Abstract
Pyroptosis is a novel regulated cell death (RCD) mode associated with inflammation and innate immunity. Gasdermin E (GSDME), a crucial component of the gasdermin (GSDM) family proteins, has the ability to convert caspase-3-mediated apoptosis to pyroptosis of cancer cells and activate anti-tumor immunity. Accumulating evidence indicates that GSDME methylation holds tremendous potential as a biomarker for early detection, diagnosis, prognosis, and treatment of tumors. In fact, GSDME-mediated pyroptosis performs a dual role in anti-tumor therapy. On the one side, pyroptotic cell death in tumors caused by GSDME contributes to inflammatory cytokines release, which transform the tumor immune microenvironment (TIME) from a ‘cold’ to a ‘hot’ state and significantly improve anti-tumor immunotherapy. However, due to GSDME is expressed in nearly all body tissues and immune cells, it can exacerbate chemotherapy toxicity and partially block immune response. How to achieve a balance between the two sides is a crucial research topic. Meanwhile, the potential functions of GSDME-mediated pyroptosis in anti-programmed cell death protein 1 (PD-1) therapy, antibody-drug conjugates (ADCs) therapy, and chimeric antigen receptor T cells (CAR-T cells) therapy have not yet been fully understood, and how to improve clinical outcomes persists obscure. In this review, we systematically summarize the latest research regarding the molecular mechanisms of pyroptosis and discuss the role of GSDME-mediated pyroptosis in anti-tumor immunity and its potential applications in cancer treatment.
Similar content being viewed by others
Facts
-
GSDME-dependent pyroptotic pathways contribute to inflammatory tumor cell death and immune response activation.
-
Assessing GSDME methylation levels is a beneficial strategy to detect cancer and distinguish between different tumor types.
-
GSDME-mediated pyroptosis is responsible for certain chemotherapeutic medications’ toxicity and side effects.
-
An in-depth understanding of the role of GSDME-mediated pyroptosis in TIME may provide novel strategies for improving anti-tumor immunotherapy.
Open Questions
-
What can be done to avoid adverse effects caused by GSDME-mediated pyroptosis?
-
What if detecting GSDME expression levels could be a promising strategy to assess the risk and severity of CRS caused by CAR T-cell therapy?
-
How do immune cells collaborate to inhibit or promote tumor progression via GSDME-mediated pyroptosis?
-
How to find a balance point between the intervention of GSDME-mediated pyroptosis through inflammatory pathways and anti-tumor immunity?
Introduction
Although tremendous advances have been made in anti-tumor therapeutic strategies over the past decade, cancer remains a pressing public health issue due to its rising incidence and mortality rates [1]. In 2020, the World Health Organization reported 19.3 million newly diagnosed cancer cases and approximately 10.0 million cancer-related deaths [2]. Conventional therapeutic approaches, such as chemotherapy, radiation therapy, and surgery, have been recognized to possess significant limitations [3]. Unfortunately, many patients with metastatic or recurrent diseases continue to experience unfavorable outcomes despite these treatments [4]. Studies have demonstrated that tumors are pathologically affected by oxidative stress, chronic inflammation, pathogen infection, immune destruction, and other factors [5,6,7]. Of note, the tumor immune microenvironment (TIME) is crucial for modulating tumor invasion, metastasis, and anti-tumor immunity [8]. Cancer treatment strategies rely heavily on inducing tumor cell death [4]. On the basis of cellular morphological characteristics, regulatory mechanisms, and biological functions, cell death can be classified into accidental cell death and regulated cell death (RCD) [9].
Pyroptosis is a type of RCD associated with inflammation. It is characterized by cell swelling, plasma membrane pores formation, intercellular content secretion, and proinflammatory effects [10]. This inflammatory cell death form is widely recognized as a biological mechanism crucial for maintaining organismal homeostasis [10]. As evidence accumulates, pyroptosis is distinguished by a faster occurrence and a more potent inflammatory response when compared to other RCD modes [11, 12]. Furthermore, pyroptosis is closely associated with inflammatory diseases caused by hypoxia, ER stress, pathogens, and tumors [13,14,15,16]. Importantly, pyroptosis plays a double-edged sword role in terms of reshaping the TIME. In one aspect, a mild inflammatory response caused by pyroptosis activates immune cells and eliminating tumor cells, maintaining the stability of the extracellular environment [10]. However, in the presence of prolonged and excessive pyroptosis effects, massive inflammatory and immune factors are released, causing normal cells to become cancerous [17].
Gasdermin (GSDM) family proteins, including GSDMs A–E, are key effectors that mediate pyroptosis [18]. In fact, pyroptosis is now recognized as an inflammatory form of RCD mediated by GSDMs [19]. In the past few years, GSDMD has been identified as the major executive protein for pyroptosis and depends on caspase-1/4/5/11 activation [20]. Recently, researchers have made noteworthy discoveries in the biological process of pyroptosis, revealing the crucial function of GSDME in the modulation of inflammation and immunology [21]. GSDME (also known as ICERE1 or DFNA5), which is present in both tumor cells and normal tissues, has the ability to convert caspase-3-mediated apoptosis to pyroptosis upon exposure to chemotherapy agents [22]. Caspase-3 was previously regarded as one of the primary apoptosis biomarkers. Interestingly, emerging evidence reveals that caspase-3 specifically splits GSDME at the linker region, thereby releasing the N-terminal domain and generate membrane holes [23]. This process leads to the release of interleukin (IL-1β/IL-18) and immunogenic molecules, disrupting ionic and water homeostasis, ultimately converting the RCD type from apoptosis to pyroptosis [23]. Furthermore, caspase-3 and GSDME-dependent pyroptotic pathways contribute to inflammatory tumor cell death and immune response activation, such as enhancing the anti-tumor activity of CD8+ T killer lymphocytes, macrophages, and tumor-infiltrating natural killer (NK) cells, which are intricately associated with anti-tumor immunity [21]. Very recently, however, it was also observed that GSDME gene methylation varied according to cancer type between tumor tissues and normal cells [24]. This indicates its potential as a detection biomarker for both pancancer and specific cancer types [24]. Overall, developing a more profound and enhanced knowledge of the role of GSDME-mediated pyroptosis in cancer may have substantial clinical utility as a diagnostic biological marker and provide novel strategies for preventing and treating tumors.
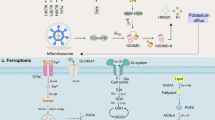
Comparison molecular mechanisms of necroptosis, apoptosis, ferroptosis, cuproptosis, and pyroptosis
Cell death plays a critical role in multiple biological mechanisms, including maintaining normal homeostasis, eliminating harmful stimuli, and killing potentially neoplastic cells [25]. In recent years, it has been increasingly recognized that accidental cell death and RCD are the two principal forms of cell death [25]. The major types of RCD include pyroptosis, necroptosis, apoptosis, ferroptosis, and cuproptosis [26, 27]. Importantly, each RCD subroutine has its own initiator, effector, and executor, which also possess distinct histological and biochemical characteristics.
Necroptosis is a lytic inflammatory RCD mode characterized by cytoplasmic membrane disruption and cellular swelling [28]. Particularly, necroptosis is considered caspase-independent [28]. Instead, upon initiation by death receptors, necroptosis is mediated by receptor-interacting protein 1 (RIP1), RIP3, and mixed lineage kinase domain-like (MLKL), leading to MLKL pores formation [29]. Apoptosis is a non-inflammatory RCD mode. Histologically, apoptosis is characterized by condensation of chromatin, formation of apoptotic bodies, fragmentation of nuclei, shrinkage and pyknosis of cells [30]. Typically, the activation of apoptosis is initiated by non-inflammatory proteases, including caspase-3/7/9 [31]. Ferroptosis is a distinctive iron-dependent RCD triggered by excessive lipid peroxides accumulation in plasma membranes [32]. This molecular event is usually accompanied by intracellular iron overload and glutathione (GSH) exhaustion, resulting in reactive oxygen species (ROS) generation and plasma membrane rupture [33]. The latest research indicates that cuproptosis is a newly discovered form of RCD triggered by excess Cu2+ [34]. Particularly, elesclomol functions as an ionophore that promotes Cu2+ transport into cells [34]. Intracellular copper ions target and bind to lipoylated components in the tricarboxylic acid (TCA) cycle [35]. This process results in the instability of Fe-S cluster proteins, which ultimately causes proteotoxic stress and cell mortality [35].
Pyroptosis is an inflammatory form of RCD. The observation of pyroptosis dates back to 1992, when Zychlinsky and colleagues first noted this new form of cell death [36]. However, at the time, it was mistakenly identified as morphological changes in apoptosis mediated by caspase-1 in macrophages infected with Shigella flexneri [36]. A decade later, in 2001, this new caspase-1-dependent RCD was termed pyroptosis by Brennan [37]. Unlike other RCD forms, pyroptosis involves the innate immune system and is characterized by its ability to induce inflammation, recruit neutrophils, and modulate the immune response [13]. Due to several newly discovered molecular events, pyroptosis has recently received increased attention [38, 39]. During the early stage of pyroptosis, a distinct type of DNA damage occurs that sets it apart from apoptosis [30, 40]. Chromatin coagulation occurs in both apoptosis and pyroptosis [40]. However, in pyroptosis, the nucleus maintains intact and does not undergo karyorrhexis [41]. In contrast to necroptosis, apoptosis, and ferroptosis, pyroptosis is characterized by inflammasome formation and GSDMs-dependent cell membrane holes generation [30, 42]. Initially, pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) are recognized intracellularly by cytoplasmic pattern recognition receptors (PRRs), triggering the assembly of inflammasome complex and the maturity of inflammatory caspases [43]. After that, cells experience pores formation in the cytoplasmic membrane, followed by swelling and eventual lysis [44]. These pore-induced intracellular traps mediated by GSDMs eventually lead to the release of cell contents and pro-inflammatory mediators, including IL-1β/18 and DAMPs [19]. Taken together, the different characteristics between pyroptosis and other RCD modalities are briefly summarized in Table 1.
Overview of the biological functions of GSDME
The wild-type GSDME is composed of 10 exons which genetically encode a protein consisting of 496 amino acids with a molecular weight of 55 kDa [45]. It consists of an autoinhibitory C-terminal domain (GSDME-CT) and an N-terminal domain (GSDME-NT) connected by an interdomain linker. GSDME-NT has been found to initiate pyroptosis through its pore-forming activities [46]. Conversely, GSDME-CT provides cellular protection against pyroptosis under non-stimulating conditions [46].
GSDME is widely expressed in most tissues and organs [47]. In humans, the expression of GSDME is predominantly observed in the heart, brain, kidneys, and placenta [48,49,50]. The ability of GSDME to trigger cell death determines its crucial role in diseases. In 1998, GSDME was identified as a deafness gene implicated in the pathogenesis of hereditary hearing loss [51]. The mutations in GSDME resulted in transcriptional skipping of exon 8 and introduced a premature stop codon which destroyed GSDME-CT, leading to the loss of GSDME self-inhibitory activity and the emergence of cytotoxicity [52, 53]. However, the relationship between pyroptosis and hearing loss caused by the GSDME mutation remains uncertain. Moreover, GSDME has been linked to various disorders including atherosclerosis, kidney diseases, cystic fibrosis, and inflammatory skin diseases through genetic association studies [54,55,56]. Coincidentally, GSDME also plays a critical role in cancer. GSDME was identified as a potential tumor suppressor gene through genomic methylation monitoring. It functions as a transcriptional target of p53 and is inhibited in various types of cancer [57]. Evidence shows that the absence of GSDME renders certain chemotherapeutic drugs ineffective [58]. However, GSDME-mediated pyroptosis is also accountable for the toxicity and adverse effects of specific chemotherapeutic medications [22].
The crosstalk between caspase-1, 4, 5, 11/GSDMD-mediated pyroptosis and caspase-3/GSDME-mediated pyroptosis
GSDMD has been extensively researched and is identified as a crucial gasdermin responsible for initiating pyroptosis [59]. Notably, GSDMD is a direct target of inflammatory caspases (caspase-1, 4, 5, and 11). In molecular terms, GSDMD-mediated pyroptosis can be classified into two distinct pathways: classical and non-classical pyroptotic pathways [60]. The classical pyroptotic pathway involves the recognition of DAMPs and PAMPs by Toll-like receptors (TLRs) and Nod-like receptors (NLRs) in response to intracellular and extracellular stimulation [61]. Upon activation of inflammasomes (particularly the NLRP3 inflammasome), caspase-1 is activated and GSDMD is cleaved to produce N-GSDMD fragments [62]. This leads to the formation of pores in the cell membrane, resulting in cell rupture and death. Additionally, cell contents such as lactate dehydrogenase (LDH) and inflammatory factors are released, exerting pro-inflammatory effects [62, 63]. In the non-classical pyroptotic pathway, caspase-4, 5, and 11 are involved instead of caspase-1. This pathway is initiated when Toll-like receptor 4 (TLR4) recognizes extracellular LPS [19, 64]. Once activated, caspase-4, 5, and 11 cleave GSDMD to generate GSDMD-NT and does not require inflammasome activation [65].
In contrast to GSDMD, GSDME is activated by caspase-3 rather than caspases-1, 4, 5, and 11 [42, 50] (Fig. 1). Particularly, caspase-3 can be activated by apoptosis initiators caspase-8 and caspase-9 [66]. Furthermore, unlike GSDMD-mediated pyroptosis, GSDME-mediated pyroptosis does not require inflammasome assembly [67, 68]. After cutting by granzyme B released by NK cells or activated caspase-3 in the interdomain linker, the GSDME-NT domain is released to initiate the formation of pyroptotic pores in cell membranes, leading to cellular contents leakage and a subsequent proinflammatory effect [69]. Intriguingly, the level of GSDME is considered a crucial factor in caspase-3-mediated cell death form [23, 48]. When GSDME expression is low, cells are more likely to undergo apoptosis rather than pyroptosis in response to intrinsic stresses or extrinsic challenges [23].
The following three pathways have been validated to trigger pyroptosis: caspase-1/GSDMD-mediated classical pyroptotic pathway, caspase-4/5/11/GSDMD-mediated non-classical pyroptotic pathway, and caspase-3/GSDME-mediated pyroptotic pathway. The classical pyroptotic pathway involves the recognition of exogenous and endogenous danger signals (e.g., pathogens). Upon NLRP3 inflammasome activation, caspase-1 is activated and GSDMD is cleaved to generate GSDMD-NT fragments. This molecular process results in membrane pores formation and cell lysis. In the non-classical pyroptotic pathway, caspase-4, 5, and 11 are involved. This pathway is initiated by extracellular LPS. Once activated, caspase-4, 5, and 11 cleave GSDMD to produce GSDMD-NT, which forms plasma membrane pores to induce cell death similar to the classical pyroptotic pathway. In the caspase-3/GSDME-mediated pyroptotic pathway, GSDME is activated by caspase-3 or granzyme B released by NK cells. Compared to GSDMD-mediated pyroptosis, GSDME-mediated pyroptosis doesn’t involve inflammasome assembly. This molecular event generates GSDME-NT and induces the formation of pyroptotic holes in cell membranes, resulting in cellular contents leakage. Notably, chemotherapy drugs could also elicit pyroptosis through the caspase-3/GSDME pathway.
GSDME convert caspase-3-dependent apoptosis into pyroptosis
Apoptosis, unlike pyroptosis, is a type of RCD not associated with inflammation [38]. It is triggered by the activation of apoptotic caspases and can occur through either an intrinsic or extrinsic pathway [65]. The intrinsic pathway is triggered by multiple intracellular events (e.g., mitochondrial damage, endoplasmic reticulum stress, and ROS formation). These stimuli then contribute to the leakage of cytochrome c into the cytoplasm. Subsequently, after interacting with apoptotic protease activating factor-1 (Apaf-1), cytochrome c activates caspase-9. In turn, caspase-9 activates caspase-3/7, which ultimately results in cell death. The extrinsic pathway begins upon the oligomerization of cell surface death receptors. Once activated, pro-caspase-8 is recruited and converted to caspase-8, which then cleaves Bid to produce tBid and activates caspase-3/7, contributing to a series of molecular alterations and initiating apoptosis. In particular, apoptosis leads to rapid phagocytic clearance of dead or damaged cells, which is generally not immunogenic [70]. Apoptosis is also critical for inhibiting tumor growth, and certain anti-cancer medications work by inducing apoptosis [71]. The presence of an apoptosis-inducing domain in GSDME suggests that it may have an intrinsic ability to induce apoptosis. Transfection of mutant GSDME into mammalian cells resulted in cell apoptosis or exhibited toxic effects, whereas transfection of wildtype GSDME did not show such a phenomenon [72]. In fact, N-terminal domain found in both wildtype and mutant GSDME induces apoptosis and cell death. On the other hand, C-terminal domain can inhibit the apoptosis-inducing ability of N-terminal domain [73]. The exclusion of exon 8 can result in a shorter C-terminal domain for the mutant GSDME [73]. This can cause the release of autoinhibition activity and activate the N-terminal domain [73]. GSDME-NT appears to target mitochondria to promote cytochrome c release, thereby forming a self-sustaining feedback loop during apoptosis [31]. The available evidence suggests that mutations in GSDME lead to apoptosis through a series of interconnected pathways, including endoplasmic reticulum stress, mitochondrial damage, and oxidative stress [23, 74, 75].
Caspase-3 executes functions by catalyzing the cleavage of peptide bonds following aspartic acid residues via the C-terminal cysteine residue [76]. Evidence suggests that caspase-3 acts as a crucial adaptor protein for caspase cascades [77]. It can be activated by intrinsic or extrinsic apoptotic pathways and is the only endogenous signal that triggers the cleavage of GSDME [78]. For decades, caspase-3 has been considered a key executor of apoptosis, but surprisingly, it can also be involved in pyroptosis in the presence of GSDME [23]. It was discovered that the absence of GSDME did not inhibit caspase-3-dependent cell death, but rather modified the form of cell death [22]. Actually, as the substrate of caspase-3, GSDME functions as a ‘transducer’ to determine whether a cell undergoes pyroptosis or apoptosis [79]. Rogers et al. discovered that activated caspase-3 could cleave GSDME and induce pyroptotic cell death following the successful induction of apoptosis by caspase-3 [31]. Additionally, GSDME-NT induces caspase-3 activation by cleaving the mitochondrial membrane, which further causes self-amplification of pyroptosis [80]. It is interesting to note that in cells with high GSDME expression, activated caspase-3 selectively cleaves GSDME to trigger pyroptosis before apoptosis, as pyroptosis is a faster process compared to apoptosis [23]. In cancer cell lines, caspase-3 can specifically cleave GSDME to induce a switch from apoptosis to pyroptosis, enhancing the sensitivity of cancer cells to chemotherapeutic agents [81, 82]. Further research found that in GSDME deletion cancer cells (e.g., Jurkat), chemotherapy drugs activate caspase-3, which cleaves the apoptotic substrate protein poly ADP-ribose polymerase (PARP) and leads to apoptosis [83]. However, in highly GSDME expressing cancer cells (e.g., SH-SY5Y), caspase-3 preferentially splits GSDME over PARP, resulting in caspase-3/GSDME-mediated pyroptosis [82]. Therefore, it can be concluded that GSDME is essential for the crosstalk between caspase-3-dependent apoptosis and pyroptosis (Fig. 2).
a The intrinsic apoptotic pathways. b The extrinsic apoptotic pathways. c The caspase-3/GSDME-mediated pyroptotic pathway. d GSDME-NT targets mitochondrial membranes to generate holes, causing the release of cytochrome c and mitochondrial DNA. GSDME works as a ‘transducer’ to determine whether a cell undergoes pyroptosis or apoptosis. Activated caspase-3 could cleave GSDME and induce pyroptotic cell death following the successful induction of apoptosis by caspase-3. Besides that, GSDME-NT cleaves the membrane of mitochondrial, which further triggers self-amplification of pyroptosis.
GSDME methylation and its role in cancer detection
Biomarkers for clinical tumor detection must exhibit high stability, sensitivity, and specificity. Additionally, they should be capable of detecting cancer in its early stages. Ideal biomarkers can be obtained from solid tissues, urine, saliva, and, most importantly, circulating DNA in the bloodstream [84]. Accumulating evidences indicate that GSDME holds promise as a cancer detection biomarker [85]. GSDME is frequently observed as epigenetic silencing through DNA methylation in various cancers [49]. Consequently, assessing its methylation levels can serve as a valuable tool for detecting cancer and distinguishing between different tumor types [86].
The widespread methylation in promoter CpGs is correlated with transcriptional silencing and is a possible mechanism for inactivating tumor suppressor genes. Methylation of CpGs in the GSDME promoter was first observed in breast cancer [87]. KIM et al. performed TaqMan-methylation specific PCR of CpGs at GSDME promoter regions in 34 primary breast adenocarcinomas samples [87]. According to the results, a specific methylation of CpGs in GSDME promoter was identified associated with breast cancer [87]. In addition, a positive correlation was found between lymph node metastasis and the degree of GSDME methylation, suggesting that breast cancer patients with higher GSDME methylation levels are more likely to experience lymph node metastasis [87]. In patients with breast ductal adenocarcinoma, a negative correlation was observed between the degree of GSDME methylation and 5-year overall survival (OS), indicating that GSDME methylation is a prospective biomarker for determining prognosis [88]. Furthermore, GSDME expression is lower in estrogen receptor (ER)-positive tumors than in ER-negative tumors, providing evidence that ER may negatively regulate the expression of the GSDME gene via epigenetic modifications in the GSDME promoter [89]. Altogether, these findings strongly imply that GSDME methylation could be an excellent candidate for breast cancer screening and detection.
In light of these findings, additional research has demonstrated that the potential of GSDME biomarkers is not restricted to breast cancer. Akino et al. discovered that GSDME promoter methylation can facilitate the transformation of normal gastric cells into malignant cells [90]. Inhibition of GSDME expression could accelerate gastric cancer development, confirming that GSDME methylation is crucial to the pathogenesis of gastric cancer and has the potential to serve as a diagnostic marker for this disease [91]. Studies conducted on colorectal cancers utilizing genome-wide methylation datasets from The Cancer Genome Atlas (TCGA) discovered that GSDME methylation could serve as a potential biomarker for detecting colorectal adenocarcinomas [92]. Furthermore, tumors with lymphatic vessel invasion and advanced tumor-node-metastasis (TNM) stage exhibited an increase in GSDME promoter methylation [92]. In a recent study, it was discovered that GSDME methylation is prevalent in various cancer types, with extensive hypermethylation in promoter and gene body CpGs [93]. According to these findings, GSDME methylation can be used as a pan-cancer biomarker and also help differentiate between different types of cancer [93]. Overview, GSDME methylation level is closely associated with the progression and organotropic metastasis of diverse types of tumors. Due to its exceptional expression and methylation properties in various cancers, GSDME is a promising gene candidate for future investigation as a tumor detection biomarker (Fig. 3).
Various body fluids (e.g., cerebrospinal fluid, saliva, pleural fluid, peripheral blood, ascites, and urine) and tumor tissue can be used to detect GSDME methylation. GSDME methylation may be a promising biomarker for distinguishing tumor types, identifying tumor stages, and detecting organotropic metastases.
Role of GSDME-mediated pyroptosis in cancer therapy
Tumorigenesis is a complex process influenced by multiple factors, such as the expression of proto- and anti-oncogenes, persistent inflammation, oxidative stress, and inflammatory TIME [94]. The elevated methylation of GSDME determines that tumor cells undergo pyroptosis rather than apoptosis, which indicates that combining methylase inhibitors and antineoplastic drugs may kill tumor cells more effectively [95]. Recent research has cast light on the crucial role of GSDME-mediated pyroptosis in treating various tumors, including melanoma, lung cancer, digestive malignancies, gynecologic malignancies, etc (Fig. 4).
GSDME-mediated pyroptosis and melanoma
Melanoma is the primary cause of cutaneous malignancy-related mortality [96]. Recent research indicates that iron combined with ROS inducers carbonyl cyanide m-chlorophenyl hydrazone can effectively inhibit melanoma progression, with GSDME-mediated pyroptosis playing a critical role in this process [97]. In melanoma cells, abundant iron boosts ROS signaling, resulting in Tom20 oxidation and oligomerization [97]. Then, oxidized Tom20 recruits Bax to mitochondria, leading to cytochrome c release and caspase-3 activation, ultimately triggering GSDME-mediated pyroptosis [97]. Lage’s study found that GSDME expression elevation enhanced melanoma cells’ sensitivity to etoposide, indicating that GSDME-mediated pyroptosis contributes to melanoma etoposide resistance [98]. BRAF inhibitors (BRAFis) represent a significant advancement in melanoma treatment; however, their effectiveness is limited by rapid resistance emergence. Wang et al. demonstrated that IRF9-STAT2 signaling enhances adaptive resistance to BRAF inhibitors (BRAFis) in melanoma cells by regulating GSDME-mediated pyroptosis, thereby elucidating the pathogenesis of resistance in targeted therapy [99]. Similarly, BRAFis and MEK inhibitors (MEKis) have been observed to induce RCD in BRAFV600E-mutant melanoma cells [100]. Mechanistically, BRAFis + MEKis treatment activates caspase-3/GSDME signaling pathways, leading to tumor-associated T cells infiltration and activating dendritic cells to kill melanoma cells [100]. Eukaryotic elongation factor-2 kinase (eEF-2K) is a protein synthesis inhibitor that acts as a negative regulator and plays a vital role in malignant cell pyroptosis under diverse conditions [101]. Evidence confirmed that eEF-2K could amplify the pyroptosis-promoting impact of doxorubicin in melanoma cell lines expressing high levels of GSDME [101]. Very recently, another study confirmed that the GSDME-mediated pyroptosis is essential for initiating antibody recognition of intracellular antigens by inducing cell pyroptosis, inhibiting liver metastasis of circulating melanoma cells [102].
GSDME-mediated pyroptosis and lung cancer
Lung cancer is the most ubiquitous and lethal malignancy worldwide [103]. Recent mechanistic work revealed that GSDME knockdown can trigger the transition from apoptosis-to-pyroptosis in lung cancer lines, confirming the hypothesis that GSDME expression determines the type of cell death in caspase-3-activated tumor cells [104]. In A549 cells, dasatinib can trigger pyroptosis and elevate GSDME levels in A549 cells without the involvement of p53 [105]. When GSDME expression is high, cisplatin and paclitaxel activate the caspase-3/GSDME pathway, convert normal chemotherapy-induced apoptosis into pyroptosis and inhibit lung cancer cell growth [105]. Han et al. revealed that myricetin could act as a pyroptosis agonist that activates caspase-3 via the ER stress pathway to facilitate GSDME cleavage and induce pyroptosis in lung cancer cells [106]. According to the latest research, GSDME inhibits EGFR signaling pathway and promotes non-small-cell lung cancer (NSCLC) cells’ survival by enhancing EGFR dimerization and activation [107]. In addition, GSDME interacts physically with EGFR, covering the EGFRY1045 site and contributing to its stability [107]. The hydrosoluble fraction of cordyceps militaris extract (CME), a dietary herb for lung cancer patients, has been shown to induce A549 cell death by simultaneously activating caspase-3/GSDME pyroptotic pathways and caspase-3/PARP apoptotic pathways [108]. Yu et al. revealed that the pyroptosis inhibitor (DSF) and apoptosis inhibitor (Z-VAD-FMK) prevented the cell mortality induced by nitidine chloride [109]. GSDME was cleaved in A549 and H1688 cells treated with nitidine chloride, accompanied increased caspase-3 cleavage [109]. Moreover, nitidine chloride substantially inhibited the PI3K/AKT pathway transduction and induced pyroptosis of tumor cells in vivo, indicating that nitidine chloride is a potential drug for lung cancer by inducing GSDME-dependent pyroptosis [109].
GSDME-mediated pyroptosis and digestive malignancies
Liver cancer is one of the most prevalent and lethal digestive malignancies [110]. Neobavaisoflavone (NBIF), a natural active compound isolated from Psoralea, has anti-cancer and anti-inflammatory activities. A recent study revealed that hepatocellular carcinoma cells treated with NBIF exhibited pyroptotic characteristics [111]. Mechanistically, NBIF suppresses hepatocellular carcinoma cell proliferation by increasing the expression of Tom20 and triggering the caspase-3/GSDME pathway [112]. Schisandrin B has multiple therapeutic effects on liver disorders, such as repairing liver damage, reducing liver fibrosis, and inhibiting liver cancer [113]. Song et al. discovered that Schisandrin B causes HepG2 cells pyroptosis while working with NK cells through the activation of the perforin-granzyme B/caspase-3/GSDME pathway, indicating that Schisandrin B is a potential immunotherapy combination agent for liver cancer treatment [114].
Gastric cancer is a malignant tumor arising from the gastric mucosa’s epithelium and is the third leading cause of cancer-related mortality worldwide [115]. In 2007, epigenetic silencing of GSDME was initially discovered in primary gastric cancer [90]. Introducing GSDME into gastric cancer tumor cells inhibited their growth, indicating that GSDME has tumor suppressor activity [90]. Recent studies show that GSDME-expressed gastric cancer cells undergo pyroptosis when treated with chemotherapeutic agents such as 5-FU [77]. However, GSDME deletion by CRISPR-Cas9 converted 5-FU-induced pyroptosis to apoptosis in SGC-7901 cells, indicating that GSDME converts chemotherapy drug-induced apoptosis to pyroptosis in gastric cancer cells [77]. Another study confirmed that the combination of BIX-01294 and cisplatin could enhance the anti-cancer chemotherapy effect in gastric cancer cells by causing GSDME-mediated pyroptosis and activating autophagic flux [116]. This indicates that GSDME plays a crucial role in inducing tumor cells pyroptosis by chemotherapeutic agents in gastric cancer treatment. Furthermore, evidence suggests that GSDME has prognostic significance in gastric cancer. Therefore, GSDME’s capacity to suppress the proliferation of gastric cancer cells suggests that it can be utilized as a predictive biomarker [117]. Recently, Xia et al. found that simvastatin inhibits cell proliferation and induces caspase-3/GSDME-mediated pyroptosis to combat gastric malignancy [91]. Restoring GSDME expression with a DNA methyltransferase inhibitor can improve the susceptibility of gastric cancer cells to simvastatin [91].
Colorectal cancer remains the top 5 leading causes of tumor-related death in the digestive tract [118]. Studies have demonstrated that GSDME-mediated pyroptosis is also essential to the development of colorectal cancer [119]. Coxsackievirus group B3 (CVB3) is able to induce pyroptosis in colon cancer cell lines both in vivo and in vitro [120]. Mechanistically, CVB3-induced pyroptosis is promoted by ROS, which activates the caspase-3/GSDME pathway to produce pores in the plasma membrane [120]. These findings suggest that CVB3 is effective in treating colon cancer through the GSDME-mediated pyroptosis pathway. Similar to CVB3, lopressor can promote ROS phosphorylation and activate the caspase-3/GSDME axis to induce colon cancer cell pyroptosis [121]. Evidence shows that lobaplatin could trigger caspase-3 activation and GSDME cleavage, as well as activate the ROS/JNK/Bax mitochondrial apoptosis pathway, which increases cytochrome c release, indicating that GSDME-dependent pyroptosis is a potential mechanism by which loplatin eliminates colon cancer cells [48]. Synthetic FXR agonist GW4064 has the potential to exert a synergistic anticancer effect via the pyroptosis pathway [122]. Notably, GW4064 could enhance the anti-tumor effect of oxaliplatin against colorectal cancer [122]. Therefore, the combination of GW4064 and oxaliplatin may represent an alternative colorectal cancer treatment strategy. Recent studies have shown that the BAK/BAX and caspase-3/GSDME axis mediates chemotherapy-induced pyroptosis in colon cancer cells [81]. Importantly, 2-bromopalmitate (2-BP) inhibits GSDME palmitoylation during chemotherapy-induced pyroptosis [81].
Esophageal cancer is the sixth most prevalent form of cancer worldwide [123]. For the treatment of esophageal cancer, high resistance to radiotherapy and chemotherapy continues to be a significant obstacle [124]. Li et al. found that Photodynamic therapy (PDT) could induce pyroptosis in esophageal squamous cell carcinoma (ESCC) by modulating the PKM2/caspase-8/caspase-3/GSDME pathway, indicating that the clinical implementation of PDT in ESCC may have significant implications [125]. A recent study revealed that elevated STAT3β expression increases cisplatin sensitivity and promotes GSDME-mediated pyroptosis in ESCC cells after cisplatin exposure [126]. Mechanistically, STAT3β causes chemosensitivity in cisplatin-treated cells by disrupting mitochondrial electron chain transport and increasing ROS expression [126]. Another study revealed that Neobractatin (NBT) could inhibit the proliferation of esophageal cancer cells via GSDME-mediated pyroptosis [127]. According to mechanistic research, NBT contribute to ROS accumulation and active caspase-3 [127]. Recently, oncolytic viruses have been demonstrated to be an effective and promising option for cancer treatment [128]. in ESCC cells, rMV-Hu191 could induce mitochondrial dysfunction, which is mediated by BAK (BCL2 antagonist/killer 1) or BAX (BCL2 associated X) [129]. Further analysis discovered that rMV-Hu191 can effectively promote caspase-3/GSDME-mediated pyroptosis, which may increase oncolytic efficacy [129].
GSDME-mediated pyroptosis and gynecologic malignancies
Breast cancer is the most prevalent malignancy among adult women [130]. Xu et al. provided preliminary evidence that GW-8510, an inhibitor of cyclin-dependent kinase (CDK) 2, could trigger GSDME-mediated pyroptosis in triple-negative breast cancer (TNBC) cells and mice models [131]. This mechanism may be associated with the potential of GW-8510 to enhance immune response and improve the efficacy of immunotherapy [131]. In a similar manner, Ganoderma lucidum extract can induce GSDME-mediated pyroptosis in TNBC cells and mouse models, while stimulating the peripheral immune system [132]. According to another study, tetraarsenic hexoxide-treated TNBC cells exhibited specific pyroptotic characteristics, inhibiting tumor formation and lung metastasis [133]. Mechanistically, tetraarsenic hexoxide significantly increased mitochondrial ROS production by preventing the phosphorylation of mitochondrial STAT3, thereby promoting GSDME-mediated pyroptosis in TNBC cells [133]. Evidence shows that the mitochondrial protein UCP1 could modulate TNBC cells proliferation and metastasis by inducing mitochondrial destruction to activate mitophagy and GSDME-mediated pyroptosis, suggesting that UCP1 may be a novel therapeutic target for TNBC [134]. Recently, Li et al. designed a carrier-free chemo-photodynamic nanoplatform (A-C/NPs) for treating breast cancer [135]. They discovered that A-C/NP treatment could trigger GSDME-mediated pyroptosis by disrupting mitochondrial homeostasis and ROS accumulation, resulting in immunogenic cell death and exhibiting potent cytotoxicity in breast cancer cells [135].
Ovarian cancer is one of the deadliest gynecological malignancies [136]. Huo et al. reported that BI 2536, a small molecule drug, effectively inhibited the proliferation of ovarian cancer cells by triggering both apoptosis and pyroptosis via the caspase-3/GSDME pathway [137]. Furthermore, BI 2536 promoted CD8+ T cell accumulation at tumor sites and exhibited anti-tumor activity. CBL0137 is a potential small molecule inhibitor that modulates p53 and nuclear factor-κB simultaneously. Recent evidence demonstrates that CBL0137 stimulates mitochondrial ROS production, BAX accumulation, and cyt c release to trigger caspase-3/GSDME-mediated pyroptosis in ovarian cancer cells [138]. Paclitaxel (PTX) combined with platinum was used as first‑line chemotherapy for ovarian cancer; however, the exact mechanism remains unclear [139]. Yang and colleagues discovered that after PTX treatment, the caspase-3/GSDME pathway was activated to disrupt cell membranes and induce tumor cells pyroptosis [140]. Bexarotene is a selective activator of the retinoid X receptor, commonly used against cutaneous T-cell lymphoma [141]. Interestingly, a study revealed that bexarotene treatment activated caspase-4 instead of caspase-3 and encouraged GSDME-dependent pyroptosis in ES2 cell line, indicating that bexarotene may be a novel agent for treating ovarian cancer [142].
GSDME-mediated pyroptosis and other cancers
Glioblastoma multiforme is an aggressive, malignant, and fatal brain tumor that is refractory to the majority of therapeutic strategies [143]. As a second-generation cyclin-dependent kinase (CDK) inhibitor, AT7519 suppresses human glioblastoma cell growth via the caspase-3/GSDME-mediated pyroptotic pathway, suggesting that AT7519 is a potential GBM treatment chemical agent [144]. Kong and co-workers discovered that the natural flavonoid Galangin exhibits potent anti-glioblastoma multiforme properties [70]. GSDME knockdown in glioblastoma multiforme cells increased nuclear DNA damage and inhibited Galangin-induced pyroptosis [70].
Nasopharyngeal carcinoma is a malignant head and neck cancer type with high morbidity, characterized by an insidious onset and difficult early diagnosis [145]. Ovarian tumor family deubiquitinase 4 (OTUD4) has been found to be down-regulated in various types of tumors, and decreased OTUD4 expression is indicative of a poor prognosis [146]. A recent study demonstrated that OTUD4 deubiquitinated and stabilized GSDME, thereby promoting GSDME-mediated pyroptosis and increasing radiosensitivity in nasopharyngeal carcinoma [146]. Consequently, targeting the OTUD4/GSDME pathway to trigger pyroptosis is a novel strategy to improve radiotherapy sensitization of nasopharyngeal carcinoma [146]. Another study revealed that the natural product triptolide effectively eradicated head and neck cancer cells via GSDME-dependent pyroptosis by inhibiting mitochondrial hexokinase-ΙΙ [147].
As the most prevalent primary bone cancer, osteosarcoma has a high propensity for local invasion and metastasis [148]. Dioscin, a steroidal saponin extracted from medicinal plants, exhibits anti-inflammatory and anti-cancer properties [149]. A recent has confirmed that dioscin suppresses osteosarcoma cell proliferation via JNK/p38-dependent apoptotic pathway and GSDME-mediated pyroptotic pathway, implying that dioscin is a potential therapeutic drug for this disease [150].
Prostate cancer is the most prevalent type of tumor in males [151]. In a recent study, Zhang et al. synthesized a novel 3’,5’-diprenylated chalcone to develop new chemotherapy agents. They discovered that 3’,5’-diprenylated chalcone treatment initiates the PKCδ/JNK axis, thereby activating caspase-3 to cleave PARP and GSDME and trigger apoptosis and pyroptosis in prostate cancer cells [152].
The role and mechanism of GSDME-mediated pyroptosis in tumor immunotherapy
The TIME plays a significant role in tumor formation, involving immune cells such as cytotoxic T lymphocytes (CTLs or CD8+ T cells), NK cells, and tumor-associated macrophages (TAMs) recruitment [153,154,155]. Increasing evidence implies that the activated state of the TIME can effectively eliminate tumor cells [156]. However, under specific conditions, TIME can aid tumor immune evasion and promote tumor growth [157].
In fact, GSDME-mediated pyroptosis has a dual effect on tumor immunotherapy. Firstly, prolonged exposure to the inflammatory TIME can stimulate tumor cell proliferation and metastasis [158]. However, GSDME overexpression can also considerably increase the quantity of NK cells and CD8+ cytotoxic T cells within tumors [21]. Granzyme B, derived from these immune cells, can directly cleave GSDME to initiate cancer cell pyroptosis, thereby transforming the TIME from a ‘noninflamed’ to an ‘inflamed’ state, which in turn increases immune cells recruitment and contributes to an improved response to immunotherapy [21]. The accumulating evidence indicates that therapeutic strategies aimed at inducing pyroptosis could trigger protective anti-tumor immune responses or expand immunotherapy responses to immune checkpoint inhibitors (ICIs) or other immunotherapies [159].
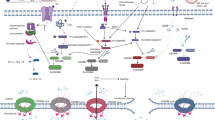
It has been confirmed that GSDME-mediated pyroptosis plays a crucial role in anti-programmed cell death protein 1 (PD-1) therapy, antibody-drug conjugates (ADCs) therapy, and chimeric antigen receptor T cells (CAR-T cells) therapy [160,161,162] (Fig. 5).
a GSDME-mediated pyroptosis and Anti-PD-1 therapy. Expression of GSDME regulates polarity changes in TAMs, Tregs, and T cells. GSDME may modulate immune infiltration via EIF2AK2 and enhance the antitumor activity of anti-PD-1 therapy. Particularly, GSDME-mediated pyroptosis is crucial for the recruitment of cytotoxic T lymphocytes in the context of VSV therapy, which can transform immunologically “cold” tumors into “hot” tumors and enhance the efficacy of anti-PD-1 therapy. b GSDME-mediated pyroptosis and ADC therapy. ADCs recognize target cells by binding to cell surface antigens, resulting in internalization of the construct and payload release inside lysosomes through the internal environment or enzymes. Notably, tubulysin has been incorporated as a cytotoxic payload in ADCs; the binding of tubulysin and dendritic cells modulates Fms-like tyrosine kinase-3 ligand (Flt3L) restores antitumor immunity in GSDME-silenced tumors. c GSDME-mediated pyroptosis and CAR-T therapy. Granzyme B released by CAR-T cells entered tumor cells and induced pyroptosis by cleaving GSDME, which activated the GSDME-mediated pyroptotic pathway and triggered cytokine release syndrome.
GSDME-mediated pyroptosis and Anti-PD-1 therapy
Immune evasion is a crucial feature of malignancies, as tumors tend to lose their immunogenicity and escape immune surveillance during division and proliferation. At present, ICIs exhibit promising potential in clinical oncology therapy [163]. ICIs bind to cytotoxic T-lymphocyte antigen-4 (CTLA-4) or PD-1, the primary targets associated with T-cell activation and exhaustion, and then eradicate tumor-induced immune suppression [164,165,166]. However, only one-third of patients respond to ICIs, thereby highlighting the urgent need to understand the mechanisms of resistance and identify suitable biomarkers that can accurately predict the clinical effectiveness of anti-PD-1 treatment [167]. The efficacy of anti-PD-1 therapy is closely related to TIME. Increasing evidence implicates the potential connection between anti-PD-1 treatment and GSDME-mediated pyroptotic cell death in tumors [168]. Hu et al. reported that GSDME expression could regulate polarity alterations in TAMs, Tregs, and T cell exhaustion [169]. GSDME might modulate immune infiltration through EIF2AK2 and augment the anti-tumor effect of anti-PD-1 therapy [169]. This study indicates that GSDME may be used as a predictive indicator of immune cell infiltration in various malignancies, including liver and lung cancers [169]. Oncolytic therapy is an emerging anti-tumor strategy that employs natural or engineered oncolytic viruses to inhibit the progression of tumors. According to a recent study, oncolytic vesicular stomatitis virus (VSV) activates GSDME-mediated pyroptosis to induce tumor cell pyroptosis [170]. Notably, GSDME is essential for recruiting cytotoxic T lymphocytes in the context of VSV therapy, which can transform immunologically ‘cool’ tumors into ‘hot’ tumors and increase the effectiveness of anti-PD-1 therapy [171]. Liang et al. designed a drug-polymer hybrid supramolecular nanoprodrug (PDNP) as a pyroptosis inducer to enhance tumor immunogenicity for an effective anti-tumor immune response [172]. In conjunction with anti-PD-1 therapy, PDNP induces GSDME-mediated pyroptosis and enhances the immune response in solid tumors, effectively halting invasive metastasis and extending survival due to its remarkable anti-tumor immunity [172].
Collectively, GSDME-mediated pyroptosis exhibits promise in augmenting anti-PD-1 treatment effectiveness and involves in tumor progression. In-depth research on this mechanism will open up novel perspectives for anti-tumor therapy.
GSDME-mediated pyroptosis and antibody drug conjugate therapy
ADCs are a novel class of drugs that exploit the specificity of monoclonal antibody (mAb) to deliver a potent cytotoxic payload to target antigens expressed on tumor cells [173, 174]. ADC therapy occupies an increasing role in the arsenal of anticancer treatments [175, 176]. Recently, research has revealed that ADC treatment stimulates pyroptotic cell death and induces anti-tumor immunity in GSDME-expressing carcinomas [172]. The binding of ADC to dendritic cells modulates Fms-like tyrosine kinase-3 ligand (Flt3L) restores anti-tumor immunity in GSDME-silenced tumors and can potentially improve clinical outcomes [162]. In mouse models of colon and breast cancer, tubulysin, a microtubule depolymerizing agent, can trigger tumor cell death by targeting the GSDME-mediated pyroptotic pathway and modulating tumor sensitivity to ADC treatment [162].
Consequently, ADC therapy may induce GSDME-mediated pyroptotic cell death in GSDME-expressing cells, thereby regulating anti-tumor immunity and therapeutic response.
GSDME-mediated pyroptosis and CAR-T therapy
Cancer immunotherapy has entered an era with the advent of CAR-T therapy [177]. Accelerated development of flexible and modular CAR-T systems enables multiple precise programming, antigen targeting, and adaptable solutions in cellular immunotherapy [178,179,180]. Due to their higher affinity for ligands compared to conventional T-cells for MHC peptide antigens, CAR-T cells have been engineered to enhance their killing ability by incorporating costimulatory domains on the receptor [181]. As a result, CAR-T cells are more effective in inducing pyroptosis compared to non-engineered CTLs [182]. Recent research indicates that CAR-T cells could activate the caspase-3/GSDME signaling pathway in B leukemia cells by releasing significant amounts of perforin and GzmB, which induce pyroptosis and enhance anti-tumor immunity [183]. Cytokine release syndrome (CRS), a consequence of extensive pyroptosis, is the most severe complication of CAR-T therapy [184]. Importantly, pyroptosisrelated factors including IL-1β and IL-6 released by macrophages play a significant role in CRS-related inflammatory responses [185, 186]. Furthermore, GSDME elimination, macrophages removal, and caspase-1/GSDMD pathway inhibition could block CRS occurrence in mice. In addition, the severity of CRS is correlated with GSDME and lactate dehydrogenase levels in leukemia patients [183]. Notably, CAR-T cells release significantly more perforin/granzyme B than nontransduced natural T cells in order to cause target cell pyroptosis [187].
In summary, given the immunomodulatory effects of GSDME in TIME, GSDME-mediated pyroptosis exhibits the potential to improve CAR-T therapy efficacy. However, when utilizing CAR-T cells for malignancy treatment, the occurrence of CRS should also be considered. It might be a promising strategy to assess the risk and severity of CRS by detecting GSDME expression levels.
Contribution Of GSDME-mediated pyroptosis to chemotherapy toxicity
Chemotherapy frequently causes severe toxic and side effects in cancer patients, which is one of the major limitations of chemotherapy medications in clinical cancer treatment [188]. Despite being silenced in most cancer cells, GSDME is expressed in many normal tissues, such as the brain, heart, gastrointestinal tract, and hematopoietic cells [49, 189,190,191]. Due to the fact that nearly all chemotherapy drugs lack tumor-specific GSDME targeting properties, toxicity and adverse effects are frequently induced during the treatment process [58, 192]. Recent research indicates that chemotherapy drugs elicit pyroptosis in human primary cells through the caspase-3/GSDME pathway [22]. While GSDME knockout significantly alleviated various tissue damage and weight loss caused by chemotherapy drugs including cisplatin, 5-Fluoruracil (5-Fu) and bleomycin in mice [193]. These findings introduce the novel concept that caspase-3 activation can induce GSDME-mediated pyroptosis and provide novel insights into cancer chemotherapy and toxic side effects [193]. In renal tubular epithelial cells, the depletion of GSDME inhibits pyroptosis induced by cisplatin, whereas 2-bromopalmitate (2-BP) suppresses chemotherapy-induced pyroptosis [81]. According to these findings, GSDME-targeted treatments may effectively overcome the nephrotoxicity associated with chemotherapy [81]. Clinical applications of doxorubicin have been hampered by severe cardiotoxicity during treatment [194]. Increasing evidence implicates that the abundant expression of GSDME in cardiomyocytes and generated mitochondrial ROS overflow may be associated with their mediated cardiotoxicity, implying that GSDME could be a potential target for mitigating the adverse effects of doxorubicin [195]. Hand-foot syndrome is a distinctive and frequent dermatological toxic effect of capecitabine-containing chemotherapy, but its exact mechanism remains unknown. Recent research indicates that it is possibly associated with elevated thymidine phosphorylase-mediated locoregional toxicity and disrupts keratinocytes via GSDME-driven pyroptosis, which induces robust and persistent inflammation [196]. It is noteworthy that macrophages also express GSDME. Chemotherapy-induced and GSDME-dependent pyroptosis of macrophages may not only contribute to the depletion of tissue-resident macrophages and impairment of innate immunity, but it may also engender a systemic inflammatory milieu that weakens the chemotherapeutic effects on cancers [197].
Overall, due to the fact that GSDME is typically methylated in tumor cells and expressed in most normal tissues, GSDME-mediated pyroptosis is a biological process responsible for the toxicity and side effects of certain chemotherapeutic medications (Table 2). Therefore, how to avoid this adverse effect caused by GSDME-mediated pyroptosis and provide tumor GSDME-specific targeted therapy is the focus of future research.
Strategies to improve capacity to target GSDME and avoid side effects
Improper management of pyroptosis induction could result in detrimental effects on adjacent normal tissue to the tumor. Therefore, achieving a balance between tumor treatment and damage to healthy tissues is a critical issue that must be resolved. Tumor-targeting nanomaterials may be a potential strategy, which combines pyroptosis inducers and certain antibodies on the surface of nanoparticles and enhances tumor targeting capacity. Recently, Ding and colleagues reported that Pluronic F127-modified CCCP-incorporated ZIF-8 NPs (F127ZIF-8CCCP NPs) induce tumor cell pyroptosis, thereby reprogramming the immunosuppressive tumor microenvironment and inhibiting tumor growth with high efficiency [198]. This facilitates a more favorable allocation of inducers within the TIME while reducing the adverse effects of pyroptosis activation [198]. Another study confirmed that MCPP, a nano-prodrug loaded with the phototoxic agent purpurin 18 (P18) and the cytotoxic agent PTX, exhibited an exceptional ability to induce GSDME-dependent pyroptosis with fewer damage to healthy tissues [199]. After administering MCPP to tumor cells and followed by laser irradiation, MCPP selectively triggered GSDME-dependent tumor cell pyroptosis and improved immune checkpoint blockade efficiency [199]. Wang et al. designed a self-supplying GSDME cooperative Nano-CRISPR scaffold (Nano-CD) intended for immunotherapy by promoting intracellular pyroptosis [200]. Mechanistically, the adjuvantic properties of the lytic intracellular content and the increased expression of GDSME facilitate cascade-amplification of the anti-tumor immune response [200]. This process ultimately leads to pyroptosis and tumor-associated antigens release [200]. Nowdays, outer membrane vesicles (OMVs) have emerged as a potential platform for the development of cancer immunotherapeutic agents and vaccines. Chen et al. developed molecularly engineered OMVs by equipping DNA aptamers on OMVs (Apt-OMVs) to facilitate secure and effective immunotherapy via targeting pyroptosis [201]. By acting as a natural carrier of LPS, Apt-OMVs stimulate cancer cell pyroptosis via the LPS-triggered pathway with high efficiency [201]. Consequently, selectively trigger GSDME-mediated pyroptotic tumor cell death could enhance tumor immunogenicity and avoid immune attacks and side effects.
Conclusion and perspectives
The study of pyroptosis in tumors has become an extensive and rapidly developing field in recent years. The newly developed tumor pyroptosis therapeutic strategy possesses considerable promise. Most malignancies exhibit positive or negative regulation of GSDMs expression, which is associated with distinct tumor prognosis. Until now, the activation mechanism of GSDMs A–C and their physiological function in cancer remained poorly understood. GSDMD is the most well-studied GSDM; however, the effects of GSDMD-mediated pyroptosis on tumors are intricate. Elevated GSDMD expression predicts poor prognosis for lung and liver cancers, promoting immune escape of tumor cells [202, 203]. Conversely, it was discovered that GSDMD acts a tumor suppressor gene in colorectal and breast cancers [204, 205]. These findings suggest that GSDMD-mediated pyroptosis might exhibit tissue specificity and perform distinct functions in various organs and tissues. Compared to other GSDMs, GSDME is more likely to act as a tumor inhibitor via activating pyroptosis and correlate with anti-cancer immunity. Evidence shows that the absence of GSDME in tumors might trigger chemotherapy drug resistance. It is noteworthy that GSDME is epigenetically inactivated by promoter DNA methylation in most cancer lines and primary cancers, and its applicability as a marker makes it more favorable for further study than other GSDMs. Therefore, evaluating GSDME methylation levels is a beneficial strategy for early detection and distinguish between different tumor types. In fact, an emerging view is that targeting GSDME-mediated pyroptosis in localized tumors has a profound effect on immune cell recruitment in TIME and the immunotherapy response.
In this review, we demonstrate extensive cross-talk between GSDME-mediated pyroptosis and anti-tumor immunity mechanisms based on existing laboratory and clinical evidence. As a potential tumor biomarker for early detection, diagnosis, prognosis, and treatment, GSDME methylation holds tremendous promise. Notably, in terms of anti-cancer treatment, GSDME-mediated pyroptosis is a ‘double-edged sword’ (Fig. 6). On the one hand, tumor suppression of GSDME is accomplished by cancer cell pyroptosis and inflammatory cytokines release, which transform the TIME from a ‘cold’ to a ‘hot’ state and considerably enhance the anti-tumor activity of CD8+ T killer lymphocytes, macrophages, and tumor-infiltrating NK cells. However, GSDME is also abundantly expressed in normal tissue cells and tumor-infiltrating macrophages, which can exacerbate chemotherapy toxicity and side effects. How to strike a balance between the two sides is an extremely critical research topic. Recent studies have identified tumor-targeting nanomaterials, photodynamic therapy, and Apt-OMVs as promising approaches to tackle these challenges by precisely targeting cancer cells. Furthermore, the role of GSDME-mediated pyroptosis in tumor immunotherapy has shown significant potential. Understanding its involvement in anti-PD-1 therapy, ADC therapy, and CAR T-cell therapy has provided innovative strategies for optimizing immunotherapy. In the near future, however, medical organizations are encouraged to conduct clinical trials in which patients are treated with approved medicines that regulate GSDME-mediated pyroptosis in conjunction with tumor immunotherapy.
On one side, tumor suppression of GSDME involves inducing cancer cell pyroptosis and releasing inflammatory cytokines. These molecular events help convert TIME from a predominantly ‘cold’ state to a ‘hot’ state. As a result, the anti-tumor activity of CD8+ T killer lymphocytes, macrophages, and tumor-infiltrating NK cells is significantly enhanced. However, because GSDME is typically methylated in tumor cells and expressed in normal tissue cells and tumor-infiltrating macrophages, GSDME-mediated pyroptosis is a biological process responsible for certain chemotherapeutic medications’ toxicity and side effects.
Clearly, it is evident that GSDME-mediated pyroptosis has yet to disclose all its secrets involved in the pathologic process of cancer. Many questions are still open to be answered. If GSDME-mediated pyroptosis interacts with other signaling pathways to regulate tumor immunotherapy? Does detecting GSDME expression levels could be a promising strategy to assess the risk and severity of CRS caused by CAR T-cell therapy? What is the distinction between the spilled cellular contents of immune cells and cancer cells during GSDME-mediated pyroptosis? How different TIME cell types (e.g., tumor, stromal, and immune cells) interact to suppress or promote tumor progression through immunity or metabolic reprogramming? Would it be feasible if tumor-specific GSDME targeting therapy could prevent chemotherapeutic medications’ toxic effects in humans? In summary, a deeper understanding of the mechanisms underlying GSDME-mediated pyroptosis in TIME will aid in the development of novel and effective anti-cancer therapies. We believe that GSDME-focused research will yield novel insights into tumor diagnosis and treatment.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Sharma P, Goswami S, Raychaudhuri D, Siddiqui BA, Singh P, Nagarajan A, et al. Immune checkpoint therapy–current perspectives and future directions. Cell. 2023;186:1652–69.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. 2021;71:209–49.
Strasser A, Vaux DL. Cell death in the origin and treatment of cancer. Mol Cell. 2020;78:1045–54.
Stine ZE, Schug ZT, Salvino JM, Dang CV. Targeting cancer metabolism in the era of precision oncology. Nat Rev Drug Discov. 2021;21:141–62.
Shan F, Somasundaram A, Bruno TC, Workman CJ, Vignali DAA. Therapeutic targeting of regulatory T cells in cancer. Trends Cancer. 2022;8:944–61.
Park JH, Pyun WY, Park HW. Cancer metabolism: phenotype, signaling and therapeutic targets. Cells. 2020;9:2308.
Dagher OK, Schwab RD, Brookens SK, Posey AD. Advances in cancer immunotherapies. Cell. 2023;186:1814–1814.e1.
Xiao Y, Yu D. Tumor microenvironment as a therapeutic target in cancer. Pharm Ther. 2021;221:107753.
Li Z, Lai X, Fu S, Ren L, Cai H, Zhang H, et al. Immunogenic cell death activates the tumor immune microenvironment to boost the immunotherapy efficiency. Adv Sci. 2022;9:2201734.
Du T, Gao J, Li P, Wang Y, Qi Q, Liu X, et al. Pyroptosis, metabolism, and tumor immune microenvironment. Clin Transl Med. 2021;11:e492.
Zhang S, Zhang Y, Feng Y, Wu J, Hu Y, Lin L, et al. Biomineralized two‐enzyme nanoparticles regulated tumor glycometabolism inducing tumor cell pyroptosis and robust anti‐tumor immunotherapy. Adv Mater. 2022;34:e2206851.
Zhu X, Li S. Ferroptosis, necroptosis, and pyroptosis in gastrointestinal cancers: the chief culprits of tumor progression and drug resistance. Adv Sci. 2023;10:e2300824.
Yu P, Zhang X, Liu N, Tang L, Peng C, Chen X. Pyroptosis: mechanisms and diseases. Sig Transduct Target Ther. 2021;6:128.
Qiu Z, He Y, Ming H, Lei S, Leng Y, Xia Z, et al. Aggravates high glucose- and hypoxia/reoxygenation-induced injury through activating ROS-dependent NLRP3 inflammasome-mediated pyroptosis in H9C2 cardiomyocytes. J Diabetes Res. 2019;2019:8151836.
Chai Q, Yu S, Zhong Y, Lu Z, Qiu C, Yu Y, et al. A bacterial phospholipid phosphatase inhibits host pyroptosis by hijacking ubiquitin. Science. 2022;378:eabq0132.
Rao Z, Zhu Y, Yang P, Chen Z, Xia Y, Qiao C, et al. Pyroptosis in inflammatory diseases and cancer. Theranostics. 2022;12:4310–29.
Sharma BR, Kanneganti T-D. NLRP3 inflammasome in cancer and metabolic diseases. Nat Immunol. 2021;22:550–59.
Kovacs SB, Miao EA. Gasdermins: effectors of pyroptosis. Trends Cell Biol. 2017;27:673–84.
Karmakar M, Minns M, Greenberg EN, Diaz-Aponte J, Pestonjamasp K, Johnson JL, et al. N-GSDMD trafficking to neutrophil organelles facilitates IL-1β release independently of plasma membrane pores and pyroptosis. Nat Commun. 2020;11:2212.
Burdette BE, Esparza AN, Zhu H, Wang S. Gasdermin D in pyroptosis. Acta Pharm Sin B. 2021;11:2768–82.
Zhang Z, Zhang Y, Xia S, Kong Q, Li S, Liu X, et al. Gasdermin E suppresses tumour growth by activating anti-tumour immunity. Nature. 2020;579:415–20.
Wang Y, Gao W, Shi X, Ding J, Liu W, He H, et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. 2017;547:99–03.
Jiang M, Qi L, Li L, Li Y. The caspase-3/GSDME signal pathway as a switch between apoptosis and pyroptosis in cancer. Cell Death Discov. 2020;6:112.
Mu M, Yu Q, Zhang Q, Guo J, Wang X, Sun X, et al. A pan-cancer analysis of molecular characteristics and oncogenic role of gasdermins. Cancer Cell Int. 2022;22:80.
Peng F, Liao M, Qin R, Zhu S, Peng C, Fu L, et al. Regulated cell death (RCD) in cancer: key pathways and targeted therapies. Sig Transduct Target Ther. 2022;7:286.
Tang R, Xu J, Zhang B, Liu J, Liang C, Hua J, et al. Ferroptosis, necroptosis, and pyroptosis in anticancer immunity. J Hematol Oncol. 2020;13:110.
Gao W, Wang X, Zhou Y, Wang X, Yu Y. Autophagy, ferroptosis, pyroptosis, and necroptosis in tumor immunotherapy. Sig Transduct Target Ther. 2022;7:196.
Yan J, Wan P, Choksi S, Liu Z-G. Necroptosis and tumor progression. Trends Cancer. 2022;8:21–27.
Yuan J, Amin P, Ofengeim D. Necroptosis and RIPK1-mediated neuroinflammation in CNS diseases. Nat Rev Neurosci. 2018;20:19–33.
Bertheloot D, Latz E, Franklin BS. Necroptosis, pyroptosis and apoptosis: an intricate game of cell death. Cell Mol Immunol. 2021;18:1106–21.
Rogers C, Erkes DA, Nardone A, Aplin AE, Fernandes-Alnemri T, Alnemri ES. Gasdermin pores permeabilize mitochondria to augment caspase-3 activation during apoptosis and inflammasome activation. Nat Commun. 2019;10:1689.
Chen X, Li J, Kang R, Klionsky DJ, Tang D. Ferroptosis: machinery and regulation. Autophagy. 2021;17:2054–81.
Chen Y, Fang Z-M, Yi X, Wei X, Jiang D-S. The interaction between ferroptosis and inflammatory signaling pathways. Cell Death Dis. 2023;14:205.
Tsvetkov P, Coy S, Petrova B, Dreishpoon M, Verma A, Abdusamad M, et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science 2022;375:1254–61.
Chen L, Min J, Wang F. Copper homeostasis and cuproptosis in health and disease. Sig Transduct Target Ther. 2022;7:378.
Zychlinsky A, Prevost MC, Sansonetti PJ. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358:167–9.
Ford HF, Spies A. Postpartum proptosis with ophthalmopathy. Optom Vis Sci. 2001;78:75–78.
Kesavardhana S, Malireddi R, Kanneganti T. Caspases in cell death, inflammation, and pyroptosis. Annu Rev Immunol. 2020;38:567–95.
Shi J, Gao W, Shao F. Pyroptosis: gasdermin-mediated programmed necrotic cell death. Trends Biochem Sci. 2017;42:245–54.
Li L, Wang S, Zhou W. Balance cell apoptosis and pyroptosis of caspase-3-activating chemotherapy for better antitumor therapy. Cancers. 2022;15:26.
Zheng M, Kanneganti T-D. The regulation of the ZBP1-NLRP3 inflammasome and its implications in pyroptosis, apoptosis, and necroptosis (PANoptosis). Immunol Rev. 2020;297:26–38.
Liu Y, Lei H, Zhang W, Xing Q, Liu R, Wu S, et al. Pyroptosis in renal inflammation and fibrosis: current knowledge and clinical significance. Cell Death Dis. 2023;14:472.
Frank D, Vince JE. Pyroptosis versus necroptosis: similarities, differences, and crosstalk. Cell Death Differ. 2018;26:99–114.
Lee S, Karki R, Wang Y, Nguyen LN, Kalathur RC, Kanneganti T-D. AIM2 forms a complex with pyrin and ZBP1 to drive PANoptosis and host defence. Nature. 2021;597:415–9.
Shen X, Wang H, Weng C, Jiang H, Chen J. Caspase 3/GSDME-dependent pyroptosis contributes to chemotherapy drug-induced nephrotoxicity. Cell Death Dis. 2021;12:186.
Wang Y, Peng J, Xie X, Zhang Z, Li M, Yang M. Gasdermin E-mediated programmed cell death: an unpaved path to tumor suppression. J Cancer. 2021;12:5241–48.
Zhang Z, Zhang H, Li D, Zhou X, Qin Q, Zhang Q. Caspase‐3‐mediated GSDME induced Pyroptosis in breast cancer cells through the ROS/JNK signalling pathway. J Cell Mol Med. 2021;25:8159–68.
Yu J, Li S, Qi J, Chen Z, Wu Y, Guo J, et al. Cleavage of GSDME by caspase-3 determines lobaplatin-induced pyroptosis in colon cancer cells. Cell Death Dis. 2019;10:193.
De Schutter E, Croes L, Ibrahim J, Pauwels P, Op de Beeck K, Vandenabeele P, et al. GSDME and its role in cancer: from behind the scenes to the front of the stage. Int J Cancer. 2021;148:2872–83.
Bhat AA, Thapa R, Afzal O, Agrawal N, Almalki WH, Kazmi I, et al. The pyroptotic role of Caspase-3/GSDME signalling pathway among various cancer: a review. Int J Biol Macromol. 2023;242:124832.
Laer LV, Huizing EH, Verstreken M, Zuijlen D, van, Wauters JG, Bossuyt PJ, et al. Nonsyndromic hearing impairment is associated with a mutation in DFNA5. Nat Genet. 1998;20:194–7.
Bischoff AMLC, Luijendijk MWJ, Huygen PLM, van Duijnhoven G, De Leenheer EMR, Oudesluijs GG, et al. A novel mutation identified in the DFNA5 gene in a Dutch family: a clinical and genetic evaluation. Audio Neurotol. 2003;9:34–46.
Yu C, Meng X, Zhang S, Zhao G, Hu L, Kong X. A 3-nucleotide deletion in the polypyrimidine tract of intron 7 of the DFNA5 gene causes nonsyndromic hearing impairment in a Chinese family. Genomics. 2003;82:575–9.
Chen Y, Lian N, Chen S, Xiao T, Ke Y, Zhang Y, et al. GSDME deficiency leads to the aggravation of UVB-induced skin inflammation through enhancing recruitment and activation of neutrophils. Cell Death Dis. 2022;13:841.
Wei Y, Lan B, Zheng T, Yang L, Zhang X, Cheng L, et al. GSDME-mediated pyroptosis promotes the progression and associated inflammation of atherosclerosis. Nat Commun. 2023;14:929.
Xia W, Li Y, Wu M, Jin Q, Wang Q, Li S, et al. Gasdermin E deficiency attenuates acute kidney injury by inhibiting pyroptosis and inflammation. Cell Death Dis. 2021;12:139.
Masuda Y, Futamura M, Kamino H, Nakamura Y, Kitamura N, Ohnishi S, et al. The potential role of DFNA5, a hearing impairment gene, in p53-mediated cellular response to DNA damage. J Hum Genet. 2006;51:652–64.
Wang S, Zhang MJ, Wu ZZ, Zhu SW, Wan SC, Zhang BX, et al. GSDME is related to prognosis and response to chemotherapy in oral cancer. J Dent Res. 2022;101:848–58.
Dai S, Ye B, Zhong L, Chen Y, Hong G, Zhao G, et al. GSDMD mediates LPS-induced septic myocardial dysfunction by regulating ROS-dependent NLRP3 inflammasome activation. Front Cell Dev Biol. 2021;9:779432.
Zhou J, Qiu J, Song Y, Liang T, Liu S, Ren C, et al. Pyroptosis and degenerative diseases of the elderly. Cell Death Dis. 2023;14:94.
Wang K, Sun Q, Zhong X, Zeng M, Zeng H, Shi X, et al. Structural mechanism for GSDMD targeting by autoprocessed caspases in pyroptosis. Cell. 2020;180:941–.e20.
Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–75.
Li S, Sun Y, Song M, Song Y, Fang Y, Zhang Q, et al. NLRP3/caspase-1/GSDMD–mediated pyroptosis exerts a crucial role in astrocyte pathological injury in mouse model of depression. JCI Insight. 2021;6:e146852.
Hu X, Chen H, Xu H, Wu Y, Wu C, Jia C, et al. Role of pyroptosis in traumatic brain and spinal cord injuries. Int J Biol Sci. 2020;16:2042–50.
Tsuchiya K. Inflammasome‐associated cell death: pyroptosis, apoptosis, and physiological implications. Microbiol Immunol. 2020;64:252–69.
Xiao F, Gao W, Wang X, Chen T. Amplification activation loop between caspase-8 and -9 dominates artemisinin-induced apoptosis of ASTC-a-1 cells. Apoptosis. 2012;17:600–11.
Kang L, Dai J, Wang Y, Shi P, Zou Y, Pei J, et al. Blocking Caspase-1/Gsdmd and Caspase-3/-8/Gsdme pyroptotic pathways rescues silicosis in mice. PLoS Genet. 2022;18:e1010515.
Wei Y, Yang L, Pandeya A, Cui J, Zhang Y, Li Z. Pyroptosis-induced inflammation and tissue damage. J Mol Biol. 2022;434:167301.
Ai Y, Wang W, Liu F, Fang W, Chen H, Wu L, et al. Mannose antagonizes GSDME-mediated pyroptosis through AMPK activated by metabolite GlcNAc-6P. Cell Res. 2023. https://doi.org/10.1038/s41422-023-00848-6
Kong Y, Feng Z, Chen A, Qi Q, Han M, Wang S, et al. The natural flavonoid galangin elicits apoptosis, pyroptosis, and autophagy in glioblastoma. Front Oncol. 2019;9:942.
Qiu N, Zhang Z, Wei X, Xu C, Jia X, Wang K, et al. Peritoneal gene transfection of tumor necrosis factor-related apoptosis-inducing ligand for tumor surveillance and prophylaxis. Nano Lett Nano Lett. 2023. https://doi.org/10.1021/acs.nanolett.3c01568
Tan G, Huang C, Chen J, Chen B, Zhi F. Gasdermin-E-mediated pyroptosis participates in the pathogenesis of Crohn’s disease by promoting intestinal inflammation. Cell Rep. 2021;35:109265.
de Beeck KO, Van Camp G, Thys S, Cools N, Callebaut I, Vrijens K, et al. The DFNA5 gene, responsible for hearing loss and involved in cancer, encodes a novel apoptosis-inducing protein. Eur J Hum Genet. 2011;19:965–73.
Zhang X, Zhang P, An L, Sun N, Peng L, Tang W, et al. Miltirone induces cell death in hepatocellular carcinoma cell through GSDME-dependent pyroptosis. Acta Pharm Sin B. 2020;10:1397–413.
Li Y-Q, Peng J-J, Peng J, Luo X-J. The deafness gene GSDME: its involvement in cell apoptosis, secondary necrosis, and cancers. Naunyn Schmiedeberg’s Arch Pharmacol. 2019;392:1043–48.
Zhou M, Liu X, Li Z, Huang Q, Li F, Li C-Y. Caspase-3 regulates the migration, invasion and metastasis of colon cancer cells. Int J Cancer. 2018;143:921–30.
Wang Y, Yin B, Li D, Wang G, Han X, Sun X. GSDME mediates caspase-3-dependent pyroptosis in gastric cancer. Biochem Biophys Res Commun. 2018;495:1418–25.
Rogers C, Fernandes-Alnemri T, Mayes L, Alnemri D, Cingolani G, Alnemri ES. Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nat Commun. 2017;8:14128.
Yao F, Jin Z, Zheng Z, Lv X, Ren L, Yang J, et al. HDAC11 promotes both NLRP3/caspase-1/GSDMD and caspase-3/GSDME pathways causing pyroptosis via ERG in vascular endothelial cells. Cell Death Discov. 2022;8:112.
Rogers C, Alnemri ES. Gasdermins: novel mitochondrial pore-forming proteins. Mol Cell Oncol. 2019;6:e1621501.
Hu L, Chen M, Chen X, Zhao C, Fang Z, Wang H, et al. Chemotherapy-induced pyroptosis is mediated by BAK/BAX-caspase-3-GSDME pathway and inhibited by 2-bromopalmitate. Cell Death Dis. 2020;11:281.
Fan C, Ye F, Peng M, Dong J, Chai W, Deng W, et al. Endogenous HMGB1 regulates GSDME-mediated pyroptosis via ROS/ERK1/2/caspase-3/GSDME signaling in neuroblastoma. Am J Cancer Res. 2023;13:436–51.
Tixeira R, Shi B, Parkes MAF, Hodge AL, Caruso S, Hulett MD, et al. Gasdermin E does not limit apoptotic cell disassembly by promoting early onset of secondary necrosis in Jurkat T cells and THP-1 monocytes. Front Immunol. 2018;9:2842.
Ibrahim J, De Schutter E, Op de Beeck K. GSDME: a potential ally in cancer detection and treatment. Trends Cancer. 2021;7:392–94.
Ma L, Bian M, Gao H, Zhou Z, Yi W. A novel 3-acyl isoquinolin-1(2H)-one induces G2 phase arrest, apoptosis and GSDME-dependent pyroptosis in breast cancer. PLoS One. 2022;17:e0268060.
Xia X, Wang X, Cheng Z, Qin W, Lei L, Jiang J, et al. The role of pyroptosis in cancer: pro-cancer or pro-“host”? Cell Death Dis. 2019;10:650.
Kim MS, Lebron C, Nagpal JK, Chae YK, Chang X, Huang Y, et al. Methylation of the DFNA5 increases risk of lymph node metastasis in human breast cancer. Biochem Biophys Res Commun. 2008;370:38–43.
Croes L, Beyens M, Fransen E, Ibrahim J, Vanden Berghe W, Suls A, et al. Large-scale analysis of DFNA5 methylation reveals its potential as biomarker for breast cancer. Clin Epigenet. 2018;10:51.
Tang J, Bei M, Zhu J, Xu G, Chen D, Jin X, et al. Acute cadmium exposure induces GSDME-mediated pyroptosis in triple-negative breast cancer cells through ROS generation and NLRP3 inflammasome pathway activation. Environ Toxicol Pharmacol. 2021;87:103686.
Akino K, Toyota M, Suzuki H, Imai T, Maruyama R, Kusano M, et al. Identification of DFNA5 as a target of epigenetic inactivation in gastric cancer. Cancer Sci. 2007;98:88–95.
Xia Y, Jin Y, Cui D, Wu X, Song C, Jin W, et al. Antitumor effect of simvastatin in combination with DNA methyltransferase inhibitor on gastric cancer via GSDME-mediated pyroptosis. Front Pharmacol. 2022;13:860546.
Ibrahim J, Op de Beeck K, Fransen E, Croes L, Beyens M, Suls A, et al. Methylation analysis of Gasdermin E shows great promise as a biomarker for colorectal cancer. Cancer Med. 2019;8:2133–45.
Khan M, Ai M, Du K, Song J, Wang B, Lin J, et al. Pyroptosis relates to tumor microenvironment remodeling and prognosis: a pan-cancer perspective. Front Immunol. 2022;13:1062225.
Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. 2019;51:27–41.
Zhai Z, Yang F, Xu W, Han J, Luo G, Li Y, et al. Attenuation of rheumatoid arthritis through the inhibition of tumor necrosis factor–induced caspase 3/Gasdermin E–mediated pyroptosis. Arthritis Rheumatol. 2022;74:427–40.
Chattopadhyay C, Kim DW, Gombos DS, Oba J, Qin Y, Williams MD, et al. Uveal melanoma: from diagnosis to treatment and the science in between. Cancer. 2016;122:2299–12.
Zhou B, Zhang J, Liu X, Chen H, Ai Y, Cheng K, et al. Tom20 senses iron-activated ROS signaling to promote melanoma cell pyroptosis. Cell Res. 2018;28:1171–85.
Lage H, Helmbach H, Grottke C, Dietel M, Schadendorf D. DFNA5 (ICERE-1) contributes to acquired etoposide resistance in melanoma cells. FEBS Lett. 2001;494:54–9.
Wang D, Fu Z, Gao L, Zeng J, Xiang Y, Zhou L, et al. Increased IRF9–STAT2 signaling leads to adaptive resistance toward targeted therapy in melanoma by restraining GSDME-dependent pyroptosis. J Investig Dermatol. 2022;142:2476–.e9.
Vernon M, Wilski NA, Kotas D, Cai W, Pomante D, Tiago M, et al. Raptinal induces gasdermin E–dependent pyroptosis in naïve and therapy-resistant melanoma. Mol Cancer Res. 2022;20:1811–21.
Yu P, Wang H, Tian M, Li A, Chen X, Wang X, et al. Eukaryotic elongation factor-2 kinase regulates the cross-talk between autophagy and pyroptosis in doxorubicin-treated human melanoma cells in vitro. Acta Pharm Sin. 2019;40:1237–44.
Dai B, Zhang R, Qi S, Liu L, Zhang X, Deng D, et al. Intravital molecular imaging reveals that ROS-caspase-3-GSDME-induced cell punching enhances humoral immunotherapy targeting intracellular tumor antigens. Theranostics. 2022;12:7603–23.
Miller KD, Nogueira L, Devasia T, Mariotto AB, Yabroff KR, Jemal A, et al. Cancer treatment and survivorship statistics, 2022. CA A Cancer J Clinicians. 2022;72:409–36.
Huang Y, Zhang G-H, Zhu Q, Wu X, Wu L-G. Role of cytokines released during pyroptosis in non-small cell lung cancer. Cancer Manag Res. 2021;13:7399–09.
Zhang J, Chen Y, He Q. Distinct characteristics of dasatinib-induced pyroptosis in gasdermin E-expressing human lung cancer A549 cells and neuroblastoma SH-SY5Y cells. Oncol Lett. 2020;20:145–54.
Han J, Cheng C, Zhang J, Fang J, Yao W, Zhu Y, et al. Myricetin activates the Caspase-3/GSDME pathway via ER stress induction of pyroptosis in lung cancer cells. Front Pharmacol. 2022;13:959938.
Xu L, Shi F, Wu Y, Yao S, Wang Y, Jiang X, et al. Gasdermin E regulates the stability and activation of EGFR in human non-small cell lung cancer cells. Cell Commun Signal. 2023;21:83.
Hu Z, Lai Y, Ma C, Zuo L, Xiao G, Gao H, et al. Cordyceps militaris extract induces apoptosis and pyroptosis via caspase‐3/PARP/GSDME pathways in A549 cell line. Food Sci Nutr. 2021;10:21–38.
Yu F, Tan W, Chen Z, Shen X, Mo X, Mo X, et al. Nitidine chloride induces caspase 3/GSDME-dependent pyroptosis by inhibting PI3K/Akt pathway in lung cancer. Chin Med. 2022;17:115.
Huang DQ, Singal AG, Kono Y, Tan DJH, El-Serag HB, Loomba R. Changing global epidemiology of liver cancer from 2010 to 2019: NASH is the fastest growing cause of liver cancer. Cell Metab. 2022;34:969–.e2.
Guo J, Shen Y, Hu S, Rui T, Liu J, Yuan Y. Neobavaisoflavone inhibits antitumor immunosuppression via myeloid-derived suppressor cells. Int Immunopharmacol. 2022;111:109103.
Li Y, Zhao R, Xiu Z, Yang X, Zhu Y, Han J, et al. Neobavaisoflavone induces pyroptosis of liver cancer cells via Tom20 sensing the activated ROS signal. Phytomedicine. 2023;116:154869.
Shi H, Yan Y, Yang H, Pu P, Tang H, Schisandrin B. Diet inhibits oxidative stress to reduce ferroptosis and lipid peroxidation to prevent pirarubicin-induced hepatotoxicity. Biomed Res Int. 2022;2022:1–11.
Song A, Ding T, Wei N, Yang J, Ma M, Zheng S, et al. Schisandrin B induces HepG2 cells pyroptosis by activating NK cells mediated anti-tumor immunity. Toxicol Appl Pharmacol. 2023;472:116574.
Ajani JA, D’Amico TA, Bentrem DJ, Chao J, Cooke D, Corvera C, et al. Gastric cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022;20:167–92.
Deng B, Jiao B, Liu Y, Li Y, Wang G. BIX‐01294 enhanced chemotherapy effect in gastric cancer by inducing GSDME‐mediated pyroptosis. Cell Biol Int. 2020;44:1890–99.
Fu L, Yonemura A, Yasuda-Yoshihara N, Umemoto T, Zhang J, Yasuda T, et al. Intracellular MUC20 variant 2 maintains mitochondrial calcium homeostasis and enhances drug resistance in gastric cancer. Gastric Cancer. 2022;25:542–57.
Baidoun F, Elshiwy K, Elkeraie Y, Merjaneh Z, Khoudari G, Sarmini MT, et al. Colorectal cancer epidemiology: recent trends and impact on outcomes. Curr Drug Targets. 2021;22:998–09.
Tan G, Lin C, Huang C, Chen B, Chen J, Shi Y, et al. Radiosensitivity of colorectal cancer and radiation-induced gut damages are regulated by gasdermin E. Cancer Lett. 2022;529:1–10.
Zhang Y, Xu T, Tian H, Wu J, Yu X, Zeng L, et al. Coxsackievirus Group B3 has oncolytic activity against colon cancer through gasdermin E-mediated pyroptosis. Cancers. 2022;14:6206.
Song W, Ren J, Xiang R, Kong C, Fu T. Identification of pyroptosis-related subtypes, the development of a prognosis model, and characterization of tumor microenvironment infiltration in colorectal cancer. OncoImmunology. 2021;10:1987636.
Guo J, Zheng J, Mu M, Chen Z, Xu Z, Zhao C, et al. GW4064 enhances the chemosensitivity of colorectal cancer to oxaliplatin by inducing pyroptosis. Biochem Biophys Res Commun. 2021;548:60–6.
Morgan E, Soerjomataram I, Rumgay H, Coleman HG, Thrift AP, Vignat J, et al. The global landscape of esophageal squamous cell carcinoma and esophageal adenocarcinoma incidence and mortality in 2020 and projections to 2040: new estimates from GLOBOCAN 2020. Gastroenterology. 2022;163:649–.e2.
Ko K-P, Huang Y, Zhang S, Zou G, Kim B, Zhang J, et al. Key genetic determinants driving esophageal squamous cell carcinoma initiation and immune evasion. Gastroenterology. 2023. https://doi.org/10.1053/j.gastro.2023.05.030.
Li L, Song D, Qi L, Jiang M, Wu Y, Gan J, et al. Photodynamic therapy induces human esophageal carcinoma cell pyroptosis by targeting the PKM2/caspase-8/caspase-3/GSDME axis. Cancer Lett. 2021;520:143–59.
Zheng Z-Y, Yang P-L, Li R-Y, Liu L-X, Xu X-E, Liao L-D, et al. STAT3β disrupted mitochondrial electron transport chain enhances chemosensitivity by inducing pyroptosis in esophageal squamous cell carcinoma. Cancer Lett. 2021;522:171–83.
Tan J-Q, Li Z, Chen G, Wu M, Feng J-L, Kong S-Y, et al. The natural compound from Garcinia bracteata mainly induces GSDME-mediated pyroptosis in esophageal cancer cells. Phytomedicine. 2022;102:154142.
Kaufman HL, Kohlhapp FJ, Zloza A. Oncolytic viruses: a new class of immunotherapy drugs. Nat Rev Drug Discov. 2015;14:642–62.
Wu A, Li Z, Wang Y, Chen Y, Peng J, Zhu M, et al. Recombinant measles virus vaccine rMV-Hu191 exerts an oncolytic effect on esophageal squamous cell carcinoma via caspase-3/GSDME-mediated pyroptosis. Cell Death Discov. 2023;9:171.
Emond R, Griffiths JI, Grolmusz VK, Nath A, Chen J, Medina EF, et al. Cell facilitation promotes growth and survival under drug pressure in breast cancer. Nat Commun. 2023;14:3851.
Xu T, Wang Z, Liu J, Wang G, Zhou D, Du Y, et al. Cyclin-dependent kinase inhibitors function as potential immune regulators via inducing pyroptosis in triple negative breast cancer. Front Oncol. 2022;12:820696.
Zhong C, Li Y, Li W, lian S, Li Y, Wu C, et al. Ganoderma lucidum extract promotes tumor cell pyroptosis and inhibits metastasis in breast cancer. Food Chem Toxicol. 2023;174:113654.
An H, Heo JS, Kim P, Lian Z, Lee S, Park J, et al. Tetraarsenic hexoxide enhances generation of mitochondrial ROS to promote pyroptosis by inducing the activation of caspase-3/GSDME in triple-negative breast cancer cells. Cell Death Dis. 2021;12:159.
Xia J, Chu C, Li W, Chen H, Xie W, Cheng R, et al. Mitochondrial protein UCP1 inhibits the malignant behaviors of triple-negative breast cancer through activation of mitophagy and pyroptosis. Int J Biol Sci. 2022;18:2949–61.
Li L, Tian H, Zhang Z, Ding N, He K, Lu S, et al. Carrier-free nanoplatform via evoking pyroptosis and immune response against breast cancer. ACS Appl Mater Interfaces. 2023;15:452–68.
Vázquez-García I, Uhlitz F, Ceglia N, Lim JLP, Wu M, Mohibullah N, et al. Ovarian cancer mutational processes drive site-specific immune evasion. Nature. 2022;612:778–86.
Huo J, Shen Y, Zhang Y, Shen LBI. 2536 induces gasdermin E-dependent pyroptosis in ovarian cancer. Front Oncol. 2022;12:963928.
Yang C, Wang Z-Q, Zhang Z-C, Lou G, Jin W-L. CBL0137 activates ROS/BAX signaling to promote caspase-3/GSDME-dependent pyroptosis in ovarian cancer cells. Biomed Pharmacother. 2023;161:114529.
Pfisterer J, Shannon CM, Baumann K, Rau J, Harter P, Joly F, et al. Bevacizumab and platinum-based combinations for recurrent ovarian cancer: a randomised, open-label, phase 3 trial. Lancet Oncol. 2020;21:699–709.
Yang X, Li C, Liao X, Liu S, Li X, Hou X, et al. Paclitaxel induces pyroptosis by inhibiting the volume-sensitive chloride channel leucine-rich repeat-containing 8a in ovarian cancer cells. Oncol Rep. 2023;49:115.
Shen D, Yu X, Wu Y, Chen Y, Li G, Cheng F, et al. Emerging roles of bexarotene in the prevention, treatment and anti-drug resistance of cancers. Expert Rev Anticancer Ther. 2018;18:487–99.
Kobayashi T, Mitsuhashi A, Hongying P, Shioya M, Kojima K, Nishikimi K, et al. Bexarotene-induced cell death in ovarian cancer cells through Caspase-4-gasdermin E mediated pyroptosis. Sci Rep. 2022;12:11123.
Anestis D, Ble C, Tsitouras V, Tsonidis C, Tsitsopoulos P. Congenital glioblastoma multiforme: an unusual and challenging tumor. Neuropediatrics. 2017;48:403–12.
Zhao W, Zhang L, Zhang Y, Jiang Z, Lu H, Xie Y, et al. The CDK inhibitor AT7519 inhibits human glioblastoma cell growth by inducing apoptosis, pyroptosis and cell cycle arrest. Cell Death Dis. 2023;14:11.
Chen Y-P, Chan ATC, Le Q-T, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394:64–80.
Di M, Miao J, Pan Q, Wu Z, Chen B, Wang M, et al. OTUD4-mediated GSDME deubiquitination enhances radiosensitivity in nasopharyngeal carcinoma by inducing pyroptosis. J Exp Clin Cancer Res. 2022;41:328.
Cai J, Yi M, Tan Y, Li X, Li G, Zeng Z, et al. Natural product triptolide induces GSDME-mediated pyroptosis in head and neck cancer through suppressing mitochondrial hexokinase-ΙΙ. J Exp Clin Cancer Res. 2021;40:190.
Chen C, Xie L, Ren T, Huang Y, Xu J, Guo W. Immunotherapy for osteosarcoma: Fundamental mechanism, rationale, and recent breakthroughs. Cancer Lett. 2021;500:1–10.
Wang P, Wang C, Liu C. Antitumor effects of dioscin in A431 cells via adjusting ATM/p53-mediated cell apoptosis, DNA damage and migration. Oncol Lett. 2021;21:59.
Ding Q, Zhang W, Cheng C, Mo F, Chen L, Peng G, et al. Dioscin inhibits the growth of human osteosarcoma by inducing G2/M‐phase arrest, apoptosis, and GSDME‐dependent cell death in vitro and in vivo. J Cell Physiol. 2019;235:2911–24.
Wilson RL, Taaffe DR, Newton RU, Hart NH, Lyons-Wall P, Galvão DA. Obesity and prostate cancer: a narrative review. Crit Rev Oncol Hematol. 2022;169:103543.
Zhang Y, Yang J, Wen Z, Chen X, Yu J, Yuan D, et al. A novel 3’,5’-diprenylated chalcone induces concurrent apoptosis and GSDME-dependent pyroptosis through activating PKCδ/JNK signal in prostate cancer. Aging. 2020;12:9103–24.
Pansy K, Uhl B, Krstic J, Szmyra M, Fechter K, Santiso A, et al. Immune regulatory processes of the tumor microenvironment under malignant conditions. IJMS. 2021;22:13311.
Fu T, Dai L-J, Wu S-Y, Xiao Y, Ma D, Jiang Y-Z, et al. Spatial architecture of the immune microenvironment orchestrates tumor immunity and therapeutic response. J Hematol Oncol. 2021;14:98.
Gajewski TF, Schreiber H, Fu Y-X. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14:1014–22.
Ma G, Zhang Z, Li P, Zhang Z, Zeng M, Liang Z, et al. Reprogramming of glutamine metabolism and its impact on immune response in the tumor microenvironment. Cell Commun Signal. 2022;20:114.
Baharom F, Ramirez-Valdez RA, Khalilnezhad A, Khalilnezhad S, Dillon M, Hermans D, et al. Systemic vaccination induces CD8+ T cells and remodels the tumor microenvironment. Cell. 2022;185:4317–.e15.
Park J, Hsueh P-C, Li Z, Ho P-C. Microenvironment-driven metabolic adaptations guiding CD8+ T cell anti-tumor immunity. Immunity. 2023;56:32–42.
Abril-Rodriguez G, Ribas A. SnapShot: immune checkpoint inhibitors. Cancer Cell. 2017;31:848–848.e1.
Lin J, Liu F, Gao F, Chen Y, Wang R, Wang X, et al. Vesicular stomatitis virus sensitizes immunologically cold tumors to checkpoint blockade by inducing pyroptosis. Biochim Biophys Acta. 2022;1868:166538.
Zhang Z, Zhang Y, Lieberman J. Lighting a fire: can we harness pyroptosis to ignite antitumor immunity? Cancer Immunol Res. 2021;9:2–7.
Wittwer NL, Staudacher AH, Liapis V, Cardarelli P, Warren H, Brown MP. An anti-mesothelin targeting antibody drug conjugate induces pyroptosis and ignites antitumor immunity in mouse models of cancer. J Immunother Cancer. 2023;11:e006274.
Shiravand Y, Khodadadi F, Kashani SMA, Hosseini-Fard SR, Hosseini S, Sadeghirad H, et al. Immune checkpoint inhibitors in cancer therapy. Curr Oncol. 2022;29:3044–60.
Martins F, Sofiya L, Sykiotis GP, Lamine F, Maillard M, Fraga M, et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019;16:563–80.
Tang Q, Chen Y, Li X, Long S, Shi Y, Yu Y, et al. The role of PD-1/PD-L1 and application of immune-checkpoint inhibitors in human cancers. Front Immunol. 2022;13:964442.
Passaro A, Brahmer J, Antonia S, Mok T, Peters S. Managing resistance to immune checkpoint inhibitors in lung cancer: treatment and novel strategies. J Clin Oncol. 2022;40:598–10.
Lentz RW, Colton MD, Mitra SS, Messersmith WA. Innate immune checkpoint inhibitors: the next breakthrough in medical oncology? Mol Cancer Ther. 2021;20:961–74.
Oura K, Morishita A, Tani J, Masaki T. Tumor immune microenvironment and immunosuppressive therapy in hepatocellular carcinoma: a review. IJMS. 2021;22:5801.
Hu J, Pei W, Jiang M, Huang Y, Dong F, Jiang Z, et al. DFNA5 regulates immune cells infiltration and exhaustion. Cancer Cell Int. 2022;22:107.
Abd-Aziz N, Poh CL. Development of oncolytic viruses for cancer therapy. Transl Res. 2021;237:98–123.
Lin J, Sun S, Zhao K, Gao F, Wang R, Li Q, et al. Oncolytic Parapoxvirus induces Gasdermin E-mediated pyroptosis and activates antitumor immunity. Nat Commun. 2023;14:224.
Liang M, Zhang M, Qiu W, Xiao Y, Ye M, Xue P, et al. Stepwise size shrinkage cascade‐activated supramolecular prodrug boosts antitumor immunity by eliciting pyroptosis. Adv Sci. 2022;9:2203353.
Yang Y, Wang S, Ma P, Jiang Y, Cheng K, Yu Y, et al. Drug conjugate-based anticancer therapy - current status and perspectives. Cancer Lett. 2023;552:215969.
Drago JZ, Modi S, Chandarlapaty S. Unlocking the potential of antibody–drug conjugates for cancer therapy. Nat Rev Clin Oncol. 2021;18:327–44.
Polakis P. Antibody drug conjugates for cancer therapy. Pharm Rev. 2015;68:3–19.
Tarantino P, Carmagnani Pestana R, Corti C, Modi S, Bardia A, Tolaney SM, et al. Antibody–drug conjugates: smart chemotherapy delivery across tumor histologies. CA A Cancer J Clinicians. 2021;72:165–82.
Wang Z, Wu Z, Liu Y, Han W. New development in CAR-T cell therapy. J Hematol Oncol. 2017;10:53.
Lu J, Jiang G. The journey of CAR-T therapy in hematological malignancies. Mol Cancer. 2022;21:194.
Wagner J, Wickman E, DeRenzo C, Gottschalk S. CAR T cell therapy for solid tumors: bright future or dark reality? Mol Ther. 2020;28:2320–39.
Huang R, Li X, He Y, Zhu W, Gao L, Liu Y, et al. Recent advances in CAR-T cell engineering. J Hematol Oncol. 2020;13:86.
Bao C, Gao Q, Li L-L, Han L, Zhang B, Ding Y, et al. The application of nanobody in CAR-T therapy. Biomolecules. 2021;11:238.
Wei J, Guo Y, Wang Y, Wu Z, Bo J, Zhang B, et al. Clinical development of CAR T cell therapy in China: 2020 update. Cell Mol Immunol. 2021;18:792–04.
Liu Y, Fang Y, Chen X, Wang Z, Liang X, Zhang T, et al. Gasdermin E–mediated target cell pyroptosis by CAR T cells triggers cytokine release syndrome. Sci Immunol. 2020;5:eaax7969.
Schubert ML, Schmitt M, Wang L, Ramos CA, Jordan K, Müller-Tidow C, et al. Side-effect management of chimeric antigen receptor (CAR) T-cell therapy. Ann Oncol. 2021;32:34–48.
Freyer CW, Porter DL. Cytokine release syndrome and neurotoxicity following CAR T-cell therapy for hematologic malignancies. J Allergy Clin Immunol. 2020;146:940–48.
Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25:625–38.
Chen P-H, Lipschitz M, Weirather JL, Jacobson C, Armand P, Wright K, et al. Activation of CAR and non-CAR T cells within the tumor microenvironment following CAR T cell therapy. JCI Insight. 2020;5:e134612.
Zhang H, Sha J, Feng X, Hu X, Chen Y, Li B, et al. Dexmedetomidine ameliorates LPS induced acute lung injury via GSK-3β/STAT3-NF-κB signaling pathway in rats. Int Immunopharmacol. 2019;74:105717.
Wang Q, Wu J, Zeng Y, Chen K, Wang C, Yang S, et al. Pyroptosis: a pro-inflammatory type of cell death in cardiovascular disease. Clin Chim Acta. 2020;510:62–72.
Liu Y, Wen D, Gao J, Xie B, Yu H, Shen Q, et al. Methamphetamine induces GSDME-dependent cell death in hippocampal neuronal cells through the endoplasmic reticulum stress pathway. Brain Res Bull. 2020;162:73–83.
Wang J, Ye T, Wang S, Wang J, Jin Y. Molecular mechanisms and therapeutic relevance of gasdermin E in human diseases. Cell Signal. 2022;90:110189.
Rioja-Blanco E, Arroyo-Solera I, Álamo P, Casanova I, Gallardo A, Unzueta U, et al. CXCR4-targeted nanotoxins induce GSDME-dependent pyroptosis in head and neck squamous cell carcinoma. J Exp Clin Cancer Res. 2022;41:49.
Li S, Yue M, Xu H, Zhang X, Mao T, Quan M, et al. Chemotherapeutic drugs-induced pyroptosis mediated by gasdermin E promotes the progression and chemoresistance of pancreatic cancer. Cancer Lett. 2023;564:216206.
Rawat PS, Jaiswal A, Khurana A, Bhatti JS, Navik U. Doxorubicin-induced cardiotoxicity: an update on the molecular mechanism and novel therapeutic strategies for effective management. Biomed Pharmacother. 2021;139:111708.
Liu Y, Zeng L, Yang Y, Chen C, Wang D, Wang H. Acyl-CoA thioesterase 1 prevents cardiomyocytes from Doxorubicin-induced ferroptosis via shaping the lipid composition. Cell Death Dis. 2020;11:756.
Yang B, Xie X, Lv D, Hu J, Chen Y, Wu Z, et al. Capecitabine induces hand-foot syndrome through elevated thymidine phosphorylase-mediated locoregional toxicity and GSDME-driven pyroptosis that can be relieved by tipiracil. Br J Cancer. 2022;128:219–31.
Mai F, He P, Ye J, Xu L, Ouyang D, Li C, et al. Caspase‐3‐mediated GSDME activation contributes to cisplatin‐ and doxorubicin‐induced secondary necrosis in mouse macrophages. Cell Prolif. 2019;52:e12663.
Ding B, Chen H, Tan J, Meng Q, Zheng P, Ma P, et al. ZIF‐8 nanoparticles evoke pyroptosis for high‐efficiency cancer immunotherapy. Angew Chem Int Ed 2023;62:e202215307.
Xiao Y, Zhang T, Ma X, Yang Q, Yang L, Yang S, et al. Microenvironment‐responsive prodrug‐induced pyroptosis boosts cancer immunotherapy. Adv Sci. 2021;8:e2101840.
Wang N, Liu C, Li Y, Huang D, Wu X, Kou X, et al. A cooperative nano-CRISPR scaffold potentiates immunotherapy via activation of tumour-intrinsic pyroptosis. Nat Commun. 2023;14:779.
Chen L, Ma X, Liu W, Hu Q, Yang H. Targeting pyroptosis through lipopolysaccharide-triggered noncanonical pathway for safe and efficient cancer immunotherapy. Nano Lett. 2023;23:8725–33.
Gao J, Qiu X, Xi G, Liu H, Zhang F, Lv T, et al. Downregulation of GSDMD attenuates tumor proliferation via the intrinsic mitochondrial apoptotic pathway and inhibition of EGFR/Akt signaling and predicts a good prognosis in non-small cell lung cancer. Oncol Rep. 2018;40:1971–84.
Yamagishi R, Kamachi F, Nakamura M, Yamazaki S, Kamiya T, Takasugi M, et al. Gasdermin D–mediated release of IL-33 from senescent hepatic stellate cells promotes obesity-associated hepatocellular carcinoma. Sci Immunol. 2022;7:eabl7209.
Xing Y, Zhang F, Ji P, Wei M, Yin C, Yang A, et al. Efficient delivery of GSDMD‐N mRNA by engineered extracellular vesicles induces pyroptosis for enhanced immunotherapy. Small. 2023;19:e2204031.
Sala R, Rioja-Blanco E, Serna N, Sánchez-García L, Álamo P, Alba-Castellón L, et al. GSDMD-dependent pyroptotic induction by a multivalent CXCR4-targeted nanotoxin blocks colorectal cancer metastases. Drug Deliv. 2022;29:1384–97.
Acknowledgements
This work is sponsored by the National Natural Science Foundation of China (No. 81473339), Hunan Provincial Natural Science Foundation of China (NO.2021JJ40549), the research project of Health Commission of Hunan Province (No. D202313019110), the research project of Xiangtan Medical Association (No. D2022xtyx20), Hunan Provincial Administration of Traditional Chinese Medicine Project (No. E2023026), and the research project of Chinese Medical Association (No. Z-2021-46-2101).
Author information
Authors and Affiliations
Contributions
Conceptualization, YH and XL; Original Draft Preparation, YH; Review & Editing, YL, HL, WZ and RL; Visualization, figures, and tables, LZ, QX, QY and WL; Supervision, YH and XL.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by Professor Boris Zhivotovsky
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hu, Y., Liu, Y., Zong, L. et al. The multifaceted roles of GSDME-mediated pyroptosis in cancer: therapeutic strategies and persisting obstacles. Cell Death Dis 14, 836 (2023). https://doi.org/10.1038/s41419-023-06382-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41419-023-06382-y