Abstract
Inflammation is a defense mechanism that can protect the host against microbe invasion. A proper inflammatory response can maintain homeostasis, but continuous inflammation can cause many chronic inflammatory diseases. To properly treat inflammatory disorders, the molecular mechanisms underlying the development of inflammation need to be fully elucidated. Pyroptosis is an inflammation-related cell death program, that is different from other types of cell death. Pyroptosis plays crucial roles in host defense against infections through the release of proinflammatory cytokines and cell lysis. Accumulating evidence indicates that pyroptosis is associated with inflammatory diseases, such as arthritis, pneumonia, and colonitis. Furthermore, pyroptosis is also closely involved in cancers that develop as a result of inflammation, such as liver cancer, esophageal cancer, pancreatic cancer, and colon cancer. Here, we review the function and mechanism of pyroptosis in inflammatory disease development and provide a comprehensive description of the potential role of pyroptosis in inflammatory diseases.
Similar content being viewed by others
Introduction to programmed cell death and pyroptosis
Acute inflammation is the host’s natural defense against external and internal infection or injury. Failure to fight an infection or injury can lead to chronic inflammation and possibly the development of cancer. The presence of a link between inflammation and tumor growth was first suggested in the 19th century by the German physician Rudolf Virchow [1] due to the infiltration of leukocytes into the tumor microenvironment [2]. Inflammation in the tumor microenvironment facilitates the proliferation and survival of malignant cells and promotes angiogenesis and tumor metastasis. It also disrupts the response of the adaptive immune system to hormone treatments and chemotherapy.
Programmed cell death (PCD) plays critical roles in resisting external infection and maintaining internal homeostasis [3]. There are six main types of PCD, including apoptosis, necrosis, efferocytosis, pyroptosis, ferroptosis, and autophagy [4]. Pyroptosis, also known as inflammatory cell necrosis, is a novel type of PCD and inflammatory caspase-dependent cell death. It is distinct from other types of PCD. When pyroptosis occurs, pores form in the cell membrane, and cell lysis occurs, leading to the release of proinflammatory cytokines, such as interleukin-1β (IL-1β) and interleukin-18 (IL-18) [5, 6]. Pyroptosis can be stimulated by noninfectious stimuli and microbes such as Salmonella, Francisella, and Legionella [5] and plays essential regulatory roles in the elimination of bacteria from specialized neutrophils, macrophages, monocytes, and dendritic cells [7]. The phenomenon of pyroptosis was first described in 1992 [8] but was first termed in 2001 when researchers observed that bacteria-infected macrophages can cause rapid lytic cell death dependent on caspase-1 activity. The word “pyroptosis” comes from the Greek term, ‘pyro’, which means fire or fever, and ‘ptosis’, which means falling or dropping [9].
In recent years, pyroptosis has received increasing attention due to its association with inflammatory diseases. Many studies have shown that pyroptosis is not only involved in the development of atherosclerosis [10, 11], Alzheimer’s disease [12] and HIV-1 infection [13, 14] but also extensively involved in the occurrence and development of a variety of inflammatory diseases, including the progression of hepatitis to liver cancer, colonitis to colon cancer, and gastritis to stomach cancer. However, a thorough understanding of pyroptosis is still lacking. Therefore, it is important to study the role of pyroptosis in both health and disease. This will help us elucidate the pathogeneses of diseases and provide new therapeutic options for inflammatory diseases.
This review aims to link inflammatory diseases with pyroptosis, determine the influence of molecular mechanisms associated with the pyroptosis pathway on the occurrence and development of inflammatory diseases, and provide new ideas and effective targets for the prevention and treatment of inflammatory diseases.
Insight into the pyroptosis pathways
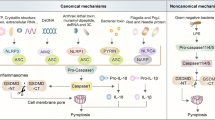
According to recent studies, there are three pyroptosis pathways, i.e., the canonical inflammasome pathway (also called the caspase-1-dependent pathway), the noncanonical inflammasome pathway (also called the caspase-1-independent pathway) and the caspase-8/3/GSDME pathway [15] (Fig. 1).
Early studies identified three pyroptosis pathways, including the caspase-1-dependent pathway, caspase-1-independent pathway and caspase-3/8/GSDMD pathway (left three panels). Recent studies have revealed a new pathway (right penal) in which granzyme A/B activate GSDMB/E to cause pyroptosis. In addition, nuclear PD-L1 can bind p-STAT3 to form a complex to activate and cleave GSDMC. Furthermore, Caspase-8 can cleave GSDMC, triggering pyroptosis. PAMPs pathogen-associated molecular patterns, NLRP1/3 NLR pyrin domain-containing 1/3, NLR nucleotide-binding oligomerization domain, leucine-rich repeat-containing protein, NLRC4 NLR CARD domain-containing 4, ASC apoptosis-associated speck-like protein containing a CARD, CARD caspase recruitment domain, AIM2 absent in melanoma 2, PYD pyrin domain, NBD nucleotide-binding domain, LRR leucine-rich repeat.
In the canonical inflammasome pathway, NLRP1, NLRP3, NLRC4, AIM2, and other inflammasome sensors can detect foreign microbial signals and recruit the adaptor protein ASC to further recruit pro-caspase-1. Activated caspase-1 has two functions: 1) caspase-1 cleaves GSDMD and produces GSDMD-NT fragments, which can bind to phosphoinositides in the plasma membrane and then generate membrane pores and 2) caspase-1 is automatically cleaved to produce caspase-1 P10/P20 and P33/P10 tetramers, which catalyze the maturation of pro-IL-18 and pro-IL-1β into IL-18 and IL-1β, respectively, which are released into the extracellular matrix, causing inflammatory responses [16].
In the noncanonical inflammasome pathway, lipopolysaccharide (LPS) from gram-negative bacteria can activate caspase 11 in mice and caspase 4 and caspase 5 in humans. Subsequently, these activated caspases cleave GSDMD, and a pore is formed in the plasma membrane. The GSDMD pores allow potassium release, resulting in the activation of the NLRP3 inflammasome and IL-1β/IL-18 maturation. Moreover, GSDMD pores cause pyroptosis which can cause the release of mature cytokines [15].
In the Caspase-3/GSDME and Caspase-8/GSDMD pathways, TNF activates caspase-8 by interacting with other substances. Activated caspase-8 has two functions: it cleaves GSDMD to induce the formation of pores in the membrane and can activate caspase-3, which cleavages GSDME to induce the formation of pores in the membrane [15].
The latest research shows that in addition to the above three pathways, pyroptosis can be caused by a transition from apoptosis mediated by GSDME and that granzyme B can directly cleave GSDME at the same site as caspase-3 [17]. Granzyme A can also directly cleave GSDMB to cause pyroptosis [18]. PD-L1 can activate GSDMC through nuclear transfer to cleave gasdermin C and cause pyroptosis [19].
The role of pyroptosis in health
Emerging evidence suggests that pyroptosis may act as an effective antimicrobial defense system in the host during infections [20]. First, pathogens can stimulate inflammasomes and cause pyroptosis, which can result in the lysis of infected cells and expose pathogens to extracellular defenses. This can expose pathogens to neutrophils to protect the host against infection [20]. Second, the inflammatory cytokines IL-1β and IL-18 can be secreted during the process of pyroptosis. IL-1β is not only a potent inducer of inflammation, vasodilation, and immune cell extravasation but also plays roles in shaping adaptive immune responses [21]. IL-18 promotes interferon (IFN)-γ production in Th1 cells, natural killer (NK) cells, and cytotoxic T cell, and promotes the development of Th2 cells and the local inflammatory response [22]. Such alarm signals also recruit immune cells to the site of infection for pathogen removal. Furthermore, pyroptosis can cause the release of extracellular inflammatory mediators, including IL-1 [5], heat-shock proteins [23] and ATP [24], which stimulate the production of proinflammatory cytokines via the activation of pattern-recognition receptors (PRRs). This “to die for life” signal helps the host control and clear microbial infections and allows tissues to return to a homeostatic state [5].
Inflammasomes are important in the process of pyroptosis. Dysfunction of inflammasomes may cause many diseases [25], such as Alzheimer’s disease and atherosclerosis [26]. The most common inflammasome is the NLRP3 inflammasome, which consists of NOD-like receptor family pyrin domain containing 3 (NLRP3), the inflammasome adaptor protein apoptosis-associated speck-like protein containing CARD (ASC), and pro-caspase-1. NLRP3 can be activated by a range of agonists, including ATP; pore-forming toxins; crystalline compounds; nucleic acids; hyaluronan; and fungal, bacterial, and viral pathogens. The formation of the NLRP3 inflammasome can cause pyroptosis and promote the maturation and secretion of IL-1β and IL-18 to protect organisms from invasive stress signals and pathogen infection and maintain health [27, 28]. A recent study reported that when pathogenic microorganisms are recognized by macrophages, these cells trigger an inflammatory response and release vimentin, which can promote the expression of Siglec-14. Siglec-14 can upregulate NLRP3 expression, promote the release of IL-1β, and cause pyroptosis [29].
Pyroptosis is related to the gasdermin (GSDM) family, which includes six members, namely, GSDMA, GSDMB, GSDMC, GSDMD, DFNA5 and DFNB59 [15]. GSDMD plays an especially important role in pyroptotic cell death. Thus, pyroptosis is also known as GSDM-mediated programmed necrotic death [16]. Therefore, GSDMs play a key role in maintaining health. Elucidating the molecular mechanism of pyroptosis is beneficial for the development of future strategies to prevent damage to the body by exogenous substances and maintain health.
Furthermore, apoptotic cell death is involved in resistance to several existing chemotherapeutic drugs. For example, decreased expression of GSDME enhances the resistance of melanoma cells to etoposide [30]. Therefore, the activation of nonapoptotic forms of programmed cell death may be an approach for the treatment apoptosis-resistant cancers. As a new form of cell death program, pyroptosis is promising as an effective target for treating apoptosis-resistant cancers. Currently, studies on the efficacy of targeting pyroptosis to treat inflammatory disease and cancer are limited.
However, some studies on pyroptosis have revealed that pyroptosis is both the cause of and the solution to the problem. On the one hand, moderate pyroptosis is helpful for eliminating infected cells in a timely manner, maintaining cell homeostasis, and effectively preventing excessive cell proliferation. In addition, inflammatory signals released as a result of pyroptosis, called “to die for life” signals, increase the immune response to further infection. On the other hand, a high level of pyroptosis may lead to aggravation of inflammatory symptoms and cause cell death and serious tissue and organ failure. These processes are related to the pathogenesis of some diseases and homeostasis disorders in vivo [11].
Biological role of pyroptosis in inflammatory disease
Studies have shown that the chronic inflammatory response is not only related to tumor progression but also plays important roles in tumor immunity and immunotherapy [31]. Excessive pyroptosis can lead to a continuous inflammatory response, increased levels of the inflammatory mediators IL-1β, IL-18, and HMGB-1, and disruption of host homeostasis. It has been reported that GSDME-mediated pyroptosis leads to activation of the ERK1/2 pathway through the release of high-mobility group box protein 1 (HMGB1), promotes tumor cell proliferation and the expression of proliferating nuclear antigen (PCNA), and subsequently induces the development of colitis-associated colorectal cancer (CAC) [32]. In addition, pyroptosis is a caspase-dependent type of cell death that can be activated by inflammasomes. Moreover, caspase-1/4/5/11 are associated with pyroptosis and are involved in the pathogenesis of a variety of diseases, including hepatitis, inflammatory bowel disease, vascular inflammation [33], and myocardial infarction [34]. Studies have shown that some cancer cells develop chemotherapy resistance, anti-apoptotic ability and the ability to survival under stress conditions [35]. It is meaningful to study the therapeutic effects of a combination of drugs and pyroptosis-targeting strategies inflammatory diseases. The research progress on the association between pyroptosis and various inflammatory diseases is briefly discussed in Table 1.
Pyroptosis in arthritis
Arthritis is a chronic inflammatory disease. Studies have shown that pyroptosis is closely related to arthritis (Table 2). In a study on gout, it was proven that BF-2 can inhibit NLRP3 inflammasome assembly and activation by blocking the binding of ASC and pro-caspase1. This reduces IL-1β secretion and inhibits pyroptosis in macrophages [36]. In study on osteoarthritis (OA), hydrogen peroxide (H2O2) treatment was found to increase the expression of ubiquitin-specific protease 7 (USP7). Experimental evidence has shown that USP7 expression is positively correlated with the level of NAD(P)H oxidase (NOX)4, which can increase reactive oxygen species (ROS) levels, stimulate NLRP3 activation and increase pyroptosis [37]. In addition, loganin inhibits NF-κB signaling and reduces pyroptosis in chondrocytes and may be used for OA treatment [38]. Additionally, licochalcone A (Lico A) inhibits the NF-κB pathway, activates Nrf2, and reduces the expression of NLRP3 inflammasome components, such as ASC and pro-caspase1. Therefore, Lico A can reduce chondrocyte pyroptosis and alleviate OA [39]. In a study on rheumatoid arthritis (RA), pentaxin 3 (PTX3) was found to promote RA monocyte pyroptosis in a C1q-dependent manner [40]. In contrast, intra-articular monosodium urate (MSU) crystals increase bromodomain-containing protein 4 (BRD4) expression, which can cause NLRP3 activation and promote macrophage pyroptosis. Furthermore, BRD4 activates the NF-κB pathway and promotes the inflammatory response. Consistently, the BRD4 inhibitor JQ-1 can be used to alleviate inflammation [41]. The presence of a large amount of acid leads to acidosis in vivo, which induces the activation of acid‐sensing ion channel 1a and causes articular chondrocyte pyroptosis through the calpain 2/calcineurin pathway, further leading to RA development and progression [42, 43].
In the MSU-induced acute gouty arthritis model, the P2Y14 receptor (P2Y14R) activates the NLRP3 inflammasome, promotes caspase-1 expression, and increases pyroptosis in macrophages. In addition, NLRP3 interacts with cAMP to promote gout flare. The adenylate cyclase activator (forskolin) also causes pyroptosis [44, 45]. Based on these findings, P2Y14R antagonists are expected to inhibit the occurrence of pyroptosis and relieve acute gouty arthritis [46]. Decreasing GSDMD expression can alleviate MSU-induced acute gouty arthritis [47]. Gallic acid increases the expression of nuclear factor Nrf2 to inhibit the activity of the NLRP3 inflammasome, reduces the release of inflammatory factors, and alleviates gouty arthritis caused by MSU accumulation [48]. Wedelolactone can phosphorylate Ser/Thr residues of NLRP3 and inhibit the activity of the NLRP3 inflammasome, thereby inhibiting the occurrence of macrophage pyroptosis and alleviating MSU-induced arthritis [49].
Pyroptosis in pneumonia
Since the global outbreak of COVID-19 at the end of 2019, numerous studies have shown that COVID-19-induced pneumonia is related to the massive accumulation of inflammatory cytokines in local organs. Therefore, an increasing number of studies are focusing on the associated between COVID-19 and pyroptosis because pyroptosis can cause inflammatory cytokine release [50,51,52]. Considering the important role of the NLRP3 inflammasome in the occurrence of pyroptosis, some researchers have proposed the use of nutrients that can inhibit inflammasome activity [53].
In a study on pneumonia, Maxing Shigan decoction (MXSG) was found to have a similar effect as the NLRP3 inhibitor INF39 and to downregulate NLRP3 expression and reduce IL-1β and IL-18 secretion [54]. In addition, activation of the IL-17 pathway can promote pyroptosis [55]. In a study on Pseudomonas aeruginosa (PA), which can induce acute lung injury, peptidylarginine deiminase (PAD)2 expression was found to be positively correlated with the expression of caspase-1. Inhibiting PAD2 expression was observed to effectively reduce macrophage pyroptosis and enhance bacterial clearance [56].
Pyroptosis promotes the progression of chronic hepatitis to hepatocellular carcinoma
Liver disease is a serious health problem worldwide. Liver disease can develop into liver fibrosis (LF), cirrhosis and hepatocellular carcinoma (HCC). Recent studies have reported that pyroptosis is involved in the development and regulation of liver diseases [57].
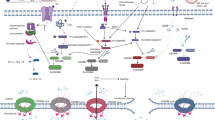
Nonalcoholic fatty liver disease (NAFLD) can be further categorized into nonalcoholic fatty liver (NAFL) and nonalcoholic steatohepatitis (NASH) according to histology. NASH is a key step in the progression of NAFLD, which can further progress to fibrosis, cirrhosis, and hepatocellular carcinoma [58, 59] (Fig. 2). In the NAFL stage, the inflammation caused by pyroptosis is not obvious, and global damage is relatively mild. NLRP3 induces caspase-1 cleavage in macrophages and adipose tissue after sensing intracellular lipid toxicity-associated ceramide [60]. In contrast, in NASH, the inflammation caused by pyroptosis is much more serious and causes greater damage to the body. Bing Xu et al. confirmed that GSDMD plays a role in NASH. GSDMD activates NF-kB by regulating the secretion of IL-1β, subsequently causing steatosis, cell pyroptosis and an inflammatory response [61]. Interestingly, pyroptosis can promote NASH, but also relieves NASH. Eun Hee Koh et al. showed that sphingomyelin synthase 1 (SMS1) is highly expressed in NASH and that free cholesterol (FC) can induce the expression of SMS1. Diacylglycerol (DAG) produced by SMS1 can activate protein kinase Cδ (PKCδ), which further activates the NLR family CARD domain-containing protein 4 (NLRC4) inflammasome. The NLRC4 inflammasome activates Caspase-1 via the canonical inflammasome pathway of pyroptosis, triggering cell pyroptosis, thus forming a novel SMS1-DAGPKCδ-NLRC4 axis, which induces hepatoma pyroptosis [62].
Hepatitis progresses to liver cancer. Initially, virus invasion, alcohol accumulation, and lack of fat consumption can lead to liver damage and inflammation. Inflammation can also be caused by pyroptosis of liver cells. In this stage, immune surveillance can effectively prevent the deterioration of inflammation. However, if inflammation is not controlled, it can further damage liver function, leading to fibrosis, cirrhosis, and liver cancer. In hepatocellular carcinoma, stimulation of pyroptosis in liver cancer cells can enhance the immune response to a certain extent. In brief, the numbers of NK cells, macrophages, B lymphocytes, T cells, and other immune cells in the tumor microenvironment increase, and the levels of immune-stimulating molecules, such as IL-2, IL-12, IL-33, TNF-α, TNF-β, and IFN-γ, also increase. Combinations of chemotherapy drugs can kill more tumor cells and relieve disease symptoms.
Hepatitis can be divided into viral hepatitis and autoimmune hepatitis. There are two main types of viral hepatitis, hepatitis B (HB) and hepatitis C (HC). In an experimental study on hepatitis B, HBx was shown to be a leading mediator of hepatic inflammation [63]. Under oxidative stress, HBx activates the NLRP3 inflammasome in liver cells by inducing mitochondrial damage and producing mitochondrial reactive oxygen species (mitoROS) to trigger pyroptosis, ultimately causing the release of the proinflammatory mediator apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC), IL-1β, IL-18, and high-mobility group box 1 (HMGB1) [57]. In a study on hepatitis C, H. M. Kofahi et al. showed that HCV-infected or bystander cells can induce pyroptosis mediated by the caspase-1 and caspase-3 signaling pathways, which is closely related to the pathogenesis of hepatitis C [64].
The most severe stage of liver disease is HCC; Thus, interventions for liver cancer are needed. Qing Wei et al. found that 17β-estradiol (E2) can induce the activation of the NLRP3 inflammasome and cause the occurrence of caspase-1-dependent pyroptosis in liver cancer cells, subsequently reducing autophagy, which is protective [65]. By combining a pyroptosis-target strategy with drugs, Carina Hage et al. showed that sorafenib, a broad-spectrum kinase inhibitor, can be used to treat hepatocellular carcinoma (HCC). Sorafenib induces macrophage pyroptosis, further releasing inflammatory signals that activate NK cells, enhance the effector function of NK cells and ultimately lead to tumor cell death [66]. Taxifolin (TAX) inhibits caspase-1 activity and IL-1β expression in steatotic liver cells, thereby inhibiting the occurrence of pyroptosis. Furthermore, TAX can regulate the expression levels of sterol regulatory element-binding protein-1 (SREBP1) and peroxisome proliferation–activated receptor gamma (PPARγ) to inhibit lipid accumulation and alleviate the inflammatory response [67]. Moreover, berberine (BBR) [68], baicalin (BA) [69], and liraglutide [70] can be used for the treatment of NASH. The commonality of these three substances is that they can inhibit the expression of NLRP3 and reduce the expression level of GSDMD, thus reducing pyroptosis and alleviating NASH [68,69,70].
Pyroptosis promotes the development of chronic colitis to intestinal cancer
Long-term exposure to chronic inflammation can cause inflammatory bowel disease (IBD) such as ulcerative colitis (UC) and Crohn’s disease (CD). Colitis-associated colon cancer (CAC), a particularly aggressive subtype of colorectal cancer (CRC), occurs in patients with IBD and is considered closely associated with chronic inflammation, which is present in the early stages of tumor onset [71].
Carvalho et al. and Carvalho et al. found that IL-1β and IL-18 are the major inflammatory factors that induce UC and that pyroptosis is closely related to UC [72, 73]. Thus, inflammation can be alleviated by inhibiting the effect of pyroptosis. Kuijieling (KJL) reduces the expression levels of NLRP3, ASC, caspase-1, GSDMD-N, IL-1β, and IL-18 [74]. Subhash Mehto et al. used dextran sodium sulfate (DSS) to induce colitis in mice and verified that IRGM/Irgm1 can limit the activity of the NLRP3 inflammasome [75]. These findings show that regulatory molecules can inhibit pyroptosis and alleviate inflammatory symptoms during pyroptosis.
In CAC, activation of GSDME causes pyroptosis and the release of HMGB1 inducing tumor cell proliferation and proliferating cell nuclear antigen (PCNA) expression through the ERK1/2 pathway and further promoting the development of CAC [32]. Liver X receptor β (LXRβ) in the cytoplasm can interact with Pannexin-1 and specifically induce caspase-1 activation, which can induce pyroptosis in colon cancer cells [76]. Lobaplatin is a third-generation platinum antineoplastic agent with the strong antitumor effect that induces pyroptosis in colorectal cancer (CRC) cells by mediating GSDME in colon cancer cells [77]. Nucleotide-binding oligomerization domain-like receptor family pyrin domain-containing 1 (NALP1) is related to cell pyroptosis, and 5-aza-2-deoxycytidine (DAC) can restore the expression of NALP1 across and inhibit the growth of colon cancer cells [78].
Pyroptosis is a key process in the progression of other inflammatory conditions to cancer
Gastrointestinal (GI) disorders occur when the balance among the microbiota in the GI tract is disrupted. In a study on esophageal adenocarcinoma (EAC), the composition of the intestinal flora of high-fat diet (HFD) induced BE model mice was altered, leading to inflammation and further progression to EAC [79]. In recent years, studies have shown that the inflammasome can protect the GI tract against invasive pathogens and maintain intestinal homeostasis. Pyroptosis occurs in esophagitis, amplifies inflammatory signals, and further accelerates esophagitis development. In addition, alcohol accumulation can activate caspase-1 and stimulate the maturation of the inflammatory cytokines IL-1β and IL-18, which causes pyroptosis in esophageal epithelial cells [80]. However, in addition to exerting these detrimental effects, pyroptosis plays roles in maintaining health by mediating the expression molecules in the pyroptosis pathway during disease. Caspase-1 mediates the inflammatory disease BE, which can further develop into EAC. Barber et al. showed that the secretion of inflammatory cytokines and chemokines is reduced in BE patient biopsies and in mouse BE organ cultures in vitro via inhibition of caspase-1 [81]. The expression of caspase-activated GSDMD is beneficial for inhibiting the proliferation of gastric cancer cells. GSDMD may regulate the cell cycle by suppressing the S to G2/M phase transition in gastric cancer cells through activation of the extracellular signal-regulated kinase (ERK), signal transducer and activator of transcription 3 (STAT3) and phosphatidylinositol 3 kinase/protein kinase B (PI3K/AKT) signaling pathways [82]. In a study on pancreatic adenocarcinoma, mammalian STE20-like kinase 1 (MST1) was found to suppress the progression of pancreatic ductal adenocarcinoma (PDAC) cells through reactive oxygen species (ROS) induced pyroptosis [83]. A study showed that the levels of inflammatory markers are correlated with the prognosis of gastric cancer [84]. Therefore, we can identify inflammatory markers associated with pyroptosis and explore the relationship between them.
The chemotherapy drug cisplatin (DDP) combined with low-dose BI2536, a polo-like Kinase 1(PLK1) inhibitor, can increase chemosensitivity by inducing pyroptosis. This leads to a better curative effect and activation of Bax (an apoptosis-related protein) and Caspase-3, further inducing a switch from apoptosis to pyroptosis via GSDME [85]. Additionally, Yubin Wang et al. demonstrated that treating gastric cancer cells SGC-7901 or MKN-45 with a combination of 5-FU can increase GSDME expression and induce a switch from caspase-3-dependent apoptosis to pyroptosis in gastric cancer cells [86]. Similar studies have shown that metformin can downregulate proline-, glutamic acid- and leucine-rich protein-1 (PELP1) expression by increasing miR-497 expression, ultimately inducing ESCC cell pyroptosis. This suggests that metformin may be a therapeutic option for diseases that are resistant to chemotherapy and radiotherapy but sensitive to pyroptosis [87].
Summary
Chronic inflammation is involved in the development of various kinds of diseases. Therefore, understanding the mechanism of inflammation progression is very important for preventing the occurrence and development of inflammatory diseases. Particularly, persistent chronic inflammation leads to the occurrence of cancer. For example, liver injury can cause hepatitis, liver fibrosis, and cirrhosis and ultimately lead to HCC [88]. Long-term damage to the esophageal mucosa results in gastroesophageal reflux disease, which further develops into Barrett’s esophagus or esophageal adenocarcinoma [89]. Prolonged colitis can lead to IBD and eventually to CRC.
Inflammasomes play essential regulatory roles in inflammation and pyroptosis [25] [28]. During pyroptosis, a series of pathogen-associated molecular patterns (PAMPs) stimulate inflammasomes, subsequently activating caspase-1/4/5/11 and inducing the production of GSDMD-N, which eventually leads to cell lysis, intracellular content release, and inflammatory cytokine production. Therefore, the release of IL-1β and IL-18 and increased GSDMD-N expression are involved in the occurrence and development of various inflammatory diseases. In addition, the NLRP3 inflammasome is one of the most important molecules in the process of pyroptosis. Accurate regulation of inflammasomes is a potential treatment strategy for inflammatory diseases. Inhibition of pyroptosis in normal cells can ameliorate inflammatory symptoms. However, pyroptosis in cancer cells can be regulated by drugs to kill these cells [68]. Therefore, it is meaningful to explore the molecular mechanism of pyroptosis in both healthy and tumor cells. Regulating key molecules involved in pyroptosis may help increase the death of tumor cells, decrease normal cell death, alleviate inflammation, and prevent the occurrence of inflammatory diseases.
Although pyroptosis has been demonstrated to be closely correlated with the progression of inflammatory diseases, many questions related to pyroptosis, such as whether NLRP3 inflammasome inhibitors are suitable for clinical application, are still unclear and need to be further explored; however, repairing membrane pores formed during pyroptosis is effective in the treatment of inflammatory diseases. Indeed, pyroptosis acts as a double-edged sword. On the one hand, inflammatory cytokines released during the process of pyroptosis can cause local inflammation, leading to the recruitment and activation of immune cells, which ultimately help the host clear the pathogen. This phenomenon also enhances immunity. In the treatment of cancer, the proliferation and migration of cancer cells can be inhibited by inducing cancer cell pyroptosis. Thus, developing activators or inhibitors of key molecules involved in pyroptosis and combining them with other immunotherapies to achieve a better therapeutic effect is a promising approach. On the other hand, during the development of inflammatory diseases, excessive activation of pyroptosis aggravates inflammatory responses and causes organ damage. NF-κB is known to be an upstream activator of the NLRP3-inflammasome and induces NLRP3 expression by triggering the priming and assembly of inflammasome [90]. Loganin inhibits chondrocyte pyroptosis and reduces aberrant angiogenesis via inhibition of NF-κB activation [38]. This suggests that activation of other pathways affects the occurrence of pyroptosis and then regulates the development of inflammatory diseases. Currently, immune checkpoint inhibitors are also used as immunotherapies [91]. As mentioned above, when hypoxia occurs, PD-L1 enters the nucleus and forms a complex with P-Y705-STAT3, resulting in apoptosis and pyroptosis [19]. Thus, should we combine drugs targeting immune checkpoint proteins (PD-L1) and pyroptosis for the treatment of cancer?
In conclusion, a comprehensively understanding of the physiological roles of pyroptosis and pyroptosis-related pathological mechanisms underlying the occurrence and development of inflammatory diseases is needed. Such an understanding will provide new strategies for the prevention and regulation of inflammatory diseases and new clinical approaches and ideas for the treatment of cancer.
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
References
RUDOLF VIRCHONV and M.D. An address on the value of pathological experiments. Br Med J. 1881;2:198–203.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74.
Jorgensen I, Rayamajhi M, Miao EA. Programmed cell death as a defence against infection. Nat Rev Immunol. 2017;17:151–64.
Kolb JP, Oguin TH, Oberst A, Martinez J. Programmed Cell Death and Inflammation: Winter Is Coming. Trends Immunol. 2017;38:705–18.
Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7:99–109.
Fanga Y, Tianb S, Pana Y, Wei Lia C, Wangd Q, Tange Y, et al. Pyroptosis A new frontier in cancer. Biomed Pharmacother. 2019;121:109595.
Taabazuing CY, Okondo MC, Bachovchin DA. Pyroptosis and Apoptosis Pathways Engage in Bidirectional Crosstalk in Monocytes and Macrophages. Cell Chem Biol. 2017;24:507–14.
Zychlinsky A, Prevost MC, Sansonetti PJ. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358:167–9 .
BT C, MA B. Pro-inflammatory programmed cell death. Trends Microbiol. 2001;9:113–4.
Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–61.
Xu Y-J, Zheng L, Hu Y-W, Wang Q. Pyroptosis and its relationship to atherosclerosis. Clin Chim Acta. 2018;476:28–37.
Tan MS, Tan L, Jiang T, Zhu XC, Wang HF, Jia CD, et al. Amyloid-beta induces NLRP1-dependent neuronal pyroptosis in models of Alzheimer’s disease. Cell Death Dis. 2014;5:e1382.
Doitsh G, Galloway NL, Geng X, Yang Z, Monroe KM, Zepeda O, et al. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature. 2014;505:509–14.
Doitsh G, Greene WC. Dissecting How CD4 T Cells Are Lost During HIV Infection. Cell Host Microbe. 2016;19:280–91.
Broz P, Pelegrín P, Shao F. The gasdermins, a protein family executing cell death and inflammation. Nat Rev Immunol. 2020;20:143–57.
Shi J, Gao W, Shao F. Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trend Biochemi Sci. 2016;42:245–54.
Zhang Z, Zhang Y, Xia S, Kong Q, Li S, Liu X, et al. Gasdermin E suppresses tumour growth by activating anti-tumour immunity. Nature. 2020;579:415–20.
Zhou Z, He H, Wang K, Shi X, Wang Y, et al. Granzyme A from cytotoxic lymphocytes cleaves GSDMB to trigger pyroptosis in target cells. Science. 2020;368:eaaz7548.
Hou J, Zhao R, Xia W, Chang CW, You Y, Hsu JM, et al. PD-L1-mediated gasdermin C expression switches apoptosis to pyroptosis in cancer cells and facilitates tumour necrosis. Nat Cell Biol. 2020;22:1264–75.
Aachoui Y, Sagulenko V, Miao EA, Stacey KJ. Inflammasome-mediated pyroptotic and apoptotic cell death, and defense against infection. Curr Opin Microbiol. 2013;16:319–26.
Joosten LA, Netea MG, Dinarello CA. Interleukin-1beta in innate inflammation, autophagy and immunity. Semin Immunol. 2013;25:416–24.
Dinarello CA, Novick D, Kim S, Kaplanski G. Interleukin-18 and IL-18 binding protein. Front Immunol. 2013;4:289.
Ohashi K, Burkart V, Flohe S, Kolb H. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J Immunol. 2000;164:558–61.
Zeng CY, Li CG, Shu JX, Xu LH, Ouyang DY, Mai FY, et al. ATP induces caspase-3/gasdermin E-mediated pyroptosis in NLRP3 pathway-blocked murine macrophages. Apoptosis. 2019;24:703–17.
Kolb R, Liu GH, Janowski AM, Sutterwala FS, Zhang W. Inflammasomes in cancer: a double-edged sword. Protein Cell. 2014;5:12–20.
Kelley N, Jeltema D, Duan Y, He Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int J Mol Sci. 2019;20:3328.
Elliott EI, Sutterwala FS. Initiation and perpetuation of NLRP3 inflammasome activation and assembly. Immunological Rev. 2015;265:35–52.
Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278–86.
Tsai CM, Riestra AM, Ali SR, Fong JJ, Liu JZ, Hughes G, et al. Siglec-14 Enhances NLRP3-Inflammasome Activation in Macrophages. J Innate Immun. 2020;12:333–43.
Lagea H, Helmbachb H, Grottkea C, Dietela M, Schadendorfb D. DFNA5 (ICERE-1) contributes to acquired etoposide resistance in melanoma cells. FEBS Lett. 2001;494:54–59.
Dunn JH, Ellis LZ, Fujita M. Inflammasomes as molecular mediators of inflammation and cancer: potential role in melanoma. Cancer Lett. 2012;314:24–33.
Tan G, Huang C, Chen J, Zhi F. HMGB1 released from GSDME-mediated pyroptotic epithelial cells participates in the tumorigenesis of colitis-associated colorectal cancer through the ERK1/2 pathway. J Hematol Oncol. 2020;13:149.
Usui F, Shirasuna K, Kimura H, Tatsumi K, Kawashima A, Karasawa T, et al. Critical role of caspase-1 in vascular inflammation and development of atherosclerosis in Western diet-fed apolipoprotein E-deficient mice. Biochem Biophys. Res Commun. 2012;425:162–8.
Frantz S. Targeted deletion of caspase-1 reduces early mortality and left ventricular dilatation following myocardial infarction. J Mol Cell Cardiol. 2003;35:685–94.
Lee S, Lee M, Kim JB, Jo A, Cho EJ, Yu SJ, et al. 17beta-estradiol exerts anticancer effects in anoikis-resistant hepatocellular carcinoma cell lines by targeting IL-6/STAT3 signaling. Biochem Biophys Res Commun. 2016;473:1247–54.
Lin X, Wang H, An X, Zhang J, Kuang J, Hou J, et al. Baeckein E suppressed NLRP3 inflammasome activation through inhibiting both the priming and assembly procedure: Implications for gout therapy. Phytomedicine. 2021;84:153521.
Liu G, Liu Q, Yan B, Zhu Z, Xu Y. USP7 Inhibition Alleviates H2O2-Induced Injury in Chondrocytes via Inhibiting NOX4/NLRP3 Pathway. Front Pharmacol. 2021;11:617270.
Hu J, Zhou J, Wu J, Chen Q, Du W, Fu F, et al. Loganin ameliorates cartilage degeneration and osteoarthritis development in an osteoarthritis mouse model through inhibition of NF-κB activity and pyroptosis in chondrocytes. J Ethnopharmacol. 2020;247:112261.
Yan Z, Qi W, Zhan J, Lin Z, Lin J, Xue X, et al. Activating Nrf2 signalling alleviates osteoarthritis development by inhibiting inflammasome activation. J Cell Mol Med. 2020;24:13046–57.
Wu XY, Li KT, Yang HX, Yang B, Lu X, Zhao LD, et al. Complement C1q synergizes with PTX3 in promoting NLRP3 inflammasome over-activation and pyroptosis in rheumatoid arthritis. J. Autoimmun. 2020;106:102336.
Hao K, Jiang W, Zhou M, Li H, Chen Y, Jiang F, et al. Targeting BRD4 prevents acute gouty arthritis by regulating pyroptosis. Int J Biol Sci. 2020;16:3163–73.
Zu SQ, Feng YB, Zhu CJ, Wu XS, Zhou RP, Li G, et al. Acid-sensing ion channel 1a mediates acid-induced pyroptosis through calpain-2/calcineurin pathway in rat articular chondrocytes. Cell Biol Int. 2020;44:2140–52.
Xu Y, Chen F. Acid-Sensing Ion Channel-1a in Articular Chondrocytes and Synovial Fibroblasts: A Novel Therapeutic Target for Rheumatoid Arthritis. Front Immunol. 2020;11:580936.
Li H, Jiang W, Ye S, Zhou M, Liu C, Yang X, et al. P2Y(14) receptor has a critical role in acute gouty arthritis by regulating pyroptosis of macrophages. Cell Death Dis. 2020;11:394.
Lu R, Wang Y, Liu C, Zhang Z, Li B, Meng Z, et al. Design, synthesis and evaluation of 3-amide-5-aryl benzoic acid derivatives as novel P2Y14R antagonists with potential high efficiency against acute gouty arthritis. Eur J Med Chem. 2021;216:113313.
Wang, W, C Liu, H Li, S Tian, Y Liu, N Wang, et al. Discovery of novel and potent P2Y14R antagonists via structure-based virtual screening for the treatment of acute gouty arthritis. J Adv Res. 2020;23:133–42.
S.-M. Ye, M.-Z. Zhou, W.-J. Jiang, C. X. Liu, Z.-W. Zhou, M.-J. Sun, et al. Silencing of gasdermin D by siRNA loaded PEI-Chol lipopolymers potently relieves acute gouty arthritis through inhibiting pyroptosis. Mol Pharmaceutics. 2020;18:667–78.
Lin Y, Luo T, Weng A, Huang X, Yao Y, Fu Z, et al. Gallic Acid Alleviates Gouty Arthritis by Inhibiting NLRP3 Inflammasome Activation and Pyroptosis Through Enhancing Nrf2 Signaling. Front Immunol. 2020;11:3197.
Pan H, Lin Y, Dou J, Fu Z, Yao Y, Ye S, et al. Wedelolactone facilitates Ser/Thr phosphorylation of NLRP3 dependent on PKA signalling to block inflammasome activation and pyroptosis. Cell Prolif. 2020;53:e12868.
Karki R, Sharma BR, Tuladhar S, Williams EP, Zalduondo L, Samir P, et al. Synergism of TNF-alpha and IFN-gamma Triggers Inflammatory Cell Death, Tissue Damage, and Mortality in SARS-CoV-2 Infection and Cytokine Shock Syndromes. Cell. 2021;184:149–68.
Yap JKY, Moriyama M, Iwasaki A. Inflammasomes and Pyroptosis as Therapeutic Targets for COVID-19. J Immunol. 2020;205:307–12.
Ratajczak MZ, Kucia M. SARS-CoV-2 infection and overactivation of Nlrp3 inflammasome as a trigger of cytokine “storm” and risk factor for damage of hematopoietic stem cells. Leukemia. 2020;34:1726–9.
McCarty, MF, SB Iloki Assanga, L Lewis Luján, JH O’Keefe, JJ Di. Nicolantonio, Nutraceutical Strategies for Suppressing NLRP3 Inflammasome Activation: Pertinence to the Management of COVID-19 and Beyond. Nutrients, 2020;13:47.
Liu F, Liu T, Sun M, Zhou J, Xue F, Chen S, et al. Maxing Shigan Decoction Mitigates Mycoplasma pneumonia-Induced Pyroptosis in A549 Cells via the NLRP3 Inflammasome. Infect Drug Resist. 2021;14:859–67.
Li LL, Dai B, Sun YH, Zhang TT. The activation of IL-17 signaling pathway promotes pyroptosis in pneumonia-induced sepsis. Ann Transl Med. 2020;8:674.
Wu Z, Tian Y, Alam HB, Li P, Duan X, Williams AM, et al. Peptidylarginine Deiminases 2 Mediates Caspase-1-Associated Lethality in Pseudomonas aeruginosa Pneumonia-Induced Sepsis. J Infect Dis. 2021;223:1093–102.
Xie WH, Ding J, Xie XX, Yang XH, Wu XF, Chen ZX, et al. Hepatitis B virus X protein promotes liver cell pyroptosis under oxidative stress through NLRP3 inflammasome activation. Inflamm Res. 2020;69:683–96.
Seitz, HK, R Bataller, H Cortez-Pinto, B Gao, A Gual, C Lackner, et al. Alcoholic liver disease. Nature Reviews Disease Primers, 2018;4:16.
Brunt, EM, VWS Wong, V Nobili, CP Day, S Sookoian, JJ Maher, et al. Nonalcoholic fatty liver disease. Nat Rev Dis Primers, 2015;1:15081.
Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179–88.
Xu B, Jiang M, Chu Y, Wang W, Chen D, Li X, et al. Gasdermin D plays a key role as a pyroptosis executor of non-alcoholic steatohepatitis in humans and mice. J Hepatol. 2018;68:773–82.
Koh EH, Yoon JE, Ko MS, Leem J, Yun JY, Hong CH, et al. Sphingomyelin synthase 1 mediates hepatocyte pyroptosis to trigger non-alcoholic steatohepatitis. Gut. 2020;70:1954–1964.
Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, et al. Hepatocellular carcinoma. Nat Rev Dis. Prim. 2016;2:16018.
Kofahi HM, Taylor NG, Hirasawa K, Grant MD, Russell RS. Hepatitis C Virus Infection of Cultured Human Hepatoma Cells Causes Apoptosis and Pyroptosis in Both Infected and Bystander Cells. Sci Rep. 2016;6:37433.
Wei Q, Zhu R, Zhu J, Zhao R, Li M. E2-Induced Activation of the NLRP3 Inflammasome Triggers Pyroptosis and Inhibits Autophagy in HCC Cells. Oncol Res. 2019;27:827–34.
Hage C, Hoves S, Strauss L, Bissinger S, Prinz Y, Poschinger T, et al. Sorafenib Induces Pyroptosis in Macrophages and Triggers Natural Killer Cell-Mediated Cytotoxicity Against Hepatocellular Carcinoma. Hepatology. 2019;70:1280–97.
Zhan ZY, Wu M, Shang Y, Jiang M, Liu J, Qiao CY, et al. Taxifolin ameliorate high-fat-diet feeding plus acute ethanol binge-induced steatohepatitis through inhibiting inflammatory caspase-1-dependent pyroptosis. Food Funct. 2021;12:362–72.
Mai W, Xu Y, Xu J, Zhao D, Ye L, Yu G, et al. Berberine Inhibits Nod-Like Receptor Family Pyrin Domain Containing 3 Inflammasome Activation and Pyroptosis in Nonalcoholic Steatohepatitis via the ROS/TXNIP Axis. Front Pharm. 2020;11:185.
Shi H, Zhang Y, Xing J, Liu L, Qiao F, Li J, et al. Baicalin attenuates hepatic injury in non-alcoholic steatohepatitis cell model by suppressing inflammasome-dependent GSDMD-mediated cell pyroptosis. Int Immunopharmacol. 2020;81:106195.
Yu X, Hao M, Liu Y, Ma X, Lin W, Xu Q, et al. Liraglutide ameliorates non-alcoholic steatohepatitis by inhibiting NLRP3 inflammasome and pyroptosis activation via mitophagy. Eur J Pharm. 2019;864:172715.
Lasry A, Zinger A, Ben-Neriah Y. Inflammatory networks underlying colorectal cancer. Nat Immunol. 2016;17:230–40.
Nowarski R, Jackson R, Gagliani N, de Zoete MarcelR, Palm NoahW, Bailis W, et al. Epithelial IL-18 Equilibrium Controls Barrier Function in Colitis. Cell. 2015;163:1444–56.
Carvalho FA, Nalbantoglu I, Ortega-Fernandez S, Aitken JD, Su Y, Koren O, et al. Interleukin-1beta (IL-1beta) promotes susceptibility of Toll-like receptor 5 (TLR5) deficient mice to colitis. Gut. 2012;61:373–84.
Jie F, Xiao S, Qiao Y, You Y, Feng Y, Long Y, et al. Kuijieling decoction suppresses NLRP3-Mediated pyroptosis to alleviate inflammation and experimental colitis in vivo and in vitro. J Ethnopharmacol. 2021;264:113243.
Mehto S, Jena KK, Nath P, Chauhan S, Kolapalli SP, Das SK, et al. The Crohn’s Disease Risk Factor IRGM Limits NLRP3 Inflammasome Activation by Impeding Its Assembly and by Mediating Its Selective Autophagy. Mol Cell. 2019;73:429–45.
Derangere V, Chevriaux A, Courtaut F, Bruchard M, Berger H, Chalmin F, et al. Liver X receptor beta activation induces pyroptosis of human and murine colon cancer cells. Cell Death Differ. 2014;21:1914–24.
Yu J, Li S, Qi J, Chen Z, Wu Y, Guo J, et al. Cleavage of GSDME by caspase-3 determines lobaplatin-induced pyroptosis in colon cancer cells. Cell Death Dis. 2019;10:193.
Chen C, Wang B, Sun J, Na H, Chen Z, Zhu Z, et al. DAC can restore expression of NALP1 to suppress tumor growth in colon cancer. Cell Death Dis. 2015;6:e1602. p
Munch NS, Fang HY, Ingermann J, Maurer HC, Anand A, Kellner V, et al. High-Fat Diet Accelerates Carcinogenesis in a Mouse Model of Barrett’s Esophagus via Interleukin 8 and Alterations to the Gut Microbiome. Gastroenterology. 2019;157:492–506.
Wang F, Li G, Ning J, Chen L, Xu H, Kong X, et al. Alcohol accumulation promotes esophagitis via pyroptosis activation. Int J Biol Sci. 2018;14:1245–55.
Barber G, Anand A, Katarzyna O, Phelan JJ, Heeran AB, Flis E, et al. Characterizing caspase-1 involvement during esophageal disease progression. Cancer Immunol Immunother. 2020;69:2635–49.
Wang WJ, Chen D, Jiang MZ, Xu B, Li XW, Chu Y, et al. Downregulation of gasdermin D promotes gastric cancer proliferation by regulating cell cycle-related proteins. J Dig Dis. 2018;19:74–83.
Cui J, Zhou Z, Yang H, Jiao F, Li N, Gao Y, et al. MST1 Suppresses Pancreatic Cancer Progression via ROS-Induced Pyroptosis. Mol. Cancer Res. 2019;17:1316–25.
Gao X, Pan Y, Han W, Hu C, Wang C, Chen L, et al. Association of systemic inflammation and body mass index with survival in patients with resectable gastric or gastroesophageal junction adenocarcinomas. Cancer Biol Med. 2021;18:283–97.
Wu M, Wang Y, Yang D, Gong Y, Rao F, Liu R, et al. A PLK1 kinase inhibitor enhances the chemosensitivity of cisplatin by inducing pyroptosis in oesophageal squamous cell carcinoma. EBioMedicine. 2019;41:244–55.
Wang Y, Yin B, Li D, Wang G, Han X, Sun X. GSDME mediates caspase-3-dependent pyroptosis in gastric cancer. Biochem Biophys Res Commun. 2018;495:1418–25.
Wang L, Li K, Lin X, Yao Z, Wang S, Xiong X, et al. Metformin induces human esophageal carcinoma cell pyroptosis by targeting the miR-497/PELP1 axis. Cancer Lett. 2019;450:22–31.
Herzer K, Sprinzl MF, Galle PR. Hepatitis viruses: live and let die. Liver Int. 2007;27:293–301.
Spechler SJ, Souza RF. Barrett’s esophagus. N Engl J Med. 2014;371:836–45.
Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21:677–87.
Jin R, Liu C, Zheng S, Wang X, Feng X, Li H, et al. Molecular heterogeneity of anti-PD-1/PD-L1 immunotherapy efficacy is correlated with tumor immune microenvironment in East Asian patients with non-small cell lung cancer. Cancer Biol Med. 2020;17:768–81.
Acknowledgements
This work was supported by the National Natural Science Foundation of China through No. 81872320, 81971540, 82173087; Prevention and Control Project for COVID-19 pneumonia (Department of Education of Guangdong Province) through No. 2020KZDZX1129; Department of Science and Technology of Guangdong Province through No. 2019A1515110740 and 2021A0505110014; Health Commission of Guangdong Province through No. A2020100.
Author information
Authors and Affiliations
Contributions
YW drafted the manuscript. YW, JZ, YL, and SY discussed the manuscript. YW and RZ designed the research and drafted the manuscript. JZ, KZ, and RZ revised the manuscript. All authors read and approved final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wu, Y., Zhang, J., Yu, S. et al. Cell pyroptosis in health and inflammatory diseases. Cell Death Discov. 8, 191 (2022). https://doi.org/10.1038/s41420-022-00998-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41420-022-00998-3
This article is cited by
-
Potential Impact of Climate Change-Induced Alterations on Pyroptotic Cell Death in Animal Cells: A Review
Molecular Biotechnology (2024)
-
Mechanically induced pyroptosis enhances cardiosphere oxidative stress resistance and metabolism for myocardial infarction therapy
Nature Communications (2023)
-
Gene expression study of host-human T-cell leukaemia virus type 1 (HTLV-1) interactions: adult T-cell leukaemia/lymphoma (ATLL)
Molecular Biology Reports (2023)
-
A positive feedback cycle between the alarmin S100A8/A9 and NLRP3 inflammasome-GSDMD signalling reinforces the innate immune response in Candida albicans keratitis
Inflammation Research (2023)
-
Systems biology approach reveals a common molecular basis for COVID-19 and non-alcoholic fatty liver disease (NAFLD)
European Journal of Medical Research (2022)