Abstract
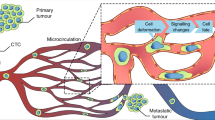
Two fields of cancer research have emerged dealing with the biology of tumour cells localised to the abluminal vascular surface: vessel co-option (VCo), a non-angiogenic mode of tumour growth and angiotropic extravascular migratory metastasis (EVMM), a non-hematogenous mode of tumour migration and metastasis. VCo is a mechanism by which tumour cells gain access to a blood supply by spreading along existing blood vessels in order to grow locally. Angiotropic EVMM involves “pericytic mimicry” (PM), which is characterised by tumour cells continuously migrating in the place of pericytes distantly along abluminal vascular surfaces. When cancer cells are engaged in PM and EVMM, they migrate along blood vessels beyond the advancing front of the tumour to secondary sites with the formation of regional and distant metastases. In the present perspective, the authors review the current scientific literature, emphasising the analogies between embryogenesis and cancer progression, the re-activation of embryonic signals by “cancer stem cells”, and the important role of laminins and epithelial-mesenchymal-transition. This perspective maintains that VCo and angiotropic EVMM constitute complementary processes and represent a continuum of cancer progression from the primary tumour to metastases and of tumour growth to EVMM, analogous to the embryonic development program.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Chitty JL, Filipe EC, Lucas MC, Herrmann D, Cox TR, Timpson P. Recent advances in understanding the complexities of metastasis. Faculty Rev-1169–1187 (2018).
Pezzella F, Pastorino U, Tagliabue E, Andreola S, Sozzi G, Gasparini G, et al. Non-small-cell lung carcinoma tumor growth without morphological evidence of neo-angiogenesis. Am J Pathol. 1997;151:1417–23.
Kuczynski EA, Vermeulen PB, Pezzella F, Kerbel RS, Reynolds AR. Vessel co-option in cancer. Nat Rev Clin Oncol. 2019;16:469–93.
Lugassy C, Eyden BP, Christensen L, Escande JP. Angio-tumoral complex in human malignant melanoma characterised by free laminin: ultrastructural and immunohistochemical observations. J Submicrosc Cytol Pathol. 1997;29:19–28.
Barnhill RL, Busam KJ, Berwick M, Blessing K, Cochran AJ, Elder DE, et al. Tumour vascularity is not a prognostic factor for cutaneous melanoma. Lancet. 1994;29:1237–8.
Lugassy C, Kleinman HK, Vermeulen PB, Barnhill RL. Angiotropism, pericytic mimicry and extravascular migratory metastasis: an embryogenesis-derived program of tumor spread. Angiogenesis. 2020;23:27–41.
Donnem T, Reynolds AR, Kuczynski EA, Gatter K, Vermeulen PB, Kerbel RS, et al. Non-angiogenic tumours and their influence on cancer biology. Nat Rev Cancer. 2018;18:323–36.
Welch DR, Hurst DR. Defining the hallmarks of metastasis. Cancer Res. 2019;79:3011–27.
Massagué J, Ganesh K. Metastasis-initiating cells and ecosystems. Cancer Discov. 2021;11:971–94.
Vandyck HH, Hillen LM, Bosisio FM, van den Oord J, Zur Hausen A, Winnepenninckx V. Rethinking the biology of metastatic melanoma: a holistic approach. Cancer Metastasis Rev. 2021;40:603–24.
Nataraj NB, Marrocco I, Yarden Y. Roles for growth factors and mutations in metastatic dissemination. Biochem Soc Trans. 2021;49:1409–23.
Haas G, Fan S, Ghadimi M, De Oliveira T, Conradi LC. Different forms of tumor vascularization and their clinical implications focusing on vessel co-option in colorectal cancer liver metastases. Front Cell Dev Biol. 2021;12:612774.
Häussinger D, Kordes C. Space of Disse: a stem cell niche in the liver. Biol Chem. 2019;18:81–95.
Montana V, Sontheimer H. Bradykinin promotes the chemotactic invasion of primary brain tumors. J Neurosci. 2011;30:4858–67.
Yadav VN, Zamler D, Baker GJ, Kadiyala P, Erdreich-Epstein A, DeCarvalho AC, et al. CXCR4 increases in-vivo glioma perivascular invasion, and reduces radiation induced apoptosis: a genetic knockdown study. Oncotarget. 2016;13:83701–19.
Griveau A, Seano G, Shelton SJ, Kupp R, Jahangiri A, Obernier K, et al. A glial signature and Wnt7 signaling regulate glioma-vascular interactions and tumor microenvironment. Cancer Cell. 2018;14:874–89.
Valiente M, Obenauf AC, Jin X, Chen Q, Zhang XH, Lee DJ, et al. Serpins promote cancer cell survival and vascular co-option in brain metastasis. Cell. 2014;27:1002–16.
Frentzas S, Simoneau E, Bridgeman VL, Vermeulen PB, Foo S, Kostaras E, et al. Vessel co-option mediates resistance to anti-angiogenic therapy in liver metastases. Nat Med. 2016;22:1294–302.
Rada M, Kapelanski-Lamoureux A, Petrillo S, Tabariès S, Siegel P. Reynolds AR, et al. Runt related transcription factor-1 plays a central role in vessel co-option of colorectal cancer liver metastases. Commun Biol. 2021;4:950.
Macklin PS, McAuliffe J, Pugh CW, Yamamoto A. Hypoxia and HIF pathway in cancer and the placenta. Placenta. 2017;56:8–13.
Gatter K, Brown D. Bone Marrow Diagnosis. An Illustrated. Guide. 3rd edn. Oxford: Wiley Blackwell; 2015.
Lugassy C, Eyden BP, Christensen L, Escande JP. Matrix interactions between tumor cells and endothelium in human malignant melanoma. J Invest Dermatol. 1996;106:894.
Lugassy C, Wadehra M, Li X, Corselli M, Akhavan D, Binder SW, et al. Pilot study on “pericytic mimicry” and potential embryonic/stem cell properties of angiotropic melanoma cells interacting with the abluminal vascular surface. Cancer Microenviron. 2013;6:19–29.
Lugassy C, Péault B, Wadehra M, Kleinman HK, Barnhill RL. Could pericytic mimicry represent another type of melanoma cell plasticity with embryonic properties? Pigment Cell Melanoma Res. 2013;26:746–54.
Lugassy C, Barnhill RL, Christensen L. Melanoma and extravascular migratory metastasis. J Cutan Pathol. 2000;27:481.
Celià-Terrassa T, Kang Y. Metastatic niche functions and therapeutic opportunities. Nat Cell Biol. 2018;20:868–77.
Moose DL, Krog BL, Kim TH, Zhao L, Williams-Perez S, Burke G, et al. Cancer cells resist mechanical destruction in circulation via rhoA/actomyosin-dependent mechano-adaptation. Cell Rep. 2020;17:3864–74.
Friedl P, Alexander S. Cancer invasion and the microenvironment: plasticity and reciprocity. Cell. 2011;23:992–1009.
Beunk L, Brown K, Nagtegaal I, Friedl P, Wolf K. Cancer invasion into musculature: mechanics, molecules and implications. Semin Cell Dev Biol. 2019;93:36–45.
Barnhill RL, Ye M, Batistella A, Stern MH, Roman-Roman S, Dendale R, et al. The biological and prognostic significance of angiotropism in uveal melanoma. Lab Invest. 2017;97:746–59.
van Dam PJ, van der Stok EP, Teuwen LA, Van den Eynden GG, Illemann M, Frentzas S, et al. International consensus guidelines for scoring the histopathological growth patterns of liver metastasis. Br J Cancer. 2017;7:1427–41.
Barnhill R, Vermeulen P, Daelemans S, van Dam PJ, Roman-Roman S, Servois V, et al. Replacement and desmoplastic histopathological growth patterns: a pilot study of prediction of outcome in patients with uveal melanoma liver metastases. J Pathol Clin Res. 2018;4:227–40.
Barnhill R, van Dam PJ, Vermeulen P, Champenois G, Nicolas A, Rawson RV, et al. Replacement and desmoplastic histopathological growth patterns in cutaneous melanoma liver metastases: frequency, characteristics, and robust prognostic value. J Pathol Clin Res. 2020;6:195–206.
Zhang Y, Wang S, Dudley AC. Models and molecular mechanisms of blood vessel co-option by cancer cells. Angiogenesis. 2020;23:17–25.
Lugassy C, Scolyer R, Long G, Menzies A, Mischel P, Barnhill RL. PDGFBR expression in anti-BRAF resistant melanoma: are angiotropic melanoma cells a source of BRAF resistance and disease progression? J Cutan Pathol. 2014;41:159–60.
Gritsenko P, Leenders W, Friedl P. Recapitulating in vivo-like plasticity of glioma cell invasion along blood vessels and in astrocyte-rich stroma. Histochem Cell Biol. 2017;148:395–406.
Arvanitis CD, Ferraro GB, Jain RK. The blood-brain barrier and blood-tumour barrier in brain tumours and metastases. Nat Rev Cancer. 2020;20:26–41.
Yao H, Price TT, Cantelli G, Ngo B, Warner MJ, Olivere L, et al. Leukaemia hijacks a neural mechanism to invade the central nervous system. Nature. 2018;560:55–60.
Levy MJ, Gleeson FC, Zhang L. Endoscopic ultrasound fine-needle aspiration detection of extravascular migratory metastasis from a remotely located pancreatic cancer. Clin Gastroenterol Hepatol. 2009;7:246–8.
Rustagi T, Gleeson FC, Chari ST, Lehrke HD, Takahashi N, Malikowski TM, et al. Safety, diagnostic accuracy, and effects of endoscopic ultrasound fine-needle aspiration on detection of extravascular migratory metastases. Clin Gastroenterol Hepatol. 2019;17:2533–40.
Shen J, Shrestha S, Rao PN, Asatrian G, Scott MA, Nguyen V, et al. Pericytic mimicry in well-differentiated liposarcoma/atypical lipomatous tumor. Hum Pathol. 2016;54:92–9.
Bald T, Quast T, Landsberg J, Rogava M, Glodde N, Lopez-Ramos D, et al. Ultraviolet-radiation-induced inflammation promotes angiotropism and metastasis in melanoma. Nature. 2014;6:109–13.
Bentolila LA, Prakash R, Mihic-Probst D, Wadehra M, Kleinman HK, Carmichael TS, et al. Imaging of angiotropism/vascular co-option in a murine model of brain melanoma: implications for melanoma progression along extravascular pathways. Sci Rep. 2016;6:23834.
Fornabaio G, Barnhill RL, Lugassy C, Bentolila LA, Cassoux N, Roman-Roman S, et al. Angiotropism and extravascular migratory metastasis in cutaneous and uveal melanoma progression in a zebrafish model. Sci Rep. 2018;11:10448.
Er EE, Valiente M, Ganesh K, Zou Y, Agrawal S, Hu J, et al. Pericyte-like spreading by disseminated cancer cells activates YAP and MRTF for metastatic colonization. Nat Cell Biol. 2018;20:966–78.
Lai CJ, Lin CY, Liao WY, Hour TC, Wang HD, Chuu CP. CD44 promotes migration and invasion of docetaxel-resistant prostate cancer cells likely via induction of Hippo-Yap signaling. Cells. 2019;30:8.
Puisieux A, Brabletz T, Caramel J. Oncogenic roles of EMT-inducing transcription factors. Nat Cell Biol. 2014;16:488–94.
Yang J, Antin P, Berx G, Blanpain C, Brabletz T, Bronner M, et al. EMT International Association (TEMTIA). Guidelines and definitions for research on epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2020;21:341–52.
Wu Z, Guan KL. Hippo signaling in embryogenesis and development. Trends Biochem Sci. 2021;46:51–63.
Paget S. The distribution of secondary growths in cancer of the breast. Lancet. 1889;133:571–3.
Dupin E, Calloni GW, Coelho-Aguiar JM, Le Douarin NM. The issue of the multipotency of the neural crest cells. Dev Biol. 2018;444:S47–59.
Burton GJ, Cindrova-Davies T, Turco MY. Review: Histotrophic nutrition and the placental-endometrial dialogue during human early pregnancy. Placenta. 2020;102:21–6.
Fares J, Fares MY, Khachfe HH, Salhab HA, Fares Y. Molecular principles of metastasis: a hallmark of cancer revisited. Signal Transduct Target Ther. 2020;12:28.
Cofre J, Saalfeld K, Abdelhay E. Cancer as an embryological phenomenon and its developmental pathways: a hypothesis regarding the contribution of the noncanonical Wnt pathway. ScientificWorldJournal. 2019;3:4714781.
Ober EA, Lemaigre FP. Development of the liver: insights into organ and tissue morphogenesis. J Hepatol. 2018;68:1049–62.
Yap L, Tay HG, Nguyen MTX, Tjin MS, Tryggvason K. Laminins in cellular differentiation. Trends Cell Biol. 2019;29:987–1000.
Sekiguchi R, Yamada KM. Basement membranes in development and disease. Curr Top Dev Biol. 2018;130:143–91.
Rousselle P, Scoazec JY. Laminin 332 in cancer: when the extracellular matrix turns signals from cell anchorage to cell movement. Semin Cancer Biol. 2020;62:149–65.
Sun T, Patil R, Galstyan A, Klymyshyn D, Ding H, Chesnokova A, et al. Blockade of a laminin-411-notch axis with CRISPR/Cas9 or a nanobioconjugate inhibits glioblastoma growth through tumor-microenvironment cross-talk. Cancer Res. 2019;79:1239–51.
Lugassy C, Vernon SE, Busam K, Engbring JA, Welch DR, Poulos EG, et al. Angiotropism of human melanoma: studies involving in transit and other cutaneous metastases and the chicken chorioallantoic membrane: implications for extravascular melanoma invasion and metastasis. Am J Dermatopathol. 2006;28:187–93.
Teuwen LA, De Rooij LPMH, Cuypers A, Rohlenova K, Dumas SJ, García-Caballero M, et al. Tumor vessel co-option probed by single-cell analysis. Cell Rep. 2021;35:109253.
Tsai HH, Niu J, Munji R, Davalos D, Chang J, Zhang H, et al. Oligodendrocyte precursors migrate along vasculature in the developing nervous system. Science. 2016;351:379–84.
Rada M, Lazaris A, Kapelanski-Lamoureux A, Mayer TZ, Metrakos P. Tumor microenvironment conditions that favor vessel co-option in colorectal cancer liver metastases: a theoretical model. Semin Cancer Biol. 2021;71:52–64.
Hutchins EJ, Bronner ME. Draxin alters laminin organization during basement membrane remodeling to control cranial neural crest EMT. Dev Biol. 2019;446:151–8.
Maeda H, Khatami M. Analyses of repeated failures in cancer therapy for solid tumors: poor tumor-selective drug delivery, low therapeutic efficacy and unsustainable costs. Clin Transl Med. 2018;1:11.
Demicheli R, Terenziani M, Valagussa P, Moliterni A, Zambetti M, Bonadonna G. Local recurrences following mastectomy: support for the concept of tumor dormancy. J Natl Cancer Inst. 1994;86:45–8.
Bushnell GG, Deshmukh AP, den Hollander P, Luo M, Soundararajan R, Jia D, et al. Breast cancer dormancy: need for clinically relevant models to address current gaps in knowledge. npj Breast Cancer. 2021;28:66.
Clark AG, Vignjevic DM. Modes of cancer cell invasion and the role of the microenvironment. Curr Opin Cell Biol Oct. 2015;36:13–22.
Barnhill RL, Lemaitre S, Lévy-Gabrielle C, Rodrigues M, Desjardins L, Dendale R, et al. Satellite in transit metastases in rapidly fatal conjunctival melanoma: implications for angiotropism and extravascular migratory. Pathology. 2016;48:166–76.
Höppener DJ, Nierop PMH, Hof J, Sideras K, Zhou G, Visser L, et al. Enrichment of the tumour immune microenvironment in patients with desmoplastic colorectal liver metastasis. Br J Cancer. 2020;123:196–206.
Acknowledgements
We sincerely thank Maud Haon who designed the diagram for Fig. 1.
Funding
N/A
Author information
Authors and Affiliations
Contributions
CL and RB designed and wrote the manuscript with essential inputs from DR, FP and PV. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lugassy, C., Vermeulen, P.B., Ribatti, D. et al. Vessel co-option and angiotropic extravascular migratory metastasis: a continuum of tumour growth and spread?. Br J Cancer 126, 973–980 (2022). https://doi.org/10.1038/s41416-021-01686-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-021-01686-2
This article is cited by
-
L1CAM and laminin vascular network: Association with the high-risk replacement histopathologic growth pattern in uveal melanoma liver metastases
Laboratory Investigation (2022)
-
The vascular outsiders
British Journal of Cancer (2022)