Abstract
Allogeneic transplantation (allo-HCT) is a curative treatment in CLL whose efficacy including the most severe forms had led to the 2006 EBMT recommendations. The advent after 2014 of targeted therapies has revolutionized CLL management, allowing prolonged control to patients who have failed immunochemotherapy and/or have TP53 alterations. We analysed the pre COVID pandemic 2009–2019 EBMT registry. The yearly number of allo-HCT raised to 458 in 2011 yet dropped from 2013 onwards to an apparent plateau above 100. Within the 10 countries who were under the EMA for drug approval and performed 83.5% of those procedures, large initial differences were found but the annual number converged to 2–3 per 10 million inhabitants during the 3 most recent years suggesting that allo-HCT remains applied in selected patients. Long-term follow-up on targeted therapies shows that most patients relapse, some early, with risk factors and resistance mechanisms being described. The treatment of patients exposed to both BCL2 and BTK inhibitors and especially those with double refractory disease will become a challenge in which allo-HCT remains a solid option in competition with emerging therapies that have yet to demonstrate their long-term effectiveness.
Similar content being viewed by others
Allogeneic hematopoietic cell transplantation (allo-HCT) in Chronic Lymphocytic Leukaemia (CLL) remains a curative option [1]. Long term analysis of the GCLLSG CLL3X trial demonstrated a 10-year overall survival (OS) and PFS of 51% and 34%, respectively [2]. A recent prospective study, with post-allo-HCT MRD management reported at 3 years 64% PFS, 9.5% NRM of 9.5% and 29.5% cumulative incidence of relapse [3].
Following the first EBMT recommendations established in 2006, the CIBMTR registered 400 to 500 cases per year in 2010 with similar numbers in the EBMT database as described below [4].
The subsequent development of B‐cell receptor signalling inhibitors (BCRi) [phosphatidylinositol‐3‐kinase inhibitors (PI3Ki) and Bruton tyrosine kinase inhibitors (BTKi)] and BCL2 inhibitors (BCL2i) led to major therapeutic advances, especially in relapse and for high-risk CLL subgroups. Ibrutinib was designated by the European Medical Agency (EMA) as an orphan medicinal product on 12 March 2013. The EMA Committee for Medicinal Products for Human (CHMP) gave a positive opinion for the use in CLL of both idelalisib and ibrutinib on 24 July 2014 and of venetoclax on 14 October 2016 (www.ema.europa.eu), followed by the marketing authorisation within the European community.
As a result, during the more recent years the majority of first-line patients with 17p/TP53 alteration have been treated with ibrutinib, with a median PFS exceeding 50% at 5 years. Patients relapsing after chemoimmunotherapy (CIT) were also mainly treated with ibrutinib with a median PFS of at least 50 months, shorter if patients had multiple treatments, TP53 alteration or a complex karyotype. In the case of relapse after prior exposure to ibrutinib, venetoclax alone or in combination with rituximab led to high overall response rates (ORR) and a median PFS of 25–30 months [5]. As a result, a new ‘category’ of patients exposed to both BCRi and BCL2i is emerging, some being truly double refractory to both agents and with a very poor prognosis and an OS below 1 year [6, 7]. Considering these new paradigms, the EBMT and the European Research Initiative on CLL (ERIC) established new recommendations in 2014 at two levels which were additionally modified in 2018 [8].
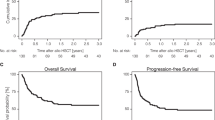
To describe the impact of the introduction of these novel drugs on the allo-HCT rate over time, we analysed data from the EBMT registry for CLL patients older than 18 years and undergoing a first allo-HCT between 2009 to 2019. From 37 EBMT participating countries, a total of 3011 allo-HCT were identified, including 23 transplants for a second cancer. For this population as a whole, the number of allo-HCT increased in the first 3 years to 458 per year in 2011, yet decreased significantly after 2013, falling below 150 from 2017 onwards (Fig. 1a). We focused our analysis on the 10 countries under the regulation of the EMA for access to drugs and having performed more than 50 allo-HCT for CLL during this period. These countries, including Germany, France, the United Kingdom, Italy, Spain, the Netherlands, the Czech Republic, Belgium, Sweden and Denmark, performed 2514 (83.5%) of these allo-HCT. The characteristics of these patients are reported in Table 1. The median follow-up, determined by means of the reverse Kaplan–Meier method, was 69 months (95% CI 65–72). Overall survival (OS) and progression free survival (PFS) were determined by Kaplan–Meier method. The OS (95% confidence interval) of this population by 3 and 5 years is 58% (56–60%) and 50% (48–52%) and the PFS (95% confidence interval) by 3 and 5 years is 46% (44–48%) and 38% (35–40%) (Fig. 2a, b). Based on Eurostat population data (https://ec.europa.eu/eurostat/) we calculated the annual incidence of CLL transplants per year and per 10 million inhabitants. This incidence has clearly decreased over the years from a median of 8–11 per 10 million inhabitants in the 3-year period 2009–2011 to 2-3 per 10 million inhabitants during the 3 most recent years (2017–2019). The overall trend for these 10 countries is the same as for all other countries reporting to EBMT (Fig. 1b).
a Number of first allo-HCT procedures for CLL patients older than 18 years, recorded in the EBMT registry between 2009 to 2019. Blue line: total number of allo-HCT; orange line: number of allo-HCT performed on the 10 countries who performed more than 50 allo-HCT during this period; grey line: number of allo-HCT performed on the remaining countries who performed less than 50 allo-HCT during this period. b Annual incidence of CLL transplants per year and per 10 million inhabitants recorded in the EBMT registry in the 10 countries who performed more than 50 allo-HCT between 2009 and 2019.
Kaplan-Meier survival curves of (a) Overall Survival (OS) and (b) Progression Free Survival (PFS) within the first 10 years after allo-HCT. The shaded regions indicate the 95% confidence intervals. The number of event free patients are indicated below the time axis. Estimates of OS and PFS including 95% CI by 3 and 5 years are given (vertical dotted lines) in (a, b) respectively.
This survey shows that following the development of new agents the number of allo-HCT dropped and seemed to have stabilised to approximately 100 procedures per year for the 2017–2019 period. We need to determine what types of CLL patients nowadays receive allo-HCT for CLL, while also considering the impact of the pandemic SARS-Cov-2 from 2020 onwards the allo-HCT indications.
The Heidelberg team reported their experience applying the ERIC/EBMT 2014 recommendations in patients at least exposed to BCRi and/or BCL2i [9]. Two recent retrospective studies have been conducted in patients exposed to the newer agents [10, 11]. One reported a PFS and NRM of 63 and 13% respectively in a population of 65 patients who had received ≥1 new drug, with exposure to ibrutinib and venetoclax in 17 cases [10]. In both studies, the number of double refractory patients was very limited. The EBMT CMWP has initiated a survey to analyse outcomes for allo-HCT after multiple pathway inhibitors including patients who discontinued ibrutinib for relapse or intolerance and treated with venetoclax before and after allo-HCT.
Without mentioning Richter’s syndrome, which is a constant concern (and the actual object of a separate EBMT survey), there is no doubt that the emergence of truly double refractory patients, including also patients who will be failing BTKi/BCL2 combinations [5] pose a challenge. For such patients allo-HCT remains the recommended option provided that a bridge can be found to obtain a disease response. This refractory group is however a moving target where allo-HCT will be put in competition with other emerging therapeutics, especially CAR-T, non-covalent BTKi, and bispecific antibodies.
While CAR-Ts have been developed for 10 years [12] with the fascinating report that long term circulating CAR-T could be demonstrated [13], no registration has been obtained yet in CLL. Despite ORR of 60–95%, including substantial MRD clearance, only a few durable responses have been reported. In the EBMT database, no CAR-T for CLL have been recorded during the 2009 to 2019 period and only 4 for the 2 following years. Barriers to the efficacy may be a reduced capacity for sustained T-cell expansion [14]. However, we believe that their underuse in Europe in CLL it is primarily due to the lack of available studies and that if access to development programmes were offered, CAR-T would be evaluated as a priority, especially in double refractory patients in competition or potentially in combination with allo-HCT.
Among reversible (non-covalent) BTKi, pirtobrutinib produces ORR of up to 62%, in previously exposed to BCRi, whatever the cause of previous interruption and independent of C481S BTK mutational status, however, the median PFS appears to be less than 2 years [15, 16] and emerging mechanisms of resistance are now described [17].
Finally, bispecifics T-cells engagers such as mosunetuzumab (NCT05091424) and epcoritamab (NCT04623541) are in development in patients pre-exposed to BCRi and/or BCL2i (but not necessarily to both) although no robust data is available yet.
In conclusion, following a clear reduction of the annual number, allo-HCT remained used for CLL at a stable rate of approximately 100/year in Europe 5 years after the approval of ibrutinib and idelalisib and 3 years after the approval of venetoclax. Further real-world evaluation to explore allo-HCT indications in the context of competing/complementary strategies is required, for BCRi/BCL2i double exposed and double refractory relapsed patients.
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
References
van Gelder M, Ziagkos D, de Wreede L, van Biezen A, Dreger P, Gramatzki M, et al. Baseline Characteristics Predicting Very Good Outcome of Allogeneic Hematopoietic Cell Transplantation in Young Patients With High Cytogenetic Risk Chronic Lymphocytic Leukemia—A Retrospective Analysis From the Chronic Malignancies Working Party of the EBMT. Clin Lymphoma Myeloma Leuk. 2017;17:667–5.e2.
Krämer I, Stilgenbauer S, Dietrich S, Böttcher S, Zeis M, Stadler M, et al. Allogeneic hematopoietic cell transplantation for high-risk CLL: 10-year follow-up of the GCLLSG CLL3X trial. Blood. 2017;130:1477–80.
Tournilhac O, Garff-Tavernier ML, Quoc SN, Forcade E, Chevallier P, Legrand-Izadifar F, et al. Efficacy of minimal residual disease driven immune-intervention after allogeneic hematopoietic stem cell transplantation for high-risk chronic lymphocytic leukemia: results of a prospective multicenter trial. Haematologica. 2021;106:1867–75.
Kharfan-Dabaja MA, El-Asmar J, Awan FT, Hamadani M, Ayala E. Current state of hematopoietic cell transplantation in CLL as smart therapies emerge. Best Pract Res Clin Haematol. 2016;29:54–66.
Hallek M, Al-Sawaf O. Chronic lymphocytic leukemia: 2022 update on diagnostic and therapeutic procedures. Am J Hematol. 2021;96:1679–705.
Mato AR, Roeker LE, Jacobs R, Hill BT, Lamanna N, Brander D, et al. Assessment of the Efficacy of Therapies Following Venetoclax Discontinuation in CLL Reveals BTK Inhibition as an Effective Strategy. Clin Cancer Res. 2020;26:3589–96.
Lew TE, Lin VS, Cliff ER, Blombery P, Thompson ER, Handunnetti SM, et al. Outcomes of patients with CLL sequentially resistant to both BCL2 and BTK inhibition. Blood Adv. 2021;5:4054–8.
Dreger P, Ghia P, Schetelig J, van Gelder M, Kimby E, Michallet M, et al. High-risk chronic lymphocytic leukemia in the era of pathway inhibitors: integrating molecular and cellular therapies. Blood. 2018;132:892–902.
Hoffmann A, Dietrich S, Hain S, Rieger M, Hegenbart U, Sellner L, et al. Allogeneic transplantation in high-risk chronic lymphocytic leukemia: a single-center, intent-to-treat analysis. Haematologica. 2019;104:e304–6.
Roeker LE, Dreger P, Brown JR, Lahoud OB, Eyre TA, Brander DM, et al. Allogeneic stem cell transplantation for chronic lymphocytic leukemia in the era of novel agents. Blood Adv. 2020;4:3977–89.
Kim HT, Shaughnessy CJ, Rai SC, Reynolds C, Ho VT, Cutler C, et al. Allogeneic hematopoietic cell transplantation after prior targeted therapy for high-risk chronic lymphocytic leukemia. Blood Adv. 2020;4:4113–23.
Tournilhac O, Dreger P Chronic Lymphocytic Leukaemia. In: Kröger N, Gribben J, Chabannon C, Yakoub-Agha I, Einsele H, editors. The EBMT/EHA CAR-T Cell Handbook. Cham (CH): Springer; 2022. http://www.ncbi.nlm.nih.gov/books/NBK584167/.
Melenhorst JJ, Chen GM, Wang M, Porter DL, Chen C, Collins MA, et al. Decade-long leukaemia remissions with persistence of CD4+ CAR T cells. Nature. 2022;602:503–9.
Griggio V, Perutelli F, Salvetti C, Boccellato E, Boccadoro M, Vitale C, et al. Immune Dysfunctions and Immune-Based Therapeutic Interventions in Chronic Lymphocytic Leukemia. Front Immunol. 2020;11:594556.
Mato AR, Shah NN, Jurczak W, Cheah CY, Pagel JM, Woyach JA, et al. Pirtobrutinib in relapsed or refractory B-cell malignancies (BRUIN): a phase 1/2 study. Lancet. 2021;397:892–901.
Mato A. Pirtobrutinib, A Next Generation, Highly Selective, Non-Covalent BTK Inhibitor in Previously Treated CLL/SLL: Updated Results from the Phase 1/2 BRUIN Study. In ASH; 2021. https://ash.confex.com/ash/2021/webprogram/Paper147599.html.
Wang E, Mi X, Thompson MC, Montoya S, Notti RQ, Afaghani J, et al. Mechanisms of Resistance to Noncovalent Bruton’s Tyrosine Kinase Inhibitors. N Engl J Med. 2022;386:735–43.
Author information
Authors and Affiliations
Contributions
OT and MvG designed the study, interpreted results and wrote the manuscript. DJE performed statistical analysis, NZ collected and managed data, PD, MB, VV, CS, JJC, TS, PJ, HS, SNQ, MS, IWB, JM, SP, PC, EF, NK, DB, JG, BN, JEJ, CK, YB, PP, AS, DPMcL, JS, PJH, and IYA and PJ delivered data and reviewed the paper.
Corresponding author
Ethics declarations
Competing interests
From: Astra-Zeneca; Abbvie; Beigene; Blueprint; Gilead; Incyte; Janssen; Roche; Sandoz; Secura-Bio: Travel grants; Research grants; Honorarium (advisory board, symposium, expertise).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tournilhac, O., van Gelder, M., Eikema, DJ. et al. The European landscape on allogeneic haematopoeietic cell transplantation in Chronic Lymphocytic Leukaemia between 2009 and 2019: a perspective from the Chronic Malignancies Working Party of the EBMT. Bone Marrow Transplant 58, 621–624 (2023). https://doi.org/10.1038/s41409-023-01955-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-023-01955-z