Abstract
Toxoplasma gondii, a widespread obligate intracellular parasite, can infect almost all warm-blooded animals, including humans. The cellular barrier of the central nervous system (CNS) is generally able to protect the brain parenchyma from infectious damage. However, T. gondii typically causes latent brain infections in humans and other vertebrates. Here, we discuss how T. gondii rhoptry proteins (ROPs) affect signaling pathways in host cells and speculate how this might affect the outcome of Toxoplasma encephalitis.
Similar content being viewed by others
Facts
-
In immunocompromised patients or in individuals with an immature immune system, Toxoplasma infection may lead to severe clinical manifestations, such as Toxoplasma encephalitis (TE), which in more extreme cases may lead to death.
-
ROP plays multiple roles in T. gondii infection, such as participating in host cell invasion, monitoring immune signals from host cells, and acting as an important virulence factor in T. gondii.
-
Studying the effect of ROP will offer broad research prospects for the treatment of toxoplasmosis.
Open questions
-
How can ROP be regulated so that ROP can have different functions under different conditions?
-
Immune cells are thought to promote the transmission of T. gondii in the brain, which may be one of the methods that the parasite uses to reach neurons.Are there other routes involved in the transmission of T. gondii in the brain?
-
Can drugs be used to treat toxoplasmosis by inhibiting the expression of ROPs?
Introduction
Toxoplasma gondii (T. gondii) is a zoonotic parasite that belongs to the phylum Apicomplexa [1]. Although the seropositivity rate has decreased in European countries over the past 20 years [2, 3], the prevalence of T. gondii remains very high in African countries [4, 5]. T. gondii can infect a host in a variety of ways. When humans and other intermediate hosts are infected via oocysts or tissue cysts from ingested parasites in contaminated food or water, parasites at the sporozoite and oocyst stages can enter the intestinal tract, pass through the blood and lymphatic system, and then gradually spread to various organs and tissues in the body to become tachyzoites [6]. Tachyzoites may transform into bradyzoites and form cysts in response to attack by the host’s immune system. Expectant mothers infected with T. gondii during pregnancy may experience vertical transmission of the parasite to the fetus, resulting in fetal malformation or abortion [7]. In addition, T. gondii is a central nervous system (CNS) parasite that can cause toxoplasmosis encephalitis (TE) after infection [8]. TE has high incidence and mortality rates [9]. Furthermore, the host may exhibit neurodegenerative diseases, such as Alzheimer’s disease, paralysis, and epilepsy, after T. gondii enters the CNS [10].
Throughout an infection, it is important to understand how the parasite reproduces within host cells and avoids host cell defenses. The early stage of invasion by T. gondii is achieved through the interaction between T. gondii transmembrane (TM) proteins and surface receptors on the host cell in a process that involves three steps: (1) Identifying host cells. T. gondii relies on its unique actin-based gliding motility to glide on the surface of the cell membrane by binding between receptors on the surface of the worm to ligands on the cell surface. (2) Forming a “moving junction” (MJ). After the parasite finds a specific location on the host cell surface for invasion, a tight junction (TJ) structure between its apical tip and the host cell membrane forms. Then, relying on the sliding system and related secreted proteins, the worm rapidly internalizes into the host cell. (3) Forming parasitophorus vacuoles (PVs). Once inside the cell, the invaginated host cell membrane wraps around the body of the parasite to form a PV. At the same time, under the action of dense granule (GRA) proteins, the composition of the PV membrane changes, preventing it from fusing with the host cell. In addition, PV membranes can be connected to parasites through the intravacuolar network (IVN) of host-derived lipid nanotubes. Parasites use secreted effectors to maintain the PV and IVN membranes, recruit nutrients into vacuoles, and evade immune recognition. Ultimately, T. gondii undergoes further development and proliferation within PVs.
Toxoplasma proliferates by endodyogeny in any nucleated cell type. This process mainly involves replication of the Golgi apparatus in late G1 phase, the replication of centrosomes in S early phase, and budding in S late phase. During this period, the division of the nucleus, endoplasmic reticulum (ER), and mitochondria also occurs. When the offspring parasite matures, the maternal membrane covers the offspring surface. The following parasite division cycle will continue until host cell resources are depleted, ultimately leading to the active exit from and destruction of the infected host cell.
During the whole invasion process, secretory organelles, including micronemes (MICs), dense granules, rhoptries and others, are all involved.
MICs
Microndosome proteins are also secreted in the free state, but their abundance may increase during invasion; microndosome proteins contain an adhesin domain, suggesting that microndosome proteins may participate in adhesion to host cells. Furthermore, microphytene proteins were found to operate in the form of complexes, such as the TgMIC1/4/6 and TgMIC2/M2AP complexes.
The TgMIC1/4/6 complex is the first and most extensively identified microfilament complex and has been extensively studied in T. gondii. This protein complex consists of the soluble adhesion proteins TgMIC1 and TgMIC4 and the TM protein TgMIC6. MIC1 and MIC4 are exposed to the tachyzoite surface and bind host cell surface receptors, and MIC6 binds the complex to the parasite surface. The interacting domains include the following: the second and third EGF-like domains of MIC6, which interact with the C-terminal galectin-like domain of MIC 1; the N-terminal microlinear adhesion repeat (MAR) domain of MIC1; and the first two domains, which bind the Apple domain of MIC4. Together, these proteins promote tachyzoite adhesion and subsequent host cell invasion while also participating in pathogenesis and immune escape.
MIC2 is a TM member of the complex whose extracellular domain consists of the whole I domain followed by five thrombospondin-like repeats (TSRs) and a degenerate sixth TSR close to the TM domain. M2AP is a soluble protein with an N-terminal propeptide, a central beta domain, and a predicted C-terminal helical domain. The hydrophobic residues on the folded side of the M2AP galectin bind the membrane-proximal sixth platelet-reactive protein type I repeat domain of MIC2. After complex formation, MIC2-M2AP can bind host cell surface receptors, which is essential for insect body movement and rapid invasion.
Rhoptries
After the micronemes participate in the attachment and penetration of T. gondii, rhoptries, which are unique apical secretory organelles shared exclusively by all apicomplexan parasites, are involved in parasite penetration and the avoidance of intracellular killing. Rhoptries also contained homogeneous dense organelles resembling dense granules (101). Rhoptries can be divided on the basis of structure into rhoptry neck proteins (RONs) and ROPs. RONs are mainly involved in forming apicomplexan and are involved in mobile junction formation during T. gondii invasion. ROPs mainly play three roles. First, ROPs are involved in invasion of the host cell, as they enhance invasion, vacuole formation and the exchange of materials and information with the host cell. Second, ROPs monitor immune signals from the host cell and avoid host cell killing. Third, some rhoptry proteins (ROPs) are important virulence factors of T. gondii. Therefore, rhoptries can be used as an entry point to study drug targets and vaccine antigen candidates. To date, approximately 54 kinds of ROPs have been identified. The role of ROPs in T. gondii infection and potential therapeutic strategies are shown in Table 1.
Dense granules (GRAs)
GRAs are unique organelles of the Apicomplexan parasite and can exist in multiple locations, for example, within the parasitophorous vacuole membrane (PVM) and on the host cytoplasmic surface as well as within the host cytoplasm and nucleus. The functions of GRAs include modification of the vacuolar membrane, parasite body growth and transcriptional regulation of host cells, and they are immunogenic in the host. At present, a variety of GRA proteins have been reported, among which GRA1 is involved in regulation of the vacuolar membrane network of the worm body. GRA2 is involved in regulating the vacuolar membrane network of Toxoplasma after invading host cells and can form multimeric complexes with GRA4 and GRA6 to maintain network structural morphology. GRA3 is secreted in T. gondii tachyzoites and contains a signal peptide and a TM region at its N-terminus, causing it to be embedded in the form of oligomers on the vacuolar membrane of parasites. GRA4, similar to GRA1 and GRA2, is secreted into the tachyzoite structure. GRA5 is secreted onto the vacuolar membrane to maintain membrane stability. GRA7 is expressed in T. gondii at both the asexual and sexual reproductive stages and is the only membrane-intrinsic protein that connects the worm somatic membrane to the host cell membrane. GRA15, GRA16 and GRA24 are among the few GRAs that can enter the host cytoplasm through the vacuolar membrane. GRA15 can enter the host nucleus and participates in regulating host transcription; studies have shown that GRA16 plays an important role in the pathogenicity of T. gondii, and GRA24 independently regulates IL-12 in the NF-κB pathway.
Structure of the blood‒brain barrier (BBB) in vertebrates
The CNS in vertebrates is protected by many cellular barriers that limit and regulate the ability of certain molecules and cells to enter the brain parenchyma [11]. Three main barriers, the BBB, blood-cerebrospinal fluid barrier, and cerebrospinal fluid-brain barrier, protect neurons from harmful substances transmitted through the bloodstream [12]. Pathogens that enter the CNS must do so via one of these routes. Recent research has not demonstrated the ability of T. gondii to invade the brain parenchyma through the cerebrospinal fluid barrier. Therefore, when T. gondii invades the CNS, it primarily does so by crossing the BBB [13].
The BBB is a special barrier structure that prevents neurotoxic plasma components, blood cells, and pathogens from entering the brain. Highlighting the relationships between cells related to the BBB, they exist in a functional unit called the neurovascular unit (NVU), which is composed of neurons, microglia, vascular endothelial cells, pericytes, astrocytes and extracellular matrix [14]. In these tissues, the factors that affect the function of the BBB mainly include cellular factors and TJs [15].
Role of the ROP of T. gondii in crossing the BBB during parasite infection
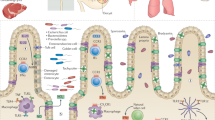
T. gondii is currently known to cross the BBB through the following mechanisms (Fig. 1): (1) by disrupting tightly coupled cellular pathways [16], (2) by infecting immune cells (the “Trojan horse” mechanism) [17], or (3) by inducing endothelial damage to the brain, leading to breakdown of the BBB [18].
Paracellular pathways that disrupt intercellular connections
TJs are important components that maintain BBB function and are mainly composed of occludin, claudin, connective adhesion molecules and endothelial cell-selective adhesion molecules. TJs are connected to the actin cytoskeleton through the TJ protein zonula occludens (ZO) [19]. The integrity of the BBB is related to dynamic changes in TJ proteins. These dynamics are also the key to the highly selective permeability of the BBB [20]. Ramírez-Flores et al. [21]. showed that proteins present in the excretory/secretory products (ESPs) of T. gondii can disrupt the TJs of adjacent cells. After ESP treatment, the expression levels of ZO-1, occludin, and claudin-1 gradually decreased. By analyzing the composition of three kinds of ESPs (ectosomes, exosomes and exosome supernatant fractions), among the detected ROPs, the ROP1, 4, 5, 7, and 8 proteins were found to be present at the highest concentrations among the three components analyzed. Other proteins, such as ROP13, ROP18, and ROP40, were expressed at low levels [21]. However, interestingly, of the few studies of the roles of the ROP1, 4, 5, 7, and 8 proteins in TJs, most of them have focused on their roles in vaccines.
The ROP1 protein is a soluble secreted antigen that plays an important role in the invasion of host cells by T. gondii [22]. ROP1 is conserved in the highly virulent RH strain of T. gondii and promotes parasite growth in mouse and human macrophages [23]. ROP1 interacts with the host cell protein C1QBP, a newly recognized innate immune signal regulator. Therefore, ROP1 has been suggested as an important innate immune system regulator in both mice and humans [23]. However, there has been relatively little research on the role of ROP1 in TJs.
The roles of ROP4 include the regulation of host cell invasion, parasitic proliferation, and parasitic virulence regulation [24]. At present, research on ROP4 has mainly focused on various vaccines, including oral recombinant vaccines [25], virus-like particle (VLP) vaccines [26], recombinant baculovirus vaccines [27], and others. Research has shown that compared to a combination of two vaccines (ROP4VLP + ROP13VLP), the single ROP (4 + 13) VLP vaccine better promoted the expression of IgG, IgG1, IgG2a, and IgA in mouse serum. Compared to mice immunized with other vaccines (ROP4 VLP, ROP13 VLP, and ROP4 VLP + ROP13 VLP), mice immunized with ROP (4 + 13) VLP exhibited higher IgG and IgA antibody responses in fecal, urine, intestinal, and vaginal samples after infection via oral attack [26].
The ROP7 and ROP4 proteins are 71% identical, including the amino acids responsible for all of the characteristics required for kinase activity. ROP7-knockout (KO) parasites exhibited a reduced inflammatory response by THP-1-derived macrophages, but the ROP7 gene has little effect on the reproductive speed of T. gondii. Other research shows that ROP7 is not a virulence factor, and the absence of ROP7 does not affect the invasion, proliferation or excretion of parasites [28].
Recent studies have shown that ROP5 is a major virulence factor, as ROP5 is necessary to cause fatal disease in mice [29]. Genetic screening showed that a polymorphism in ROP5 underlies the differences in virulence between T. gondii strains in infected laboratory mice [30]. The study found that deleting type II ROP5 essentially eliminated chronic infections and significantly reduced the virulence mortality rate by >100-fold, indicating that type II ROP5 is a virulence factor [31]. Moreover, a recent study analyzed the interaction between the parasite virulence proteins ROP5B and ROP39. In addition, the direct binding of ROP5B to Irga6 and the inhibition of the IRG resistance system by enhancing ROP18 kinase activity were found to be related to ROP39. Functional analysis of ROP39 showed that it is located on the PVM. Although separate RHΔROP18 T. gondii type I exhibited a relatively small impact on virulence in vivo, compared with the RHΔROP18 strain, the toxicity of the double deletion mutant RHΔROP18/ROP39 (double KO T. gondii strain) was significantly decreased [32].
ROP8, which belongs to the ROP2 protein family, is the most abundant protein in the ectosome and exosome [21]. Although only a few studies have used ROP8 as a diagnostic marker for toxoplasmosis [33], studies have used ROP8 as a potential candidate vaccine [34]. Research has shown that vaccination with a ROP8 DNA vaccine produced significant humoral and cell-mediated immune responses and conferred significant protective effects to mice against deadly parasitic attack [35]. Mice immunized with ROP8 pVAX-1 DNA had a 100% survival rate before 9 days postinfection (dpi), while all control mice died [36]. However, the mechanism by which ROP8 affects TJs is not yet clear.
Trojan horse mechanism
The “Trojan horse” mechanism refers to the use of infected cells as carriers to help pathogens spread and cross biological barriers without being detected [37, 38]. Studies have shown that various pathogens can cross biological barriers using the “Trojan horse” mechanism. For example, Listeria monocytogenes (Lm) causes bacterial encephalitis by crossing the BBB using the “Trojan horse” mechanism [39], and flaviviruses spread into the nervous system using the “Trojan horse” mechanism [40]. Leukocytes play an important role in the “Trojan horse” mechanism.
The migratory function of leukocytes makes them a suitable vector for T. gondii [41]. Dendritic cells (DCs) in T. gondii-infected leukocytes, but not in macrophages, show enhanced migration after parasitism by T. gondii associated with the GABA/L-VDCC/Cav1.3 motogenic signaling axis [42]. However, when endothelial cells come into contact with DCs, DCs adhere to the endothelium and move in an integrin-dependent manner [43]. This causes a significant reduction in the motility of DCs. Furthermore, CD11b+ cells were shown to play a very important role in this process [44]. CD11b+/CD11c+ cells exhibited the highest infection rate among all peripheral blood mononuclear cells (PBMCs), whereas the majority of the infected cells that migrated across the BBB were CD11b+/CD11c+ cells. Two types of ROPs play an important role in this process.
TgWIP, a newly identified secreted ROPs that is not harmful to host cells during parasitic invasion, plays an important role in parasite transmission from the infection site to the brain by inducing the excessive movement of DCs [45]. The KO of TgWIP affected the transmission of T. gondii to distal organs. In addition, this protein can regulate actin dynamics by interacting with the WAVE regulatory complex (WRC) and SHP2 phosphatase in host cells [46, 47]. The WRC promotes actin polymerization and the formation of pseudopodia, which are closely related to cytoskeletal regulation [48]. SHP2 is a key regulatory factor for the integrity of the blood‒testis barrier (BTB) and BBB [49, 50]. Because TgWIP can affect the morphology of DCs and promote their adhesion to the extracellular matrix, TgWIP enhances the movement and migration of parasitic DCs and may facilitate a “Trojan horse” mechanism for parasites to spread through the host.
TgROP17, which belongs to the ROP2 family, is a serine/threonine kinase [51]. Previous studies have shown that ROP17 can control acute toxicity in mice by synergistically forming complexes with T. gondii ROP5 and ROP18 [52]. However, the functions of ROP17 in different genotypes of T. gondii differ [53]. Research has shown that ROP17 in genotype I T. gondii contributes to cancer immune regulation [54], possibly due to its ability to inhibit the innate immune response of host cells and promote their survival [55]. Deletion of ROP17 in the type II T. gondii Pru strain was shown to almost completely eliminate the formation of cysts in the brain [56]. This is mainly because ROP17 promoted the growth of T. gondii type II in vitro and, in synergy with ROP18, protected parasitic vacuoles from blocking the host’s immune-related GTPase (IRG)-mediated immune responses [54].
Recent research shows that the main roles of ROP17 in the BBB are to activate Rho/ROCK-dependent processes, to promote monocyte migration in tissues, and to help monocytes quickly reach the BBB [57], rather than helping parasites penetrate the BBB through exosmosis. The migration of parasitic monocytes and macrophages requires host Rho/ROCK signaling and secretion of the parasitic kinase ROP17, which is also necessary for effective transmission in vivo [57]. During the infection of monocytes with T. gondii, Rho upregulates interstitial migration to promote the rapid transmission of infected cells within tissues and systemic parasitic transmission [58]. Conversely, the inability of infected monocytes to effectively complete integrin-mediated adhesion or endothelial migration processes indicates that they are not suitable to act as “Trojan horses” to transmit parasites in the BBB. Therefore, the activation of ROP17-dependent interstitial tissue migration in infected monocytes can lead to the inhibition of integrin-dependent processes in endothelial cells.
Induced disruption of the BBB by the induction of brain endothelial damage
Previous studies have shown that T. gondii can cross biological barriers using paracellular migration or by infecting host cells and then crossing the BBB. Recent studies show that parasites can replicate in endothelial cells before they invade the CNS [59, 60]. In these parasites, the ROPs toxofilin and Rab11 also play a very important role in cell invasion.
Toxofilin is a 27 kDa protein that can regulate actin dynamics through monomeric actin. Through protein sequence analysis, toxofilin was found to contain an N-terminal signaling sequence for secretion, mainly present in rhoptries, which plays a crucial role in host invasion [61]. Research shows that toxofilin promotes correct vacuole folding by targeting the host cortical actin cytoskeleton, thus facilitating the invasion process [62].
T. gondii Rab11 proteins (Rab11A, Rab11B, and Rab11C/Rab25) are present in different cell compartments, such as the trans-Golgi network (TGN), posterior Golgi vesicles, and circulating nuclear endosomes around the centriole (RE) [63]. Rab11A is involved in phagocytosis, synaptic function, and cell migration. Additionally, Rab11A regulates not only eukaryotic cell exocytosis but also the movement of extracellular parasites and adhesion to host cells [64].
Notably, secretion of the microneme protein MIC2 is altered in Rab11A-deficient parasites, which also exhibit severe morphological defects [65]. Shield-1 was used to treat extracellular Rab11A-WT and KO (Rab11A-DN) parasites for 2 h, and their ability to adhere to host cells was then monitored. Compared to the Rab11A-WT parasite, the Rab11A-DN tachyzoites were severely damaged in terms of their ability to adhere to the monolayer surface of human foreskin fibroblasts (HFFs). In addition, parasites that successfully adhered exhibited strong motor deficits. Importantly, compared to that of the Rab11A-WT parasite, the morphology of the adherent motile Rab11A-DN parasite became wider and shorter, and these parasites were without the typical arc. These defects of Rab11A-DN parasites in terms of host cell adhesion and movement may be due to impaired transmission of MIC2 to the plasma membrane (PM) [65]. This suggests that Rab11 may also play a crucial role in cell invasion.
The role of T. gondii ROPs in the infected brain parenchyma
Previous studies have shown that T. gondii enters the brain through direct infection and lysis of BBB endothelial cells or through the “Trojan horse” mechanism, but little is known about the transmission of parasites within the brain. Although various cells can infect parasites, current research generally supports the concept that carrier immune cells can mediate transport [66].
According to recent research by Schneider et al. [67], although extracellular T. gondii generally moves faster than cells infected with parasites in vitro, the migration of extracellular T. gondii in the brain parenchyma is slower than that of cells infected with parasites. The movement of cells infected with T. gondii can be divided into two types. One type of cell movement, which was seen in microglia in vitro, may involve excessive migration after infection with T. gondii [68], but movement remained also static in vivo [67]. The movement of other types of cells, such as peripheral infiltrating immune CD45+ cells, can transport T. gondii tachyzoites through the brain and increase their transmission speed; these parasite-carrying CD45+ cells still have a slower average transmission rate in the brain than uninfected CD45+ cells [68, 69]. Because microglia play a protective role in maintaining the health of myelin, the presence of fewer microglia will lead to the disintegration of myelin and thus affect cognitive function [70]; this study also suggests that immune cells play dual roles in neuroprotection and promoting the spread of parasites in the brain.
Although astrocytes, microglia and neurons are easily infected in vitro, Cabral et al. [71]. found that parasites tend to be located in neurons. It was not previously clear how T. gondii reached neurons, but because immune cells have been suggested to promote the spread of parasites in the brain, this may be one of the methods by which parasites reach neurons. The infection process may involve the initial infection of immune cells followed by transmission to neurons, causing neuronal infection. ROP18 may play a role throughout this process.
In response to various brain injuries, microglia are activated and polarize to form M1-type microglia (proinflammatory) or M2-type microglia (anti-inflammatory) [72, 73]. The immunoregulatory molecules produced by M1 and M2 microglia, which include various inflammatory factors and chemokines, are closely related to brain injury and brain repair, respectively [74,75,76]. Studies have shown that T. gondii ROP18 interacts with purinergic P2X1, a purinergic receptor in SF268 human nerve cells, and suppresses ATP-induced apoptosis via the mitochondrial pathway [77]. This helps to clarify the potential mechanism underlying neuroinflammation mediated by activated microglia. In addition, because the ER-related protein RTN1-C is the substrate for phosphorylation by T. gondii ROP18, when ROP18 phosphorylates RTN1-C, this promotes neuronal apoptosis through inducing ER stress. Studies have shown that ROP18 can phosphorylate the serine residues at sites 7 and 134 and threonine residues at sites 4, 8 and 118 in RTN1-C. Phosphorylated RTN1-C can inhibit histone deacetylase (HDAC) activity and promote the acetylation of glucose-regulated protein 78 (GRP78), and highly acetylated GRP78 can upregulate the unfolded protein response, leading to ER stress-related apoptosis [78]. This may be an important mechanism of neuronal apoptosis during the process of toxoplasmosis encephalitis (TE).
ROP 18 is a T. gondii kinase that phosphorylates IRGA6, thereby preventing worm body death caused by the aggregation of IRGA6 on the PV. ROP18 can form a complex with ROP5, and this complex can bind the GRA protein GRA7. Furthermore, the indirect interaction between an effector immunity-related GTPase (IRG), IRGA6, is mediated by ROP5. Thus, ROP18 should regulate IRGA6 by binding ROP5, and the presence of ROP5 in this complex causes IRGA6 to be phosphorylated after binding by G7 [79]. In neural cells, worm strains expressing high levels of ROP18 did not contain IRGA6 on the PV regardless of IFN-γ stimulation. Other insect strains stimulated by IFN-γ showed the activation of IRGs, which eventually removed intracellular parasites in vitro and in vivo [80].
In ROP16 plays an additional very important role. In a vesicular stress model and in mouse primary neuronal cell culture (PNC), the absence of ROP16 did not affect type II parasite encystation but significantly reduced type III parasite encystation. Furthermore, the phosphorylation and activation of the host cell transcription factor STAT6 by the parasitic kinase ROP16 were found to be necessary for encystation in T. gondii type III strains. Research has shown that compared to WT type III and ROP16 type III parasites, IIIΔ ROP16 and ROP16 type II parasites showed a significant reduction in cysts (30-50%) at 4-8 dpi [81]. Meanwhile, the loss of ROP 16 in type III parasites caused a significantly reduction in parasite numbers and enhanced parasite-specific T-cell responses, suggesting that ROP16 is a virulence factor [82].
Currently, the size and number of cysts in the brain are weighted indicators used to measure the quality of T. gondii vaccines. Therefore, vaccines prepared with various ROPs have shown various impacts on the size and quantity of cysts in the brain [1, 83]. For example, ROP4 and ROP13 VLP vaccines play a specific role in inducing intracranial antibody responses [26]. Furthermore, intracranial IgG and IgA responses in groups treated with ROP4 VLP and ROP13 VLP vaccines were significantly stronger than those in the infected group. Meanwhile, the IFN-γ and IL-6 levels in the ROP4 VLP treatment group were significantly lower than those in the infected group. In addition, cyst sizes in the ROP4 and ROP13 VLP treatment groups were significantly smaller than those in the infected group. Furthermore, the number of cysts in mice immunized with the vaccine were also significantly decreased. The efficacy of the vaccine was evaluated by the attack of infected and immunized mice with T. gondii ME49. Immunization with VLPs could prevent severe weight loss and ensured 100% survival, while nonimmunized mice gradually lost body weight and eventually died.
Conclusion
Encephalitis is the most important manifestation of toxoplasmosis in immunosuppressed patients, as it causes the most severe damage to patients. Infection can occur in any organ. Patients may experience headaches, disorientation, drowsiness, hemiplegia, altered reflexes, and convulsions, with many falling into a coma. Most lesions in the brain are necrotic, especially in the thalamus. Although recent progress has contributed to a better understanding of the role of certain T. gondii ROPs in the outcome of TE, many issues still need to be addressed. For example, there are many ways to transport parasites through the BBB, but more work needs to be done to elucidate the role of each mechanism. In addition, it should be noted that the same ROP can have different functions under different conditions. Because some ROPs have been mainly investigated in terms of their extracellular effects, their functions have not yet been fully explored. However, ROP antigens are strong vaccine candidates, and future research on ROPs might therefore be a major direction in addressing T. gondii infection.
References
Foroutan M, Ghaffarifar F, Sharifi Z, Dalimi A, Jorjani O. Rhoptry antigens as Toxoplasma gondii vaccine target. Clin Exp Vaccin Res. 2019;8:4–26.
Kovačević G, Cvjetković IH, Patić A, Radovanov J, Kovačević B. Negative trend in seroprevalence of anti-Toxoplasma gondii IgG antibodies among the general population of the province of Vojvodina, Serbia, 2008-2021. Parasitol Int. 2023;92:102689.
Dubey JP, Murata FHA, Cerqueira-Cézar CK, Kwok OCH, Villena I. Congenital toxoplasmosis in humans: an update of worldwide rate of congenital infections. Parasitology. 2021;148:1406–16.
Odeniran PO, Omolabi KF, Ademola IO. Risk factors associated with seropositivity for Toxoplasma gondii in population-based studies among immunocompromised patients (pregnant women, HIV patients and children) in West African countries, Cameroon and Gabon: a meta-analysis. Acta Trop. 2020;209:105544.
Belkacemi M, Heddi B. Toxoplasmosis immunity status of blood donors in Sidi Bel Abbès, West Algeria. Cureus. 2022;14:e28826.
Tong WH, Pavey C, O’Handley R, Vyas A. Behavioral biology of Toxoplasma gondii infection. Parasit Vectors. 2021;14:77.
Valladares-Garrido MJ, Failoc-Rojas VE, Ichiro-Peralta C, Astudillo-Rueda D, Silva-Díaz H. Toxoplasma gondii infection and threatened abortion in women from Northern Peru. Infect Dis Obstet Gynecol. 2022;2022:1163655.
Matta SK, Rinkenberger N, Dunay IR, Sibley LD. Toxoplasma gondii infection and its implications within the central nervous system. Nat Rev Microbiol. 2021;19:467–80.
Martins-Duarte ES, Lemgruber L, De Souza W, Vommaro RC. Toxoplasma gondii: fluconazole and itraconazole activity against toxoplasmosis in a murine model. Exp Parasitol. 2010;124:466–9.
Mendez OA, Koshy AA. Toxoplasma gondii: entry, association, and physiological influence on the central nervous system. PLoS Pathog. 2017;13:e1006351.
Mancinelli E, Takuma M, Fujie T, Pensabene V. Recreating cellular barriers in human microphysiological systems in vitro. Annu Int Conf IEEE Eng Med Biol Soc. 2022;2022:3923–6.
Zhang S, Gan L, Cao F, Wang H, Gong P, Ma C, et al. The barrier and interface mechanisms of the brain barrier, and brain drug delivery. Brain Res Bull. 2022;190:69–83.
Ross EC, Olivera GC, Barragan A. Early passage of Toxoplasma gondii across the blood-brain barrier. Trends Parasitol. 2022;38:450–61.
Zhao M, Jiang XF, Zhang HQ, Sun JH, Pei H, Ma LN, et al. Interactions between glial cells and the blood-brain barrier and their role in Alzheimer’s disease. Ageing Res Rev. 2021;72:101483.
Yang C, Hawkins KE, Doré S, Candelario-Jalil E. Neuroinflammatory mechanisms of blood-brain barrier damage in ischemic stroke. Am J Physiol Cell Physiol. 2019;316:C135–C153.
Soldati D, Dubremetz JF, Lebrun M. Microneme proteins: structural and functional requirements to promote adhesion and invasion by the apicomplexan parasite Toxoplasma gondii. Int J Parasitol. 2001;31:1293–302.
Bhandage AK, Barragan A. Calling in the Cavalry-Toxoplasma gondii hijacks GABAergic signaling and voltage-dependent calcium channel signaling for trojan horse-mediated dissemination. Front Cell Infect Microbiol. 2019;9:61.
Franklin-Murray AL, Mallya S, Jankeel A, Sureshchandra S, Messaoudi I, Lodoen MB. Toxoplasma gondii dysregulates barrier function and mechanotransduction signaling in human endothelial cells. mSphere. 2020;5:e00550–19.
Kuo WT, Odenwald MA, Turner JR, Zuo L. Tight junction proteins occludin and ZO-1 as regulators of epithelial proliferation and survival. Ann N. Y Acad Sci. 2022;1514:21–33.
Kadry H, Noorani B, Cucullo L. A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS. 2020;17:69.
Ramírez-Flores CJ, Cruz-Mirón R, Lagunas-Cortés N, Mondragón-Castelán M, Mondragon-Gonzalez R, González-Pozos S, et al. Toxoplasma gondii excreted/secreted proteases disrupt intercellular junction proteins in epithelial cell monolayers to facilitate tachyzoites paracellular migration. Cell Microbiol. 2021;23:e13283.
Sonaimuthu P, Ching XT, Fong MY, Kalyanasundaram R, Lau YL. Induction of protective immunity against toxoplasmosis in BALB/c mice vaccinated with Toxoplasma gondii rhoptry-1. Front Microbiol. 2016;7:808.
Butterworth S, Torelli F, Lockyer EJ, Wagener J, Song OR, Broncel M, et al. Toxoplasma gondii virulence factor ROP1 reduces parasite susceptibility to murine and human innate immune restriction. PLoS Pathog. 2022;18:e1011021.
Carey KL, Jongco AM, Kim K, Ward GE. The Toxoplasma gondii rhoptry protein ROP4 is secreted into the parasitophorous vacuole and becomes phosphorylated in infected cells. Eukaryot Cell. 2004;3:1320–30.
Yoon KW, Chu KB, Kang HJ, Kim MJ, Eom GD, Quan FS. Orally administrated recombinant vaccinia virus displaying ROP4 induces protection against Toxoplasma gondii challenge infection. Vaccines. 2022;10:152.
Kang HJ, Lee SH, Kim MJ, Chu KB, Lee DH, Chopra M, et al. Influenza virus-like particles presenting both Toxoplasma gondii ROP4 and ROP13 enhance protection against T. gondii infection. Pharmaceutics. 2019;11:342.
Yoon KW, Chu KB, Kang HJ, Kim MJ, Eom GD, Lee SH, et al. Mucosal administration of recombinant baculovirus displaying Toxoplasma gondii ROP4 confers protection against T. gondii challenge infection in mice. Front Cell Infect Microbiol. 2021;11:735191.
Zhu L, Qi W, Yang G, Yang Y, Wang Y, Zheng L, et al. Toxoplasma gondii rhoptry protein 7 (ROP7) interacts with NLRP3 and promotes inflammasome hyperactivation in THP-1-derived macrophages. Cells. 2022;11:1630.
Bernstein M, Pardini L, Castro BBP, Unzaga JM, Venturini MC, Moré G. ROP18 and ROP5 alleles combinations are related with virulence of T. gondii isolates from Argentina. Parasitol Int. 2021;83:102328.
Behnke MS, Khan A, Lauron EJ, Jimah JR, Wang Q, Tolia NH, et al. Rhoptry proteins ROP5 and ROP18 are major murine virulence factors in genetically divergent South American strains of Toxoplasma gondii. PLoS Genet. 2015;11:e1005434.
Fox BA, Rommereim LM, Guevara RB, Falla A, Triana MAH, Sun Y, et al. The Toxoplasma gondii rhoptry kinome is essential for chronic infection. mBio. 2016;7:e00193–16.
Singh S, Murillo-León M, Endres NS, Soto AFA, Gómez-Marín JE, Melbert F, et al. ROP39 is an Irgb10-specific parasite effector that modulates acute Toxoplasma gondii virulence. PLoS Pathog. 2023;19:e1011003.
Sonaimuthu P, Fong MY, Kalyanasundaram R, Mahmud R, Lau YL. Sero-diagnostic evaluation of Toxoplasma gondii recombinant Rhoptry antigen 8 expressed in E. coli. Parasit Vectors. 2014;7:297.
Foroutan M, Ghaffarifar F, Sharifi Z, Dalimi A, Pirestani M. Bioinformatics analysis of ROP8 protein to improve vaccine design against Toxoplasma gondii. Infect Genet Evol. 2018;62:193–204.
Foroutan M, Ghaffarifar F, Sharifi Z, Dalimi A. Vaccination with a novel multi-epitope ROP8 DNA vaccine against acute Toxoplasma gondii infection induces strong B and T cell responses in mice. Comp Immunol Microbiol Infect Dis. 2020;69:101413.
Parthasarathy S, Fong MY, Ramaswamy K, Lau YL. Protective immune response in BALB/c mice induced by DNA vaccine of the ROP8 gene of Toxoplasma gondii. Am J Trop Med Hyg. 2013;88:883–7.
Benmimoun B, Papastefanaki F, Périchon B, Segklia K, Roby N, Miriagou V, et al. An original infection model identifies host lipoprotein import as a route for blood-brain barrier crossing. Nat Commun. 2020;11:6106.
Strickland AB, Shi M. Mechanisms of fungal dissemination. Cell Mol Life Sci. 2021;78:3219–38.
Ireton K, Mortuza R, Gyanwali GC, Gianfelice A, Hussain M. Role of internalin proteins in the pathogenesis of Listeria monocytogenes. Mol Microbiol. 2021;116:1407–19.
Marshall EM, Koopmans MPG, Rockx B. A Journey to the central nervous system: routes of flaviviral neuroinvasion in human disease. Viruses. 2022;14:2096.
Drewry LL, Sibley LD. The Hitchhiker’s guide to parasite dissemination. Cell Microbiol. 2019;21:e13070.
Yin K, Xu C, Zhao G, Xie H. Epigenetic manipulation of psychiatric behavioral disorders induced by Toxoplasma gondii. Front Cell Infect Microbiol. 2022;12:803502.
Ross EC, Ten Hoeve AL, Barragan A. Integrin-dependent migratory switches regulate the translocation of Toxoplasma-infected dendritic cells across brain endothelial monolayers. Cell Mol Life Sci. 2021;78:5197–212.
Courret N, Darche S, Sonigo P, Milon G, Buzoni-Gâtel D, Tardieux I. CD11c- and CD11b-expressing mouse leukocytes transport single Toxoplasma gondii tachyzoites to the brain. Blood. 2006;107:309–16.
Ross EC, Hoeve ALT, Saeij JPJ, Barragan A. Toxoplasma effector-induced ICAM-1 expression by infected dendritic cells potentiates transmigration across polarised endothelium. Front Immunol. 2022;13:950914.
Ólafsson EB, Barragan A. The unicellular eukaryotic parasite Toxoplasma gondii hijacks the migration machinery of mononuclear phagocytes to promote its dissemination. Biol Cell. 2020;112:239–50.
Sangaré LO, Ólafsson EB, Wang Y, Yang N, Julien L, Camejo A, et al. In vivo CRISPR screen identifies TgWIP as a Toxoplasma modulator of dendritic cell migration. Cell Host Microbe. 2019;26:478–492.e8.
Rottner K, Stradal TEB, Chen B. WAVE regulatory complex. Curr Biol. 2021;31:R512–R517.
Hu X, Tang Z, Li Y, Liu W, Zhang S, Wang B, et al. Deletion of the tyrosine phosphatase Shp2 in Sertoli cells causes infertility in mice. Sci Rep. 2015;5:12982.
Coyne CB, Kim KS, Bergelson JM. Poliovirus entry into human brain microvascular cells requires receptor-induced activation of SHP-2. EMBO J. 2007;26:4016–28.
Zhao Y, Yap GS. Toxoplasma’s arms race with the host interferon response: a ménage à trois of ROPs. Cell Host Microbe. 2014;15:517–8.
Etheridge RD, Alaganan A, Tang K, Lou HJ, Turk BE, Sibley LD. The Toxoplasma pseudokinase ROP5 forms complexes with ROP18 and ROP17 kinases that synergize to control acute virulence in mice. Cell Host Microbe. 2014;15:537–50.
Hamilton CM, Black L, Oliveira S, Burrells A, Bartley PM, Melo RPB, et al. Comparative virulence of Caribbean, Brazilian and European isolates of Toxoplasma gondii. Parasit Vectors. 2019;12:104.
Fox BA, Sanders KL, Rommereim LM, Guevara RB, Bzik DJ. Secretion of rhoptry and dense granule effector proteins by nonreplicating Toxoplasma gondii uracil auxotrophs controls the development of antitumor immunity. PLoS Genet. 2016;12:e1006189.
Li JX, He JJ, Elsheikha HM, Chen D, Zhai BT, Zhu XQ, et al. Toxoplasma gondii ROP17 inhibits the innate immune response of HEK293T cells to promote its survival. Parasitol Res. 2019;118:783–92.
Jones NG, Wang Q, Sibley LD. Secreted protein kinases regulate cyst burden during chronic toxoplasmosis. Cell Microbiol. 2017;19:e12651.
Drewry LL, Jones NG, Wang Q, Onken MD, Miller MJ, Sibley LD. The secreted kinase ROP17 promotes Toxoplasma gondii dissemination by hijacking monocyte tissue migration. Nat Microbiol. 2019;4:1951–63.
Mrass P, Oruganti SR, Fricke GM, Tafoya J, Byrum JR, Yang L, et al. ROCK regulates the intermittent mode of interstitial T cell migration in inflamed lungs. Nat Commun. 2017;8:1010.
Konradt C, Ueno N, Christian DA, Delong JH, Pritchard GH, Herz J, et al. Endothelial cells are a replicative niche for entry of Toxoplasma gondii to the central nervous system. Nat Microbiol. 2016;1:16001.
Harun MSR, Marsh V, Elsaied NA, Webb KF, Elsheikha HM. Effects of Toxoplasma gondii infection on the function and integrity of human cerebrovascular endothelial cells and the influence of verapamil treatment in vitro. Brain Res. 2020;1746:147002.
Czimbalek L, Kollár V, Kardos R, Lőrinczy D, Nyitrai M, Hild G. The effect of toxofilin on the structure and dynamics of monomeric actin. FEBS Lett. 2015;589:3085–9.
Delorme-Walker V, Abrivard M, Lagal V, Anderson K, Perazzi A, Gonzalez V, et al. Toxofilin upregulates the host cortical actin cytoskeleton dynamics, facilitating Toxoplasma invasion. J Cell Sci. 2012;125:4333–42.
Kelly EE, Horgan CP, McCaffrey MW. Rab11 proteins in health and disease. Biochem Soc Trans. 2012;40:1360–7.
Kremer K, Kamin D, Rittweger E, Wilkes J, Flammer H, Mahler S, et al. An overexpression screen of Toxoplasma gondii Rab-GTPases reveals distinct transport routes to the micronemes. PLoS Pathog. 2013;9:e1003213.
Venugopal K, Chehade S, Werkmeister E, Barois N, Periz J, Lafont F, et al. Rab11A regulates dense granule transport and secretion during Toxoplasma gondii invasion of host cells and parasite replication. PLoS Pathog. 2020;16:e1008106.
Reyes J, Yap GS. Macrophage to dendritic cell transitioning induced by Toxoplasma. Trends Parasitol. 2023;39:10–11.
Schneider CA, Velez DXF, Orchanian SB, Shallberg LA, Agalliu D, Hunter CA, et al. Toxoplasma gondii dissemination in the brain is facilitated by infiltrating peripheral immune cells. mBio. 2022;13:e0283822.
Bhandage AK, Kanatani S, Barragan A. Toxoplasma-induced hypermigration of primary cortical microglia implicates GABAergic signaling. Front Cell Infect Microbiol. 2019;9:73.
Ploix CC, Noor S, Crane J, Masek K, Carter W, Lo DD, et al. CNS-derived CCL21 is both sufficient to drive homeostatic CD4+ T cell proliferation and necessary for efficient CD4+ T cell migration into the CNS parenchyma following Toxoplasma gondii infection. Brain Behav Immun. 2011;25:883–96.
McNamara NB, Munro DAD, Bestard-Cuche N, Uyeda A, Bogie JFJ, Hoffmann A, et al. Microglia regulate central nervous system myelin growth and integrity. Nature. 2023;613:120–9.
Cabral CM, Tuladhar S, Dietrich HK, Nguyen E, MacDonald WR, Trivedi T, et al. Neurons are the primary target cell for the brain-tropic intracellular parasite Toxoplasma gondii. PLoS Pathog. 2016;12:e1005447.
Yang X, Xu S, Qian Y, Xiao Q. Resveratrol regulates microglia M1/M2 polarization via PGC-1α in conditions of neuroinflammatory injury. Brain Behav Immun. 2017;64:162–72.
Wang G, Li X, Li N, Wang X, He S, Li W, et al. Icariin alleviates uveitis by targeting peroxiredoxin 3 to modulate retinal microglia M1/M2 phenotypic polarization. Redox Biol. 2022;52:102297.
Kirkley KS, Popichak KA, Afzali MF, Legare ME, Tjalkens RB. Microglia amplify inflammatory activation of astrocytes in manganese neurotoxicity. J Neuroinflammation. 2017;14:99.
Lan X, Han X, Li Q, Yang QW, Wang J. Modulators of microglial activation and polarization after intracerebral haemorrhage. Nat Rev Neurol. 2017;13:420–33.
Liu XL, Sun DD, Zheng MT, Li XT, Niu HH, Zhang L, et al. Maraviroc promotes recovery from traumatic brain injury in mice by suppression of neuroinflammation and activation of neurotoxic reactive astrocytes. Neural Regen Res. 2023;18:141–9.
Zhou LJ, Chen M, Puthiyakunnon S, He C, Xia J, He CY, et al. Toxoplasma gondii ROP18 inhibits human glioblastoma cell apoptosis through a mitochondrial pathway by targeting host cell P2X1. Parasit Vectors. 2019;12:284.
An R, Tang Y, Chen L, Cai H, Lai DH, Liu K, et al. Encephalitis is mediated by ROP18 of Toxoplasma gondii, a severe pathogen in AIDS patients. Proc Natl Acad Sci USA. 2018;115:E5344–E5352.
Hermanns T, Müller UB, Könen-Waisman S, Howard JC, Steinfeldt T. The Toxoplasma gondii rhoptry protein ROP18 is an Irga6-specific kinase and regulated by the dense granule protein GRA7. Cell Microbiol. 2016;18:244–59.
Chandrasekaran S, Kochanowsky JA, Merritt EF, Lagas JS, Swannigan A, Koshy AA. IFN-γ stimulated murine and human neurons mount anti-parasitic defenses against the intracellular parasite Toxoplasma gondii. Nat Commun. 2022;13:4605.
Kochanowsky JA, Chandrasekaran S, Sanchez JR, Thomas KK, Koshy AA. ROP16-mediated activation of STAT6 enhances cyst development of type III Toxoplasma gondii in neurons. PLoS Pathog. 2023;19:e1011347.
Schulte K, Pawlowski N, Faelber K, Fröhlich C, Howard J, Daumke O. The immunity-related GTPase Irga6 dimerizes in a parallel head-to-head fashion. BMC Biol. 2016;14:14.
Faridnia R, Daryani A, Sarvi S, Sharif M, Kalani H. Vaccination against Toxoplasma gondii using rhoptry antigens: a systematic review. Comp Immunol Microbiol Infect Dis. 2018;59:32–40.
Funding
This work was supported by the Bethune Research Plan of Jilin University (grant number: 2020-31).
Author information
Authors and Affiliations
Contributions
XW, LQ, JC and JTZ performed most of the research and data analysis and helped draft the manuscript. YJ, KH, ZZ, JZ,and YA analyzed and interpreted the raw data. All authors read and approved the final paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, X., Qu, L., Chen, J. et al. Toxoplasma rhoptry proteins that affect encephalitis outcome. Cell Death Discov. 9, 439 (2023). https://doi.org/10.1038/s41420-023-01742-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41420-023-01742-1