Abstract
Second allogeneic stem cell transplantation (alloSCT2) is among the most effective treatments for acute myeloid leukemia (AML) relapse after first alloSCT (alloSCT1). Long-term EBMT registry data were used to provide large scale, up-to-date outcome results and to identify factors for improved outcome. Among 1540 recipients of alloSCT2, increasing age, better disease control and performance status before alloSCT2, more use of alternative donors and higher conditioning intensity represented important trends over time. Between the first (2000–2004) and last (2015–2019) period, two-year overall and leukemia-free survival (OS/LFS) increased considerably (OS: 22.5–35%, LFS: 14.5–24.5%). Cumulative relapse incidence (RI) decreased from 64% to 50.7%, whereas graft-versus-host disease and non-relapse mortality (NRM) remained unchanged. In multivariable analysis, later period of alloSCT2 was associated with improved OS/LFS (HR = 0.47/0.53) and reduced RI (HR = 0.44). Beyond, remission duration, disease stage and patient performance score were factors for OS, LFS, RI, and NRM. Myeloablative conditioning for alloSCT2 decreased RI without increasing NRM, leading to improved OS/LFS. Haploidentical or unrelated donors and older age were associated with higher NRM and inferior OS. In summary, outcome after alloSCT2 has continuously improved over the last two decades despite increasing patient age. The identified factors provide clues for the optimized implementation of alloSCT2.
Similar content being viewed by others
Introduction
Allogeneic stem cell transplantation (alloSCT) represents a potentially curative treatment for patients suffering from acute myeloid leukemia (AML). However, leukemia relapse after the first alloSCT (alloSCT1) occurs in 30–50% of patients [1, 2]. The prognosis of these patients is generally poor, especially if they relapse within six months from alloSCT1 [3, 4]. A second alloSCT (alloSCT2) has become one of the most frequently applied therapy in patients who are considered fit enough to tolerate the procedure [5]. In a recent study, the Acute Leukaemia Working Party (ALWP) of the European Society for Blood and Marrow Transplantation (EBMT) has analysed trends in treatment and outcome in more than 8000 patients experiencing AML relapse post alloSCT1 [6]. AlloSCT2, although performed only in a minority of patients, contributed considerably to long-term remission and improved outcome over time. However, a detailed analysis of alloSCT2 was not the focus of this study.
Therefore, we performed a detailed retrospective registry-based analysis among adults receiving alloSCT2 for AML relapse after alloSCT1 between the years 2000 and 2019. The study aimed to analyze trends in patient characteristics, transplant settings and outcome over the last two decades and to identify factors associated with improved results after alloSCT2, that might allow to define strategies for an optimized implementation of the procedure.
Methods
Study design
Data were extracted from the EBMT registry, which comprises >600 transplant centers providing reports and annual follow-up on all consecutive SCT. Audits are routinely performed to determine data accuracy. Since 1990, patients have provided informed consent, authorizing the use of their personal information for research purposes. The study was approved by the general assembly and review board of the ALWP and complied with country-specific regulatory requirements.
Considering all types of conditioning, all donors apart from cord blood, and all disease stages, 1540 consecutive patients ≥18 years receiving alloSCT2 for hematological AML relapse after alloSCT1 were included. AlloSCT2 applied for graft failure were excluded. For evaluation of changes over time, patients were grouped by period of transplant into 5-year intervals. Variables of interest included patient and donor characteristics (age, patient and donor sex, Karnofsky performance score (KPS), donor switch for alloSCT2, cytomegalovirus (CMV) serostatus of patient and donor), disease-related (de novo/secondary AML, cytogenetics, remission duration after alloSCT1, disease status at alloSCT2) and transplant-related factors (year of transplant, delay between relapse post alloSCT1 and alloSCT2, graft source, donor type, female donor to male recipient, conditioning regime, use of myeloablative or reduced intensity conditioning (MAC/RIC), total body irradiation (TBI), in-vivo or in-vitro T-cell depletion (TCD) or use of post-transplant cyclophosphamide (PTCY) and graft-versus-host disease (GVHD) prophylaxis). Analyzed outcome variables comprised overall survival (OS), leukemia-free survival (LFS), cumulative incidence of relapse (RI), non-relapse mortality (NRM), acute and chronic graft-versus-host disease (GVHD), and GVHD-free/relapse-free survival (GRFS).
Definitions
As recommended [7], complete remission (CR) was defined by <5% bone marrow blasts, absence of circulating blasts and extramedullary disease. Failure to achieve CR after two courses of standard induction chemotherapy was defined as primary induction failure (PIF) [8]. Relapse was defined as more than 5% bone marrow blasts or reappearance of circulating blasts after a documented CR or development of extramedullary disease. OS was defined as the interval between day of alloSCT2 and day of death or last follow-up, LFS as interval between alloSCT2 and date of leukemia persistence, relapse, progression or death. NRM was defined as death from any cause without relapse or progression. GRFS was defined as survival without acute GVHD grades III–IV, chronic GVHD requiring systemic treatment, relapse, or death [9]. Reduced intensity conditioning (RIC) was defined using EBMT guidelines [8]. Cytogenetic subgroups were defined according to European Leukemia Net (ELN) criteria [10].
Statistics
Descriptive statistics were presented using median, range (from minimum to maximum) and inter-quartile range for continuous data, frequency and percentages for categorical data. Survivors were censored at last contact. Cumulative incidence was used to estimate the endpoints of NRM, RI, acute and chronic GVHD to accommodate for competing risks. Relapse and death were considered competing events for acute and chronic GVHD. Probabilities of OS, LFS, and GRFS were calculated using the Kaplan–Meier method. The median follow-up has been estimated using the reverse Kaplan-Meier method. All outcomes have been censored at two years according to the median follow-up of the most recent period.
A Cox proportional-hazards model was performed for multivariable regressions. Period of transplant, type of alloSCT2, age, Karnofsky performance score (KPS) and disease stage at alloSCT2, cytogenetics, recipient/donor gender, cytomegalovirus (CMV) status, in-vivo T-cell depletion (TCD), conditioning, interval alloSCT1 to relapse, and interval relapse to alloSCT2 were included. Results were expressed as hazard ratio (HR) and 95% confidence intervals (CI). All tests were 2-sided. Center effect was considered as frailty. Type I error rate was fixed at 0.05 for factors associated with time-to-event outcomes. Analyses were performed using R 4.3.2 (https://www.R-project.org/).
Results
Trends in patient and transplant characteristics
Over time, the number of patients reported to have received an alloSCT2 in EBMT centers increased from 144 in the period 2000–2004 to 619 between 2015 and 2019, with remarkable changes with respect to patient characteristics and transplant settings: In recent time periods, patients were older (median age 43.4 years between 2000 and 2004 and 48.6 years between 2015 and 2019, p = 0.012), received alloSCT2 more frequently in CR (37.5% between 2000 and 2004, increase to 52.7% in 2015–2019, p < 0.0001) and had a better performance status before alloSCT2 (2000–2004: KPS ≥90% in 25.5% patients, 2015–2019: increase to 58.5%, p < 0.001). Moreover, the interval between alloSCT1 and relapse increased from 9.1 to 12.3 months (p < 0.0001) and the interval between relapse after alloSCT1 and alloSCT2 increased from 2.5 to 3.5 months (p < 0.001). In the more recent time periods, a different donor, especially unrelated (increase from 30.6% [2000–2004] to 61.7% [2015–2019]) and haploidentical (increase from 0.7% [2000–2004] to 22.9% [2015–2019]), was used more frequently for alloSCT2 (p < 0.0001 each). Finally, over time, a more frequent use of myeloablative conditioning (MAC; p = 0.045), in-vivo TCD (p < 0.001), as well as post-transplant cyclophosphamide (PTCY) (p < 0.001) and cyclosporin A/mycophenolate mofetil (MMF) for GVHD prophylaxis (p < 0.001) for alloSCT2 was observed. Cytogenetic subgroups were not equally distributed over the years, however without a clear trend. No differences were found regarding stem cell source, sex of patient and donor, frequency of female donors for male recipients, number of secondary AML, CMV status of donors, cell source, or use of total body irradiation (TBI) (see Table 1 and supplemental Table 1 for details).
Outcomes
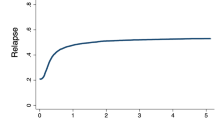
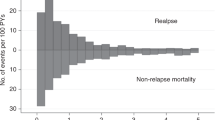
Median follow-up from alloSCT2 was 15.1 years in the cohort transplanted between 2000 and 2004, and 2.5 years in the most recent cohort. Concerning clinical results after alloSCT2, RI decreased over time, whereas OS, LFS, and GRFS improved: Between 2000 and 2019, two-year OS increased from 22.5% to 35%, LFS increased from 14.5% to 24.5% and GRFS increased from 10.5% to 17%. Whereas no clear trend was observed for NRM and incidence of acute and chronic GVHD, RI decreased from 64% to 50.7% (Fig. 1, Table 2).
Overall survival, leukemia-free survival, non-relapse mortality, the cumulative incidence of relapse and graft-versus-host disease/ relapse-free survival after second allogeneic stem cell transplantation for patients transplanted between 2000 and 2004, 2005 and 2009, 2010 and 2014, and 2015 and 2019.
Multivariable analysis of risk factors
In the multivariable analysis (Table 3 and supplemental Table 2), a later period of alloSCT2 was significantly associated with improved OS (2015–2019: HR 0.47, p < 0.001; 2010–2014: HR 0.48, p = 0.002; 2005–2009: HR 0.61, p = 0.04), LFS (2015–2019: HR 0.53, p = 0.01; 2010–2014: HR 0.5, p = 0.003; 2005–2009: HR 0.62, p = 0.046) and GRFS (2015–2019: HR 0.52, p = 0.003; 2010–2014: HR 0.47, p < 0.001; 2005–2009: HR 0.58, p = 0.02). These findings came along with a markedly lower RI (HR 0.44, p = 0.003 for the period 2015–2019), whereas NRM and acute GVHD remained unchanged. Disease status at alloSCT2 had significant effects on OS, LFS, RI and NRM (HR for active disease at alloSCT2 1.59, p < 0.001 for OS; HR 1.63, p < 0.001 for LFS; HR 1.91, p < 0.001 for RI; HR 1.35, p = 0.04 for NRM). Similarly, longer duration of CR after alloSCT1 was associated with better OS and LFS, lower RI and lower rates of NRM and acute GVHD (HR for lower remission duration 1.83, p < 0.001 for OS; HR 1.72, p < 0.001 for LFS; HR 1.86, p < 0.001 for RI; HR 1.46, p = 0.01 for NRM; HR 1.32, p = 0.02 for acute GVHD). In contrast, alloSCT2 from haploidentical or unrelated donors, and older patient age were associated with higher NRM (HR 1.88, p = 0.01 for haploidentical donor; HR 2.14, p < 0.001 for unrelated donor; HR 1.1, p < 0.001 for increasing age by 5-year intervals) and therefore inferior OS (HR 1.34, p = 0.02 for haploidentical donor; HR 1.32, p = 0.02 for unrelated donor; HR 1.05, p = 0.002 for increasing age).
MAC was associated with decreased RI without increasing NRM, leading to increased OS and LFS (HR 0.7, p < 0.001 for RI; HR 1.1, p = 0.47 for NRM; HR 0.82, p = 0.02 for OS; HR 0.79, p = 0.002 for LFS). LFS and OS were positively affected by a better KPS (HR 0.84, p = 0.02 for LFS; HR 0.74, p < 0.001 for OS). In-vivo TCD was associated with lower NRM and lower rates of GVHD (HR 0.7, p = 0.02 for NRM; HR 0.52, p < 0.001 for acute GVHD grades II–IV; HR 0.64, p = 0.003 for chronic GVHD).
Role of donor switch for alloSCT2
Switch to another donor could not be included in the main multivariable model due to missing information in about one third of patients. However, an exploratory analysis of 1006 informative patients revealed no signal of a significant influence (data not shown).
Discussion
This large registry analysis describes trends in patient characteristics, transplant strategies, and outcome after alloSCT2 for patients with AML relapsing after a first alloSCT over the last two decades. As shown for alloSCT1 [11], the number of second transplants has increased considerably over time, which may be due to an easier availability of alternative donors such as matched unrelated (MUD) and haploidentical donors. The formation of different RIC regimen [12] and improved supportive care might be other reasons [13]. Further significant changes over time comprised increasing patient age, longer remission after alloSCT1, more frequent use of alternative donors and donor change for alloSCT2, changes in GVHD prophylaxis, more intensive conditioning as well as improved disease control and improved KPS at alloSCT2. Fortunately, 2-year OS after alloSCT2 has continuously increased over time, reaching 35% in the most recent cohort. This was mainly due to a marked decrease in 2-year RI. In contrast, both rates of 2-year NRM and GVHD remained stable over the years, despite increased patient age and more frequent use of alternative donors. Better performance score at alloSCT2 might have counterbalanced the increased risk for NRM associated with increasing age and alternative donors. Besides alloSCT2 in earlier years, identified risk factors for inferior outcome after alloSCT2 included established variables such as older age, shorter remission after alloSCT1, donor type other than a matched sibling donor (MSD), RIC for alloSCT2, as well as active disease and lower KPS at time of alloSCT2.
Considering the analysis of changes over time together with risk factors for outcome, some lessons can be learned for planning and performing alloSCT2 for AML relapse after alloSCT1. These strategies include both measures to be taken before, during, and after alloSCT2:
The duration of remission between alloSCT1 and relapse is probably the most relevant risk factor for outcome, as seen in our analysis as well as in previous studies [5, 6, 14,15,16]. Early relapse is thought to mainly represent an aggressive nature of the leukemia with low sensitivity to both the conditioning and the allogeneic immune reaction [17]. However, due to the limited time for recovery from the physical and mental toxicities of alloSCT1, the need for salvage therapy shortly after alloSCT1 might also increase toxicity, decrease patients’ general conditions and hence diminish the resilience to another transplant, as well as their motivation to undergo the procedure for a second time. Therefore, prolongation of the remission after alloSCT1 is of benefit also for those finally developing relapse. More frequent use of maintenance therapy in recent years might be an explanation for the prolonged remission observed in the later period of our study, although we cannot prove this from our data, since the treatment applied between alloSCT1 and 2 is not covered by the EBMT registry. Nevertheless, both targeted therapies [18,19,20], unspecific pharmaceutical approaches [21,22,23,24] and prophylactic or preemptive donor lymphocyte infusion [25,26,27] have been increasingly used for maintenance post-transplant after showing their potential to reduce the relapse rate and lengthen remission duration after alloSCT.
Second, our data as well as observations from other studies [14, 17, 28] including a recent meta-analysis [16], showed that initial disease control and, at best, achieving CR after post-transplant relapse is a major factor for final success of alloSCT2. Therefore, timely and effective medical treatment shortly after diagnosis of post-transplant relapse is mandatory, which, however, must avoid disproportionate side effects, to which patients in this situation are highly sensitive. Carefully adapting treatment toxicity to the individual patient is of particular relevance, given that a better KPS was another factor independently associated with outcome from alloSCT2 in the present study. As a limitation of our analysis, we did not have enough details on therapies applied between relapse and alloSCT2 in the registry to draw meaningful conclusions concerning the optimized strategy. Fortunately, modern antileukemic therapies offer a better balance between efficacy and toxicity than classical chemotherapy. In particular, the use of hypomethylating agents±venetoclax [29,30,31] or application of targeted therapies [32,33,34,35,36,37] represent promising options in that sense.
Third, since despite recent achievements, long-term remission is a rarity after conventional treatment for post-transplant relapse, the identification of a donor for an eventual alloSCT2 should be part of the management immediately after relapse, to allow alloSCT2 at the optimal time point, defined by best disease response and a high KPS. Although intuitive, change to an alternative donor for alloSCT2 does not seem to be mandatory, since a variety of studies including our own could not demonstrate an advantage (although no disadvantage either) after alloSCT2 from a different donor [14,15,16]. Due to missing information in one third of patients, donor switch could not be included in the multivariate analysis in our study. However, an exploratory analysis of 1006 patients revealed no hint of a significant influence, suggesting that the observed trend to more frequent donor change in recent years did not decisively contribute to improved outcome over time. In contrast, an improved outcome was observed among patients receiving alloSCT2 from a MSD, who in the vast majority did not undergo donor change. This indirectly might suggest that at least after alloSCT1 from a MSD, using the same donor for alloSCT2 remains an option. Nevertheless, beyond unavailability of the original donor, switching to a different one might be reasonable under certain conditions. For instance, the situation of HLA loss by the malignant blasts, particularly after haploidentical or mismatched unrelated alloSCT1 [38], justifies change to an alternative donor [39], although unequivocal clinical evidence is missing. Furthermore, as described above, optimized timing for alloSCT2 to the point of maximum control of the leukemia and the patient being in good clinical condition, is mandatory. In that sense, the most easily available donor, including mismatched relatives, might be preferable, given that the general feasibility of alloSCT2 from a haploidentical donor has been demonstrated [40, 41].
Forth, according to our data, a MAC regimen should be considered for alloSCT2 in all patients who might tolerate it. Similar findings have been described by others [28] and are supported by data obtained both after alloSCT1 [42] and in a prospective trial in alloSCT2 [43]. Recent developments in the field of conditioning have identified myeloablative regimens with reduced toxicity and hence improved outcome [44]. Beyond, although not validated for alloSCT2, the EBMT transplant conditioning intensity (TCI) score [12] may support the selection of less toxic, but still myeloablative regimen also for alloSCT2. Unfortunately, the huge variety of conditioning regimens applied in our cohort precluded a reasonable comparison and hence the definition of an optimized regimen.
Finally, an optimized GVHD prophylaxis might improve the results after alloSCT2. In our analysis, the use of in-vivo TCD was associated with a markedly decreased incidence of both acute and chronic GVHD, leading to a reduction of NRM. Although this did not translate into improved survival, using in-vivo TCD might be preferable in alloSCT2, rather than omitting it in the interest of an eventually stronger graft-versus-leukemia effect. The use of PTCY might be an alternative to in-vivo TCD, even in the matched donor setting. However, since this strategy has only been introduced very recently, data are not mature enough to draw any conclusion on its use in the setting of alloSCT2.
Apart from the retrospective nature, several limitations of our study need to be considered. As discussed above, we lack sufficient data on maintenance therapy after alloSCT1 and initial disease control strategies after post-transplant relapse. Hence, the higher percentage of patients receiving alloSCT2 in CR and good clinical status in the later periods might be a consequence of the more frequent use of modern targeted therapies. Beyond, we missed information on the quality of remission before alloSCT2, since minimal residual disease (MRD) status was reported only recently and therefore in about 20% of our patients. Data from alloSCT1 have shown an advantage for patients transplanted in MRD-negative CR [45, 46], although this seems not to be true for all molecular markers used for MRD detection [47]. Hence the role of MRD negativity before alloSCT2 remains to be elucidated. As discussed above, the influence of PTCY as GVHD prophylaxis could not be evaluated either in the multivariable analysis because it was mainly used in the later period. Last, the contribution of maintenance treatment after alloSCT2 to overall outcome cannot be estimated since these data have not been captured in the registry for many years.
In summary, according to this large registry analysis on >1500 patients, outcome after alloSCT2 has continuously improved over the last two decades, despite increasing patient age. In particular, decreased RI did not come at the cost of increased toxicity. This might be a result of better disease control and improved performance score at time of alloSCT2, as well as an increasing use of MAC, in-vivo TCD and eventually PTCY in alloSCT2 over time. These data encourage to perform alloSCT2 in relapsed AML after first transplant. Factors that were associated with improved outcome may help to optimize the procedure, which at present represents the most effective therapy in this setting. In detail, maintenance strategies after alloSCT1, early identification of a donor for alloSCT2, application of less toxic strategies for initial disease control, timely implementation of alloSCT2 when the best response status has been achieved, as well as the use of intensive, but toxicity-reduced conditioning regimen and in-vivo T-cell depletion for GVHD prophylaxis are treatment elements that might contribute to improved results.
Nevertheless, with still half of the patients relapsing again and only one third being cured by alloSCT2, there is still a lot of room for improvement. Preclinical research has largely increased our understanding of the biology of post-transplant relapse [48, 49]. It is hoped that specific and individualized treatment will be based on this knowledge in the future to improve outcomes after relapse after alloSCT.
Data availability
The data were allocated by the EBMT registry in Paris. The datasets are available upon data-specific request.
References
Arfons LM, Tomblyn M, Rocha V, Lazarus HM. Second hematopoietic stem cell transplantation in myeloid malignancies. Curr Opin Hematol. 2009;16:112–23.
de Lima M, Porter DL, Battiwalla M, Bishop MR, Giralt SA, Hardy NM, et al. Proceedings from the National Cancer Institute’s Second international workshop on the biology, prevention, and treatment of relapse after hematopoietic stem cell transplantation: Part III. Prevention and treatment of relapse after allogeneic transplantation. Biol Blood Marrow Transpl. 2014;20:4–13.
Bejanyan N, Weisdorf DJ, Logan BR, Wang H-L, Devine SM, de Lima M, et al. Survival of patients with acute myeloid leukemia relapsing after allogeneic hematopoietic cell transplantation: a center for international blood and marrow transplant research study. Biol Blood Marrow Transpl. 2015;21:454–9.
Schmid C, De Wreede LC, Van Biezen A, Finke J, Ehninger G, Ganser A, et al. Outcome after relapse of myelodysplastic syndrome and secondary acute myeloid leukemia following allogeneic stem cell transplantation: a retrospective registry analysis on 698 patients by the Chronic Malignancies Working Party of the European Society of Blood and Marrow Transplantation. Haematologica 2018;103:237–45.
Kharfan-Dabaja MA, Labopin M, Polge E, Nishihori T, Bazarbachi A, Finke J, et al. Association of second allogeneic hematopoietic cell transplant vs donor lymphocyte infusion with overall survival in patients with acute myeloid leukemia relapse. JAMA Oncol. 2018;4:1245.
Bazarbachi A, Schmid C, Labopin M, Beelen D, Wolfgang Blau I, Potter V, et al. Evaluation of trends and prognosis over time in patients with AML relapsing after allogeneic hematopoietic cell transplant reveals improved survival for young patients in recent years. Clin Cancer Res. 2020;26:6475–82.
Döhner H, Wei AH, Appelbaum FR, Craddock C, DiNardo CD, Dombret H, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood 2022;140:1345–77.
EBMT. Med-AB Forms Manual. 2019.https://www.ebmt.org/sites/default/files/2019-11/MED-ABFormsManual.pdf. Accessed 28 Feb 2024.
Ruggeri A, Labopin M, Ciceri F, Mohty M, Nagler A. Definition of GvHD-free, relapse-free survival for registry-based studies: an ALWP–EBMT analysis on patients with AML in remission. Bone Marrow Transpl. 2016;51:610–1.
Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017. https://doi.org/10.1182/blood-2016-08-733196.
Bertoli S, Tavitian S, Huynh A, Borel C, Guenounou S, Luquet I, et al. Improved outcome for AML patients over the years 2000–2014. Blood Cancer J. 2017;7:635.
Spyridonidis A, Labopin M, Savani BN, Niittyvuopio R, Blaise D, Craddock C, et al. Redefining and measuring transplant conditioning intensity in current era: a study in acute myeloid leukemia patients. Bone Marrow Transpl. 2020;55:1114–25.
Kassim AA, Savani BN. Hematopoietic stem cell transplantation for acute myeloid leukemia: a review. Hematol Oncol Stem Cell Ther. 2017;10:245–51.
Christopeit M, Kuss O, Finke J, Bacher U, Beelen DW, Bornhäuser M, et al. Second allograft for hematologic relapse of acute leukemia after first allogeneic stem-cell transplantation from related and unrelated donors: the role of donor change. J Clin Oncol. 2013;31:3259–71.
Yanada M, Konuma T, Yamasaki S, Kondo T, Fukuda T, Shingai N, et al. Relapse of acute myeloid leukemia after allogeneic hematopoietic cell transplantation: clinical features and outcomes. Bone Marrow Transpl. 2021;56:1126–33.
Kharfan-Dabaja MA, Reljic T, Yassine F, Nishihori T, Kumar A, Tawk MM, et al. Efficacy of a second allogeneic hematopoietic cell transplant in relapsed acute myeloid. Leukemia: Results a Syst Rev Meta-Anal Transpl Cell Ther. 2022;28:767.e1–767.e11.
Yerushalmi Y, Shem-Tov N, Danylesko I, Canaani J, Avigdor A, Yerushalmi R, et al. Second hematopoietic stem cell transplantation as salvage therapy for relapsed acute myeloid leukemia/myelodysplastic syndromes after a first transplantation. Haematologica 2022;108:1782–92.
Perl AE, Larson RA, Podoltsev NA, Strickland S, Wang ES, Atallah E, et al. Outcomes in patients with FLT3-mutated relapsed/ refractory acute myelogenous leukemia who underwent transplantation in the Phase 3 ADMIRAL trial of gilteritinib versus salvage chemotherapy. Transpl Cell Ther. 2023;29:265.e1–265.e10.
Burchert A, Bug G, Fritz LV, Finke J, Stelljes M, Röllig C, et al. Sorafenib maintenance after allogeneic hematopoietic stem cell transplantation for acute myeloid leukemia with FLT3 –internal tandem duplication mutation (SORMAIN). J Clin Oncol. 2020;38:2993–3002.
Bazarbachi A, Bug G, Baron F, Brissot E, Ciceri F, Dalle IA, et al. Clinical practice recommendation on hematopoietic stem cell transplantation for acute myeloid leukemia patients with FLT3 -internal tandem duplication: a position statement from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Haematologica 2020;105:1507–16.
Bug G, Burchert A, Wagner E-M, Kröger N, Berg T, Güller S, et al. Phase I/II study of the deacetylase inhibitor panobinostat after allogeneic stem cell transplantation in patients with high-risk MDS or AML (PANOBEST trial). Leukemia 2017;31:2523–5.
De Lima M, Giralt S, Thall PF, De Padua Silva L, Jones RB, Komanduri K, et al. Maintenance therapy with low‐dose azacitidine after allogeneic hematopoietic stem cell transplantation for recurrent acute myelogenous leukemia or myelodysplastic syndrome: A dose and schedule finding study. Cancer 2010;116:5420–31.
Platzbecker U, Wermke M, Radke J, Oelschlaegel U, Seltmann F, Kiani A, et al. Azacitidine for treatment of imminent relapse in MDS or AML patients after allogeneic HSCT: results of the RELAZA trial. Leukemia 2012;26:381–9.
Bewersdorf JP, Allen C, Mirza A-S, Grimshaw AA, Giri S, Podoltsev NA, et al. Hypomethylating agents and FLT3 inhibitors as maintenance treatment for acute myeloid leukemia and myelodysplastic syndrome after allogeneic hematopoietic stem. Cell Transplant–A Syst Rev Meta-Anal Transpl Cell Ther. 2021;27:997.e1–997.e11.
Schmid C, Labopin M, Schaap N, Veelken H, Schleuning M, Stadler M et al. Prophylactic donor lymphocyte infusion after allogeneic stem cell transplantation in acute leukaemia – a matched pair analysis by the Acute Leukaemia Working Party of EBMT. Br J Haematol. 2019. https://doi.org/10.1111/bjh.15691.
Schmid C, Labopin M, Schaap N, Veelken H, Brecht A, Stadler M, et al. Long-term results and GvHD after prophylactic and preemptive donor lymphocyte infusion after allogeneic stem cell transplantation for acute leukemia. Bone Marrow Transpl. 2022;57:215–23.
Dholaria B, Savani BN, Labopin M, Luznik L, Ruggeri A, Mielke S, et al. Clinical applications of donor lymphocyte infusion from an HLA-haploidentical donor: consensus recommendations from the Acute Leukemia Working Party of the EBMT. Haematologica 2020;105:47–58.
Nagler A, Peczynski C, Dholaria B, Labopin M, Valerius T, Dreger P, et al. Impact of conditioning regimen intensity on outcomes of second allogeneic hematopoietic cell transplantation for secondary acute myelogenous leukemia. Bone Marrow Transpl. 2022;57:1116–23.
Rautenberg C, Bergmann A, Germing U, Fischermanns C, Pechtel S, Kaivers J, et al. Prediction of response and survival following treatment with azacitidine for relapse of acute myeloid leukemia and myelodysplastic syndromes after allogeneic hematopoietic stem cell transplantation. Cancers 2020;12:2255.
DiNardo CD, Rausch CR, Benton C, Kadia T, Jain N, Pemmaraju N, et al. Clinical experience with the BCL 2‐inhibitor venetoclax in combination therapy for relapsed and refractory acute myeloid leukemia and related myeloid malignancies. Am J Hematol. 2018;93:401–7.
Schuler E, Wagner-Drouet E-M, Ajib S, Bug G, Crysandt M, Dressler S, et al. Treatment of myeloid malignancies relapsing after allogeneic hematopoietic stem cell transplantation with venetoclax and hypomethylating agents—a retrospective multicenter analysis on behalf of the German Cooperative Transplant Study Group. Ann Hematol. 2021;100:959–68.
Metzelder SK, Schroeder T, Finck A, Scholl S, Fey M, Götze K, et al. High activity of sorafenib in FLT3-ITD-positive acute myeloid leukemia synergizes with allo-immune effects to induce sustained responses. Leukemia 2012;26:2353–9.
Rautenberg C, Nachtkamp K, Dienst A, Schmidt PV, Heyn C, Kondakci M, et al. Sorafenib and azacitidine as salvage therapy for relapse of FLT 3‐ ITD mutated AML after allo‐ SCT. Eur J Haematol. 2017;98:348–54.
Davids MS, Kim HT, Bachireddy P, Costello C, Liguori R, Savell A, et al. Ipilimumab for Patients with Relapse after Allogeneic Transplantation. N. Engl J Med. 2016;375:143–53.
Stein EM, DiNardo CD, Fathi AT, Pollyea DA, Stone RM, Altman JK, et al. Molecular remission and response patterns in patients with mutant-IDH2 acute myeloid leukemia treated with enasidenib. Blood 2019;133:676–87.
DiNardo CD, Stein EM, De Botton S, Roboz GJ, Altman JK, Mims AS, et al. Durable Remissions with Ivosidenib in IDH1 -Mutated Relapsed or Refractory AML. N. Engl J Med. 2018;378:2386–98.
Daver NG, Dail M, Garcia JS, Jonas BA, Yee KWL, Kelly KR, et al. Venetoclax and idasanutlin in relapsed/refractory AML: a nonrandomized, open-label phase 1b trial. Blood 2023;141:1265–76.
Vago L, Perna SK, Zanussi M, Mazzi B, Barlassina C, Stanghellini MTL, et al. Loss of Mismatched HLA in Leukemia after Stem-Cell Transplantation. N. Engl J Med. 2009;361:478–88.
Crucitti L, Crocchiolo R, Toffalori C, Mazzi B, Greco R, Signori A, et al. Incidence, risk factors and clinical outcome of leukemia relapses with loss of the mismatched HLA after partially incompatible hematopoietic stem cell transplantation. Leukemia 2015;29:1143–52.
Tischer J, Engel N, Fritsch S, Prevalsek D, Hubmann M, Schulz C, et al. Second haematopoietic SCT using HLA-haploidentical donors in patients with relapse of acute leukaemia after a first allogeneic transplantation. Bone Marrow Transpl. 2014;49:895–901.
Filippini Velázquez G, Labopin M, Tischer J, Raiola AM, Angelucci E, Kulagin AD, et al. Second haploidentical stem cell transplantation (HAPLO-SCT2) after relapse from a first HAPLO-SCT in acute leukaemia—a study on behalf of the Acute Leukaemia Working Party (ALWP) of the European Society for Blood and Marrow Transplantation (EBMT). Bone Marrow Transpl. 2023;58:907–15.
Sengsayadeth S, Gatwood KS, Boumendil A, Labopin M, Finke J, Ganser A, et al. Conditioning intensity in secondary AML with prior myelodysplastic syndrome/myeloproliferative disorders: an EBMT ALWP study. Blood Adv. 2018. https://doi.org/10.1182/bloodadvances.2018019976.
Finke J, Schmoor C, Stelljes M, Burchert A, Dreger P, Hegenbart U, et al. Thiotepa–fludarabine–treosulfan conditioning for 2nd allogeneic HCT from an alternative unrelated donor for patients with AML: a prospective multicenter phase II trial. Bone Marrow Transpl. 2022;57:1664–70.
Beelen DW, Stelljes M, Reményi P, Wagner‐Drouet E, Dreger P, Bethge W, et al. Treosulfan compared with reduced‐intensity busulfan improves allogeneic hematopoietic cell transplantation outcomes of older acute myeloid leukemia and myelodysplastic syndrome patients: final analysis of a prospective randomized trial. Am J Hematol. 2022;97:1023–34.
Freeman SD, Hills RK, Virgo P, Khan N, Couzens S, Dillon R, et al. Measurable residual disease at induction redefines partial response in acute myeloid leukemia and stratifies outcomes in patients at standard risk without NPM1 mutations. J Clin Oncol. 2018;36:1486–97.
Thol F, Gabdoulline R, Liebich A, Klement P, Schiller J, Kandziora C, et al. Measurable residual disease monitoring by NGS before allogeneic hematopoietic cell transplantation in AML. Blood 2018;132:1703–13.
Jongen-Lavrencic M, Grob T, Hanekamp D, Kavelaars FG, Al Hinai A, Zeilemaker A, et al. Molecular minimal residual disease in acute myeloid leukemia. N Engl J Med. 2018;378:1189–99.
Zeiser R, Vago L. Mechanisms of immune escape after allogeneic hematopoietic cell transplantation. Blood 2019;133:1290–7.
Sauerer T, Velázquez GF, Schmid C. Relapse of acute myeloid leukemia after allogeneic stem cell transplantation: immune escape mechanisms and current implications for therapy. Mol Cancer. 2023;22:180.
Acknowledgements
The outstanding contribution of all EBMT centers whose patients could be included in this analysis is highly appreciated, as the excellent work done by the EBMT data managers.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
AKS analyzed and interpreted data, and drafted the manuscript, MN and JEG performed statistical analysis, AB, JF, NK, MB, MS, FS, JT, TS, PD, IWB, BS, and SG contributed to data collection and revised the manuscript, JE, AN, FC and MM contributed to collecting data, concepted and designed study, revised manuscript, CS contributed to collecting data, concepted and designed study, analyzed and interpreted data and drafted manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schmälter, AK., Ngoya, M., Galimard, JE. et al. Continuously improving outcome over time after second allogeneic stem cell transplantation in relapsed acute myeloid leukemia: an EBMT registry analysis of 1540 patients. Blood Cancer J. 14, 76 (2024). https://doi.org/10.1038/s41408-024-01060-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41408-024-01060-4