatty acid catabolism of immune cells [136]. There is a great deal of evidence that the intestinal microflora is closely related to response to immunotherapy [137,138,139,140]. Short-chain fatty acids (SCFAs), such as acetate, propionic acid and butyrate, are the main end products of intestinal microflora. High levels of single-chain fatty acids are significantly associated with better outcomes in patients receiving anti-PD-1 therapy [141, 142]. The ability of SCFAs to regulate immune function may explain the ability to predict treatment outcome, but whether it is also involved in a change in treatment outcome needs to be revealed by more studies.
Tumour growth was significantly inhibited after a patient’s white blood cells and tumour cells were infused back into the body. This treatment, which was first carried out in 1966, was later called adoptive cell therapy (ACT) [143]. Recently, many studies have suggested that fatty acid metabolism can regulate the memory stroke of T cells and play a vital role in maintaining the long-term efficacy of transplanted cells [45, 144, 145]. In vivo, TILs cultured with AKT inhibitors enhance memory characteristics and show enhanced persistence after adoptive transfer [146]. Mechanistically, accumulation of long-chain and polyunsaturated fatty acids leads to enhanced fatty acid oxidation in AKI-treated CD8+ T cells, and up-regulation of FAO enhances mitochondrial respiration. The use of AKI enhances anti-tumour immunity by promoting\differentiation of T cells into memory subsets and improving the efficiency of adoptive TIL [146]. On the other hand, activation of PPAR δ and PPAR α promotes FAO in activated CD8+ T cells, and enhanced production of IFN γ maintains the persistence and good function of T cells in the ACT model [60]. This is consistent with the report that CPT1 inhibitors combined with adoptive cell therapy show more significant anti-tumour function [147].
Chimeric antigen receptor T-cell (CAR-T) therapy is a kind of adoptive T-cell therapy that has been proven to have excellent therapeutic potential in haematological cancer [148, 149], but its application in solid tumours still faces great challenges [150]. Recent studies have found that the short-chain fatty acids (SCFAs) valerate and butyrate inhibit histone deacetylase (HDACs), increase production of effector factors (CD25, IFN-γ and TNF-α) and enhance the antitumor activity of receptor tyrosine kinase-like orphan receptor 1 (ROR1) targeting CAR T cells and CTLs in mouse melanoma and pancreatic cancer adoptive metastasis models [55], which has a positive effect on the clinical application of adoptive T-cell therapy.
The first batch of therapeutic cancer vaccines has been approved, and although they have performed well in enough preclinical trials, their clinical application in cancer patients has not yielded gratifying results [151]. The therapeutic cancer vaccine did not improve the overall and disease-free survival of patients with MAGE-A3-positive non-small cell lung cancer (MAGRIT) during phase 3 treatment [152]. Although we still do not fully understand the causes of tumour immune escape and immunosuppressive TME formation, considering the role of fatty acid metabolism in affecting immune capacity, we may be able to focus on combining therapeutic cancer vaccines with drugs targeting fatty acid metabolism of immune cells in the TME to enhance immune activity. Studies have shown that overexpression of PGC-1α increases differentiation of CD8 central memory T cells and that increased fatty acid oxidation in T cells enhances SRC, mediates stronger peptide vaccine memory ability and promotes anti-tumour immunity [153]. In a mouse melanoma model, tumour progression was delayed by the combination of a PPARα agonist and a cancer vaccine. Among them, the increased demand for fatty acids by tumour and stromal cells increases the chance of glucose uptake by T cells, which in turn enhances anti-tumour immunity [154]. According to the above experiments, the combination of therapeutic cancer vaccines and immune cell FA metabolic drugs appears to be a feasible strategy for cancer treatment and should be considered together with other immunotherapy methods; further research should be carried out (Table 2).
Conclusion
There may be metabolic competition for limited nutrients between tumour cells and immune cells, which is an important feature of the TME and has a great impact on immunotherapy and the clinical response of patients. Through metabolic adaptation to a variety of adverse and dynamic environments, tumour cells gradually show the ability to absorb a variety of nutrients to meet their growth needs. There has been increasing evidence that cancer provides energy and even information to tumour cells in harsh environments through fatty acid metabolism.
In this review, we summarize the latest research on fatty acid metabolism in immune cells in the TME and the application and prospects of fatty acid metabolism in tumour immunotherapy. Overall, the synthesis, decomposition, uptake and transmission of fatty acids participate in all levels of immune cell functions (including killing, activation and differentiation) through a large number of substrates from their own or other sources through metabolic pathways rather than simply providing energy. The metabolic characteristics of the tumour microenvironment characterized by acidification, hypoxia and energy depletion reveal the application of fatty acids, and performance in immune cells is even more impressive. For example, the use of short-chain fatty acids (SCFAs) can enhance the anti-tumour activity of CAR T cells and CTL cells by increasing the production of effector factors, which has unique therapeutic potential.
Considering the improvement of immunotherapy strategies, inhibition of a single pathway or a single enzyme does not reflect the outstanding potential of targeted fatty acid metabolism in cancer immunotherapy. Therefore, the combination of fatty acids and their synthesis, functional pathway and even the whole tumour immune microenvironment as the target may provide a direction for the implementation of the new combined therapy strategy. Hence, tumour immunotherapy strategies, including immune checkpoint blocking, adoptive cell therapy and cancer vaccines, can target the dependence of tumour cells on fatty acids in the form of drug targeting. This approach was combined with the traditional model to exert a synergistic anti-tumour effect. However, due to the different types of cancer and the uniqueness of the TME, it is still a problem to find accurate targets and reduce possible side effects. Therefore, it is of great significance to explore the target of fatty acid metabolism of immune cells in the TME to enhance the ability of anti-tumour immunotherapy, and in the future, more researchers will devote efforts to understanding the function and interaction of immune cells and fatty acid metabolism in the TME to find a new way to improve the efficiency of tumour immunotherapy.
References
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74.
Pavlova NN, Thompson CB. The emerging hallmarks of cancer metabolism. Cell Metab. 2016;23:27–47.
Bader JE, Voss K, Rathmell JC. Targeting metabolism to improve the tumor microenvironment for cancer immunotherapy. Mol Cell. 2020;78:1019–33.
Luo Y, Wang H, Liu B, Wei J. Fatty acid metabolism and cancer immunotherapy. Curr Oncol Rep. 2022;24:659–70.
Li Y, Wan YY, Zhu B. Immune cell metabolism in tumor microenvironment. Adv Exp Med Biol. 2017;1011:163–96.
Zeng W, Yin X, Jiang Y, Jin L, Liang W. PPARalpha at the crossroad of metabolic-immune regulation in cancer. FEBS J. 2022;289:7726–39.
Yang Y. Cancer immunotherapy: harnessing the immune system to battle cancer. J Clin Invest. 2015;125:3335–7.
Chen X, Chen S, Yu D. Metabolic reprogramming of chemoresistant cancer cells and the potential significance of metabolic regulation in the reversal of cancer chemoresistance. Metabolites. 2020;10:289.
Corazao-Rozas P, Guerreschi P, Andre F, Gabert PE, Lancel S, Dekiouk S, et al. Mitochondrial oxidative phosphorylation controls cancer cell’s life and death decisions upon exposure to MAPK inhibitors. Oncotarget. 2016;7:39473–85.
Warburg O. On the origin of cancer cells. Science. 1956;123:309–14.
Altman BJ, Stine ZE, Dang CV. From Krebs to clinic: glutamine metabolism to cancer therapy. Nat Rev Cancer. 2016;16:619–34.
Bacci M, Lorito N, Smiriglia A, Morandi A. Fat and furious: lipid metabolism in antitumoral therapy response and resistance. Trends Cancer. 2021;7:198–213.
Baron A, Migita T, Tang D, Loda M. Fatty acid synthase: a metabolic oncogene in prostate cancer? J Cell Biochem. 2004;91:47–53.
Germain N, Dhayer M, Boileau M, Fovez Q, Kluza J, Marchetti P. Lipid metabolism and resistance to anticancer treatment. Biology (Basel). 2020;9:474.
Hay N. Reprogramming glucose metabolism in cancer: can it be exploited for cancer therapy? Nat Rev Cancer. 2016;16:635–49.
Luo X, Cheng C, Tan Z, Li N, Tang M, Yang L, et al. Emerging roles of lipid metabolism in cancer metastasis. Mol Cancer. 2017;16:76.
Currie E, Schulze A, Zechner R, Walther TC, Farese RV Jr. Cellular fatty acid metabolism and cancer. Cell Metab. 2013;18:153–61.
Huang B, Song BL, Xu C. Cholesterol metabolism in cancer: mechanisms and therapeutic opportunities. Nat Metab. 2020;2:132–41.
Zaidi N, Lupien L, Kuemmerle NB, Kinlaw WB, Swinnen JV, Smans K. Lipogenesis and lipolysis: the pathways exploited by the cancer cells to acquire fatty acids. Prog Lipid Res. 2013;52:585–9.
Bian X, Liu R, Meng Y, Xing D, Xu D, Lu Z. Lipid metabolism and cancer. J Exp Med. 2021;218:e20201606.
Snaebjornsson MT, Janaki-Raman S, Schulze A. Greasing the wheels of the cancer machine: the role of lipid metabolism in cancer. Cell Metab. 2020;31:62–76.
Kuhajda FP, Pizer ES, Li JN, Mani NS, Frehywot GL, Townsend CA. Synthesis and antitumor activity of an inhibitor of fatty acid synthase. Proc Natl Acad Sci USA. 2000;97:3450–4.
Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007;7:763–77.
Zhou W, Han WF, Landree LE, Thupari JN, Pinn ML, Bililign T, et al. Fatty acid synthase inhibition activates AMP-activated protein kinase in SKOV3 human ovarian cancer cells. Cancer Res. 2007;67:2964–71.
Kuemmerle NB, Rysman E, Lombardo PS, Flanagan AJ, Lipe BC, Wells WA, et al. Lipoprotein lipase links dietary fat to solid tumor cell proliferation. Mol Cancer Ther. 2011;10:427–36.
Nomura DK, Long JZ, Niessen S, Hoover HS, Ng SW, Cravatt BF. Monoacylglycerol lipase regulates a fatty acid network that promotes cancer pathogenesis. Cell. 2010;140:49–61.
Zhang S, Peng X, Yang S, Li X, Huang M, Wei S, et al. The regulation, function, and role of lipophagy, a form of selective autophagy, in metabolic disorders. Cell Death Dis. 2022;13:132.
Rohrig F, Schulze A. The multifaceted roles of fatty acid synthesis in cancer. Nat Rev Cancer. 2016;16:732–49.
Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, et al. Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature. 2013;496:238–42.
Qu Q, Zeng F, Liu X, Wang QJ, Deng F. Fatty acid oxidation and carnitine palmitoyltransferase I: emerging therapeutic targets in cancer. Cell Death Dis. 2016;7:e2226.
Ma Y, Temkin SM, Hawkridge AM, Guo C, Wang W, Wang XY, et al. Fatty acid oxidation: an emerging facet of metabolic transformation in cancer. Cancer Lett. 2018;435:92–100.
Su X, Abumrad NA. Cellular fatty acid uptake: a pathway under construction. Trends Endocrinol Metab. 2009;20:72–7.
Yin X, Zeng W, Wu B, Wang L, Wang Z, Tian H, et al. PPARalpha inhibition overcomes tumor-derived exosomal lipid-induced dendritic cell dysfunction. Cell Rep. 2020;33:108278.
Calder PC. Fuel utilization by cells of the immune system. Proc Nutr Soc. 1995;54:65–82.
Calder PC. Feeding the immune system. Proc Nutr Soc. 2013;72:299–309.
Zhang X, Zink F, Hezel F, Vogt J, Wachter U, Wepler M, et al. Metabolic substrate utilization in stress-induced immune cells. Intensive Care Med Exp. 2020;8:28.
Boothby M, Rickert RC. Metabolic regulation of the immune humoral response. Immunity. 2017;46:743–55.
MacIver NJ, Michalek RD, Rathmell JC. Metabolic regulation of T lymphocytes. Annu Rev Immunol. 2013;31:259–83.
Van den Bossche J, Saraber DL. Metabolic regulation of macrophages in tissues. Cell Immunol. 2018;330:54–9.
O’Neill LA, Kishton RJ, Rathmell J. A guide to immunometabolism for immunologists. Nat Rev Immunol. 2016;16:553–65.
Howie D, Ten Bokum A, Necula AS, Cobbold SP, Waldmann H. The role of lipid metabolism in T lymphocyte differentiation and survival. Front Immunol. 2017;8:1949.
Hubler MJ, Kennedy AJ. Role of lipids in the metabolism and activation of immune cells. J Nutr Biochem. 2016;34:1–7.
Endo Y, Kanno T, Nakajima T. Fatty acid metabolism in T-cell function and differentiation. Int Immunol. 2022;34:579–87.
Zhang Y, Kurupati R, Liu L, Zhou XY, Zhang G, Hudaihed A, et al. Enhancing CD8(+) T cell fatty acid catabolism within a metabolically challenging tumor microenvironment increases the efficacy of melanoma immunotherapy. Cancer Cell. 2017;32:377–91.e9.
Lochner M, Berod L, Sparwasser T. Fatty acid metabolism in the regulation of T cell function. Trends Immunol. 2015;36:81–91.
Raud B, McGuire PJ, Jones RG, Sparwasser T, Berod L. Fatty acid metabolism in CD8(+) T cell memory: challenging current concepts. Immunol Rev. 2018;283:213–31.
Zhang C, Yue C, Herrmann A, Song J, Egelston C, Wang T, et al. STAT3 activation-induced fatty acid oxidation in CD8(+) T effector cells is critical for obesity-promoted breast tumor growth. Cell Metab. 2020;31:148–61.e5.
Patsoukis N, Bardhan K, Chatterjee P, Sari D, Liu B, Bell LN, et al. PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation. Nat Commun. 2015;6:6692.
Brown RE, Steele RW, Marmer DJ, Hudson JL, Brewster MA. Fatty acids and the inhibition of mitogen-induced lymphocyte transformation by leukemic serum. J Immunol. 1983;131:1011–6.
Kleinfeld AM, Okada C. Free fatty acid release from human breast cancer tissue inhibits cytotoxic T-lymphocyte-mediated killing. J Lipid Res. 2005;46:1983–90.
Lin R, Zhang H, Yuan Y, He Q, Zhou J, Li S, et al. Fatty acid oxidation controls CD8(+) tissue-resident memory T-cell survival in gastric adenocarcinoma. Cancer Immunol Res. 2020;8:479–92.
van der Windt GJ, Everts B, Chang CH, Curtis JD, Freitas TC, Amiel E, et al. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity. 2012;36:68–78.
Klein Geltink RI, O’Sullivan D, Corrado M, Bremser A, Buck MD, Buescher JM, et al. Mitochondrial priming by CD28. Cell. 2017;171:385–97.e11.
Sun P, Zhang X, Wang RJ, Ma QY, Xu L, Wang Y, et al. PI3Kalpha inhibitor CYH33 triggers antitumor immunity in murine breast cancer by activating CD8(+)T cells and promoting fatty acid metabolism. J Immunother Cancer. 2021;9:e003093.
Luu M, Riester Z, Baldrich A, Reichardt N, Yuille S, Busetti A, et al. Microbial short-chain fatty acids modulate CD8(+) T cell responses and improve adoptive immunotherapy for cancer. Nat Commun. 2021;12:4077.
Navarro MN, Cantrell DA. Serine-threonine kinases in TCR signaling. Nat Immunol. 2014;15:808–14.
Pollizzi KN, Patel CH, Sun IH, Oh MH, Waickman AT, Wen J, et al. mTORC1 and mTORC2 selectively regulate CD8(+) T cell differentiation. J Clin Invest. 2015;125:2090–108.
Basingab FS, Ahmadi M, Morgan DJ. IFNgamma-dependent interactions between ICAM-1 and LFA-1 counteract prostaglandin E2-mediated inhibition of antitumor CTL responses. Cancer Immunol Res. 2016;4:400–11.
Chowdhury PS, Chamoto K, Kumar A, Honjo T. PPAR-induced fatty acid oxidation in T cells increases the number of tumor-reactive CD8(+) T cells and facilitates anti-PD-1 therapy. Cancer Immunol Res. 2018;6:1375–87.
Saibil SD, St Paul M, Laister RC, Garcia-Batres CR, Israni-Winger K, Elford AR, et al. Activation of peroxisome proliferator-activated receptors alpha and delta synergizes with inflammatory signals to enhance adoptive cell therapy. Cancer Res. 2019;79:445–51.
Franco F, Jaccard A, Romero P, Yu YR, Ho PC. Metabolic and epigenetic regulation of T-cell exhaustion. Nat Metab. 2020;2:1001–12.
Sen DR, Kaminski J, Barnitz RA, Kurachi M, Gerdemann U, Yates KB, et al. The epigenetic landscape of T cell exhaustion. Science. 2016;354:1165–9.
Thommen DS, Koelzer VH, Herzig P, Roller A, Trefny M, Dimeloe S, et al. A transcriptionally and functionally distinct PD-1(+) CD8(+) T cell pool with predictive potential in non-small-cell lung cancer treated with PD-1 blockade. Nat Med. 2018;24:994–1004.
Ma X, Xiao L, Liu L, Ye L, Su P, Bi E, et al. CD36-mediated ferroptosis dampens intratumoral CD8(+) T cell effector function and impairs their antitumor ability. Cell Metab. 2021;33:1001–12.e5.
Xu S, Chaudhary O, Rodriguez-Morales P, Sun X, Chen D, Zappasodi R, et al. Uptake of oxidized lipids by the scavenger receptor CD36 promotes lipid peroxidation and dysfunction in CD8(+) T cells in tumors. Immunity. 2021;54:1561–77.e7.
Maciolek JA, Pasternak JA, Wilson HL. Metabolism of activated T lymphocytes. Curr Opin Immunol. 2014;27:60–74.
El Hage A, Dormond O. Combining mTOR inhibitors and T cell-based immunotherapies in cancer treatment. Cancers (Basel). 2021;13:1359.
Berod L, Friedrich C, Nandan A, Freitag J, Hagemann S, Harmrolfs K, et al. De novo fatty acid synthesis controls the fate between regulatory T and T helper 17 cells. Nat Med. 2014;20:1327–33.
Hradilkova K, Maschmeyer P, Westendorf K, Schliemann H, Husak O, von Stuckrad ASL, et al. Regulation of fatty acid oxidation by twist 1 in the metabolic adaptation of T helper lymphocytes to chronic inflammation. Arthritis Rheumatol. 2019;71:1756–65.
Cluxton D, Petrasca A, Moran B, Fletcher JM. Differential regulation of human treg and Th17 cells by fatty acid synthesis and glycolysis. Front Immunol. 2019;10:115.
Michalek RD, Gerriets VA, Jacobs SR, Macintyre AN, MacIver NJ, Mason EF, et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol. 2011;186:3299–303.
Pacella I, Procaccini C, Focaccetti C, Miacci S, Timperi E, Faicchia D, et al. Fatty acid metabolism complements glycolysis in the selective regulatory T cell expansion during tumor growth. Proc Natl Acad Sci USA. 2018;115:E6546–E55.
Xu C, Fu Y, Liu S, Trittipo J, Lu X, Qi R, et al. BATF regulates t regulatory cell functional specification and fitness of triglyceride metabolism in restraining allergic responses. J Immunol. 2021;206:2088–100.
Sun Y, Jing J, Xu H, Xu L, Hu H, Tang C, et al. N-cadherin inhibitor creates a microenvironment that protect TILs from immune checkpoints and Treg cells. J Immunother Cancer. 2021;9:e002138.
Kumagai S, Togashi Y, Sakai C, Kawazoe A, Kawazu M, Ueno T, et al. An oncogenic alteration creates a microenvironment that promotes tumor progression by conferring a metabolic advantage to regulatory T cells. Immunity. 2020;53:187–203.e8.
Lim SA, Wei J, Nguyen TM, Shi H, Su W, Palacios G, et al. Lipid signalling enforces functional specialization of Treg cells in tumours. Nature. 2021;591:306–11.
Angelin A, Gil-de-Gomez L, Dahiya S, Jiao J, Guo L, Levine MH, et al. Foxp3 reprograms T cell metabolism to function in low-glucose, high-lactate environments. Cell Metab. 2017;25:1282–93.e7.
Miska J, Lee-Chang C, Rashidi A, Muroski ME, Chang AL, Lopez-Rosas A, et al. HIF-1alpha is a metabolic switch between glycolytic-driven migration and oxidative phosphorylation-driven immunosuppression of tregs in glioblastoma. Cell Rep. 2019;27:226–37.e4.
Pollizzi KN, Powell JD. Integrating canonical and metabolic signalling programmes in the regulation of T cell responses. Nat Rev Immunol. 2014;14:435–46.
Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao B, et al. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–44.
Kang J, Huddleston SJ, Fraser JM, Khoruts A. De novo induction of antigen-specific CD4+CD25+Foxp3+ regulatory T cells in vivo following systemic antigen administration accompanied by blockade of mTOR. J Leukoc Biol. 2008;83:1230–9.
Wang H, Franco F, Tsui YC, Xie X, Trefny MP, Zappasodi R, et al. CD36-mediated metabolic adaptation supports regulatory T cell survival and function in tumors. Nat Immunol. 2020;21:298–308.
Liu X, Zhang J, Zeigler AC, Nelson AR, Lindsey ML, Saucerman JJ. Network analysis reveals a distinct axis of macrophage activation in response to conflicting inflammatory cues. J Immunol. 2021;206:883–91.
Williams JW, Giannarelli C, Rahman A, Randolph GJ, Kovacic JC. Macrophage biology, classification, and phenotype in cardiovascular disease: JACC macrophage in CVD series (Part 1). J Am Coll Cardiol. 2018;72:2166–80.
Cassetta L, Fragkogianni S, Sims AH, Swierczak A, Forrester LM, Zhang H, et al. Human tumor-associated macrophage and monocyte transcriptional landscapes reveal cancer-specific reprogramming, biomarkers, and therapeutic targets. Cancer Cell. 2019;35:588–602.e10.
Pan Y, Yu Y, Wang X, Zhang T. Tumor-associated macrophages in tumor immunity. Front Immunol. 2020;11:583084.
Silveira LS, Antunes Bde M, Minari AL, Dos Santos RV, Neto JC, Lira FS. Macrophage polarization: implications on metabolic diseases and the role of exercise. Crit Rev Eukaryot Gene Expr. 2016;26:115–32.
Cook J, Hagemann T. Tumour-associated macrophages and cancer. Curr Opin Pharm. 2013;13:595–601.
Wang F, Zhang S, Vuckovic I, Jeon R, Lerman A, Folmes CD, et al. Glycolytic stimulation is not a requirement for M2 macrophage differentiation. Cell Metab. 2018;28:463–75.e4.
Namgaladze D, Brune B. Fatty acid oxidation is dispensable for human macrophage IL-4-induced polarization. Biochim Biophys Acta. 2014;1841:1329–35.
Su P, Wang Q, Bi E, Ma X, Liu L, Yang M, et al. Enhanced lipid accumulation and metabolism are required for the differentiation and activation of tumor-associated macrophages. Cancer Res. 2020;80:1438–50.
Wu H, Han Y, Rodriguez Sillke Y, Deng H, Siddiqui S, Treese C, et al. Lipid droplet-dependent fatty acid metabolism controls the immune suppressive phenotype of tumor-associated macrophages. EMBO Mol Med. 2019;11:e10698.
Leone RD, Powell JD. Metabolism of immune cells in cancer. Nat Rev Cancer. 2020;20:516–31.
Menegaut L, Thomas C, Lagrost L, Masson D. Fatty acid metabolism in macrophages: a target in cardio-metabolic diseases. Curr Opin Lipido. 2017;28:19–26.
Sica A, Larghi P, Mancino A, Rubino L, Porta C, Totaro MG, et al. Macrophage polarization in tumour progression. Semin Cancer Biol. 2008;18:349–55.
Johnson AR, Qin Y, Cozzo AJ, Freemerman AJ, Huang MJ, Zhao L, et al. Metabolic reprogramming through fatty acid transport protein 1 (FATP1) regulates macrophage inflammatory potential and adipose inflammation. Mol Metab. 2016;5:506–26.
Cui J, Shan K, Yang Q, Qi Y, Qu H, Li J, et al. Prostaglandin E3 attenuates macrophage-associated inflammation and prostate tumour growth by modulating polarization. J Cell Mol Med. 2021;25:5586–601.
Albakri MM, Huang SC, Tashkandi HN, Sieg SF. Fatty acids secreted from head and neck cancer induce M2-like macrophages. J Leukoc Biol. 2022;112:617–28.
Liang P, Henning SM, Grogan T, Elashoff D, Ye H, Cohen P, et al. Effects of dietary omega-3 fatty acids on orthotopic prostate cancer progression, tumor associated macrophages, angiogenesis and T-cell activation-dependence on GPR120. Prostate Cancer Prostatic Dis. 2022;25:539–46.
Liu D, Chang C, Lu N, Wang X, Lu Q, Ren X, et al. Comprehensive proteomics analysis reveals metabolic reprogramming of tumor-associated macrophages stimulated by the tumor microenvironment. J Proteome Res. 2017;16:288–97.
Wu H, Weidinger C, Schmidt F, Keye J, Friedrich M, Yerinde C, et al. Oleate but not stearate induces the regulatory phenotype of myeloid suppressor cells. Sci Rep. 2017;7:7498.
Nelson VL, Nguyen HCB, Garcia-Canaveras JC, Briggs ER, Ho WY, DiSpirito JR, et al. PPARgamma is a nexus controlling alternative activation of macrophages via glutamine metabolism. Genes Dev. 2018;32:1035–44.
Schug TT, Li X. PPARdelta-mediated macrophage activation: a matter of fat. Dis Model Mech. 2009;2:421–2.
Niu Z, Shi Q, Zhang W, Shu Y, Yang N, Chen B, et al. Caspase-1 cleaves PPARgamma for potentiating the pro-tumor action of TAMs. Nat Commun. 2017;8:766.
Odegaard JI, Ricardo-Gonzalez RR, Red Eagle A, Vats D, Morel CR, Goforth MH, et al. Alternative M2 activation of Kupffer cells by PPARdelta ameliorates obesity-induced insulin resistance. Cell Metab. 2008;7:496–507.
Wu L, Zhang X, Zheng L, Zhao H, Yan G, Zhang Q, et al. RIPK3 Orchestrates fatty acid metabolism in tumor-associated macrophages and hepatocarcinogenesis. Cancer Immunol Res. 2020;8:710–21.
Schumann T, Adhikary T, Wortmann A, Finkernagel F, Lieber S, Schnitzer E, et al. Deregulation of PPARbeta/delta target genes in tumor-associated macrophages by fatty acid ligands in the ovarian cancer microenvironment. Oncotarget. 2015;6:13416–33.
Bozza PT, Magalhaes KG, Weller PF. Leukocyte lipid bodies—biogenesis and functions in inflammation. Biochim Biophys Acta. 2009;1791:540–51.
Bozza PT, Viola JP. Lipid droplets in inflammation and cancer. Prostaglandins Leukot Ess Fat Acids. 2010;82:243–50.
den Brok MH, Raaijmakers TK, Collado-Camps E, Adema GJ. Lipid droplets as immune modulators in myeloid cells. Trends Immunol. 2018;39:380–92.
Walther TC, Farese RV Jr. Lipid droplets and cellular lipid metabolism. Annu Rev Biochem. 2012;81:687–714.
Yang Q, Guo N, Zhou Y, Chen J, Wei Q, Han M. The role of tumor-associated macrophages (TAMs) in tumor progression and relevant advance in targeted therapy. Acta Pharm Sin B. 2020;10:2156–70.
Ramesh A, Malik V, Brouillard A, Kulkarni A. Supramolecular nanotherapeutics enable metabolic reprogramming of tumor-associated macrophages to inhibit tumor growth. J Biomed Mater Res A. 2022;110:1448–59.
Cao S, Saw PE, Shen Q, Li R, Liu Y, Xu X. Reduction-responsive RNAi nanoplatform to reprogram tumor lipid metabolism and repolarize macrophage for combination pancreatic cancer therapy. Biomaterials. 2022;280:121264.
Bonapace L, Coissieux MM, Wyckoff J, Mertz KD, Varga Z, Junt T, et al. Cessation of CCL2 inhibition accelerates breast cancer metastasis by promoting angiogenesis. Nature. 2014;515:130–3.
Keklikoglou I, De Palma M. Cancer: Metastasis risk after anti-macrophage therapy. Nature. 2014;515:46–7.
Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol. 2013;31:563–604.
Everts B, Amiel E, Huang SC, Smith AM, Chang CH, Lam WY, et al. TLR-driven early glycolytic reprogramming via the kinases TBK1-IKKvarepsilon supports the anabolic demands of dendritic cell activation. Nat Immunol. 2014;15:323–32.
Krawczyk CM, Holowka T, Sun J, Blagih J, Amiel E, DeBerardinis RJ, et al. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood. 2010;115:4742–9.
Wu D, Sanin DE, Everts B, Chen Q, Qiu J, Buck MD, et al. Type 1 interferons induce changes in core metabolism that are critical for immune function. Immunity. 2016;44:1325–36.
Kobayashi S, Wannakul T, Sekino K, Takahashi Y, Kagawa Y, Miyazaki H, et al. Fatty acid-binding protein 5 limits the generation of Foxp3(+) regulatory T cells through regulating plasmacytoid dendritic cell function in the tumor microenvironment. Int J Cancer. 2022;150:152–63.
Herber DL, Cao W, Nefedova Y, Novitskiy SV, Nagaraj S, Tyurin VA, et al. Lipid accumulation and dendritic cell dysfunction in cancer. Nat Med. 2010;16:880–6.
Ramakrishnan R, Tyurin VA, Veglia F, Condamine T, Amoscato A, Mohammadyani D, et al. Oxidized lipids block antigen cross-presentation by dendritic cells in cancer. J Immunol. 2014;192:2920–31.
Ibrahim J, Nguyen AH, Rehman A, Ochi A, Jamal M, Graffeo CS, et al. Dendritic cell populations with different concentrations of lipid regulate tolerance and immunity in mouse and human liver. Gastroenterology. 2012;143:1061–72.
Jiang L, Fang X, Wang H, Li D, Wang X. Ovarian cancer-intrinsic fatty acid synthase prevents anti-tumor immunity by disrupting tumor-infiltrating dendritic cells. Front Immunol. 2018;9:2927.
Santos PM, Menk AV, Shi J, Tsung A, Delgoffe GM, Butterfield LH. Tumor-derived alpha-fetoprotein suppresses fatty acid metabolism and oxidative phosphorylation in dendritic cells. Cancer Immunol Res. 2019;7:1001–12.
Bottcher JP, Bonavita E, Chakravarty P, Blees H, Cabeza-Cabrerizo M, Sammicheli S, et al. NK cells stimulate recruitment of cDC1 into the tumor microenvironment promoting cancer immune control. Cell. 2018;172:1022–37.e14.
Gierlich P, Lex V, Technau A, Keupp A, Morper L, Glunz A, et al. Prostaglandin E2 in a TLR3- and 7/8-agonist-based DC maturation cocktail generates mature, cytokine-producing, migratory DCs but impairs antigen cross-presentation to CD8(+) T cells. Cancer Immunol Immunother. 2020;69:1029–42.
Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol 2018;4:e180013.
Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. Updated analysis of KEYNOTE-024: pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer With PD-L1 tumor proportion score of 50% or greater. J Clin Oncol. 2019;37:537–46.
Wu YL, Lu S, Cheng Y, Zhou C, Wang J, Mok T, et al. Nivolumab versus docetaxel in a predominantly Chinese patient population with previously treated advanced NSCLC: CheckMate 078 randomized phase III clinical trial. J Thorac Oncol. 2019;14:867–75.
Chamoto K, Chowdhury PS, Kumar A, Sonomura K, Matsuda F, Fagarasan S, et al. Mitochondrial activation chemicals synergize with surface receptor PD-1 blockade for T cell-dependent antitumor activity. Proc Natl Acad Sci USA. 2017;114:E761–E70.
Scharping NE, Menk AV, Moreci RS, Whetstone RD, Dadey RE, Watkins SC, et al. The tumor microenvironment represses T cell mitochondrial biogenesis to drive intratumoral T cell metabolic insufficiency and dysfunction. Immunity. 2016;45:374–88.
Wan H, Xu B, Zhu N, Ren B. PGC-1alpha activator-induced fatty acid oxidation in tumor-infiltrating CTLs enhances effects of PD-1 blockade therapy in lung cancer. Tumori. 2020;106:55–63.
Liu C, Chikina M, Deshpande R, Menk AV, Wang T, Tabib T, et al. Treg cells promote the SREBP1-dependent metabolic fitness of tumor-promoting macrophages via repression of CD8(+) T cell-derived interferon-gamma. Immunity. 2019;51:381–97.e6.
Mock A, Zschabitz S, Kirsten R, Scheffler M, Wolf B, Herold-Mende C, et al. Serum very long-chain fatty acid-containing lipids predict response to immune checkpoint inhibitors in urological cancers. Cancer Immunol Immunother. 2019;68:2005–14.
Lurienne L, Cervesi J, Duhalde L, de Gunzburg J, Andremont A, Zalcman G, et al. NSCLC immunotherapy efficacy and antibiotic use: a systematic review and meta-analysis. J Thorac Oncol. 2020;15:1147–59.
Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillere R, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359:91–7.
Wilson BE, Routy B, Nagrial A, Chin VT. The effect of antibiotics on clinical outcomes in immune-checkpoint blockade: a systematic review and meta-analysis of observational studies. Cancer Immunol Immunother. 2020;69:343–54.
Yu Y, Zheng P, Gao L, Li H, Tao P, Wang D, et al. Effects of antibiotic use on outcomes in cancer patients treated using immune checkpoint inhibitors: a systematic review and meta-analysis. J Immunother. 2021;44:76–85.
Botticelli A, Vernocchi P, Marini F, Quagliariello A, Cerbelli B, Reddel S, et al. Gut metabolomics profiling of non-small cell lung cancer (NSCLC) patients under immunotherapy treatment. J Transl Med. 2020;18:49.
Nomura M, Nagatomo R, Doi K, Shimizu J, Baba K, Saito T, et al. Association of short-chain fatty acids in the gut microbiome with clinical response to treatment with nivolumab or pembrolizumab in patients with solid cancer tumors. JAMA Netw Open. 2020;3:e202895.
Southam CM, Brunschwig A, Levin AG, Dizon QS. Effect of leukocytes on transplantability of human cancer. Cancer. 1966;19:1743–53.
Busch DH, Frassle SP, Sommermeyer D, Buchholz VR, Riddell SR. Role of memory T cell subsets for adoptive immunotherapy. Semin Immunol. 2016;28:28–34.
Chodon T, Comin-Anduix B, Chmielowski B, Koya RC, Wu Z, Auerbach M, et al. Adoptive transfer of MART-1 T-cell receptor transgenic lymphocytes and dendritic cell vaccination in patients with metastatic melanoma. Clin Cancer Res. 2014;20:2457–65.
Crompton JG, Sukumar M, Roychoudhuri R, Clever D, Gros A, Eil RL, et al. Akt inhibition enhances expansion of potent tumor-specific lymphocytes with memory cell characteristics. Cancer Res. 2015;75:296–305.
Hossain F, Al-Khami AA, Wyczechowska D, Hernandez C, Zheng L, Reiss K, et al. Inhibition of fatty acid oxidation modulates immunosuppressive functions of myeloid-derived suppressor cells and enhances cancer therapies. Cancer Immunol Res. 2015;3:1236–47.
Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl J Med. 2014;371:1507–17.
Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N. Engl J Med. 2017;377:2531–44.
Martinez M, Moon EK. CAR T cells for solid tumors: new strategies for finding, infiltrating, and surviving in the tumor microenvironment. Front Immunol. 2019;10:128.
Song Q, Zhang CD, Wu XH. Therapeutic cancer vaccines: from initial findings to prospects. Immunol Lett. 2018;196:11–21.
Vansteenkiste JF, Cho BC, Vanakesa T, De Pas T, Zielinski M, Kim MS, et al. Efficacy of the MAGE-A3 cancer immunotherapeutic as adjuvant therapy in patients with resected MAGE-A3-positive non-small-cell lung cancer (MAGRIT): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2016;17:822–35.
Dumauthioz N, Tschumi B, Wenes M, Marti B, Wang H, Franco F, et al. Enforced PGC-1alpha expression promotes CD8 T cell fitness, memory formation and antitumor immunity. Cell Mol Immunol. 2021;18:1761–71.
Chekaoui A, Ertl HCJ. PPARalpha agonist fenofibrate enhances cancer vaccine efficacy. Cancer Res. 2021;81:4431–40.
Zhao F, Xiao C, Evans KS, Theivanthiran T, DeVito N, Holtzhausen A, et al. Paracrine Wnt5a-beta-catenin signaling triggers a metabolic program that drives dendritic cell tolerization. Immunity. 2018;48:147–60.e7.
Acknowledgements
I gratefully acknowledge the assistance of Fusheng Zhang who helped me wrote the figures.
Funding
This study was supported by grants from the National Natural Science Foundation of China (81960043), the Science and Technology Innovation Base Construction Project of Jiangxi Province (20212BCG74001) and the Program for Academic and Technical Leaders of Jiangxi Province (20213BCJ22016).
Author information
Authors and Affiliations
Contributions
SZ performed a literature investigation and wrote the manuscript. All authors have reviewed the paper and all approved of the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, S., Lv, K., Liu, Z. et al. Fatty acid metabolism of immune cells: a new target of tumour immunotherapy. Cell Death Discov. 10, 39 (2024). https://doi.org/10.1038/s41420-024-01807-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41420-024-01807-9