Abstract
Study design
Review of the literature and semi-structured interviews.
Objective
To explore the possible use of topical analgesics for the treatment of neuropathic pain (NP) in spinal cord injury (SCI).
Setting
Institute for Neuropathic Pain, Soest, The Netherlands.
Methods
A review was performed of studies on topical analgesics for SCI-related NP published up to May 2019. In addition, eight persons with SCI-related NP who were treated with topical analgesics were interviewed in a semi-structured interview on their experience with topical analgesics.
Results
Seven studies (five case reports and two case series) were found that evaluated the use of topical analgesics for SCI-related NP. None of the studies used a control treatment. Topical analgesics included baclofen, ketamine, lidocaine, capsaicin, and isosorbide dinitrate. All studies reported a decrease in NP over time. Persons interviewed were 49–72 years of age and all but one had an incomplete SCI. They used topical agents containing phenytoin, amitriptyline, baclofen, ketamine or loperamide. All showed a decrease in pain of at least 3 points on the 11-point numeric rating scale during this treatment.
Discussion/conclusions
Evidence on the use of topical analgesics in SCI is scarce. Case reports, case series and interviews suggest that the use of topical analgesics can be beneficial in treating SCI-related NP. Placebo-controlled studies are required to investigate the effect of topical analgesics on SCI-related NP.
Similar content being viewed by others
Introduction
Neuropathic pain (NP) is a common condition among people with spinal cord injury (SCI). The prevalence of NP in SCI is estimated at 53% [1]. Chronic pain in SCI is known to have a negative effect on quality of life [2,3,4]. NP has been described as the most severe type of pain in SCI [5]. Health care professionals and people with SCI point out NP as one of the most important challenges in SCI [6, 7].
Systemic anticonvulsants (pregabalin and gabapentin) are the most evidence-based pharmacotherapies in treating SCI-related NP to date. Other common pharmacological treatment options include antidepressants, opioids, and cannabinoids [8, 9]. However, the effectiveness of pharmacological treatments is suboptimal [8, 9]. Also, their long-term use is associated with the risk of developing adverse events on the central nervous system, like somnolence, fatigue, and drowsiness [9, 10]. Therefore, many people with SCI use non-pharmacological interventions, such as physical therapy, psychological therapy, or homeopathy as main or supplementary treatment of NP. However, the use of non-pharmacological treatments for SCI-related NP is not supported by scientific evidence and, consequently, current guidelines on SCI-related NP rarely include such treatments [11, 12].
The use of topical analgesics has been studied in peripheral NP. A recent single-blind placebo-controlled trial showed a positive effect of phenytoin 10% cream compared to placebo in persons affected with several types of peripheral neuropathies [13]. It is hypothesized that changes in peripheral nerves influence SCI-related NP [14, 15]. Also, clinical practice does show positive results of the use of topical analgesics for SCI-related NP [16, 17]. Therefore, the aim of this study is to explore the possible use of topical analgesics as a treatment for SCI-related NP by a literature review and interviews with persons with SCI treated with topical analgesics for NP.
Methods
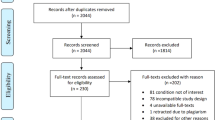
First, a literature search was performed in Pubmed, Embase, and Web of Science using the following keywords: “spinal cord injury”, “neuropathic pain”, and “topical”. Titles and abstracts were screened for relevance to the subject. The participant’s characteristics, location of pain and type of treatment were extracted from the included studies. As it was expected that the search would produce only few and low-quality studies, a systematic review approach with meta-analysis was considered not applicable.
In addition to the literature search, a convenience sample of people from the Institute for Neuropathic Pain in the Netherlands were invited for a telephone interview. The Institute for Neuropathic Pain is an outpatient clinic specialized in treating persons with NP, not associated to a University Medical Center. Inclusion criteria were: having SCI, suffering from NP and being treated with topical analgesics at the time of the interview or in the past. The topical analgesic containing an active pharmaceutical ingredient (API) was administered at the outpatient clinic. If the pain decreased in 30 min, the treatment was considered effective and the treatment was prescribed. A structured interview scheme was developed including questions on demographics, level and etiology of SCI, years affected, use of topical analgesics and pain score on the 11-point numeric rating scale (NRS) before and after application of topical analgesics. Ratings of pain severity before and after application were asked on the most recent time the participants used the treatment. The interview questions were asked for the most effective type of API.
Results
Literature review
Five case reports and two case series (two and five participants) describe the use of topical analgesics in SCI-related NP [16,17,18,19,20,21,22]. Characteristics and results of the case reports are shown in Table 1 and those of the case series are shown in Table 2. All studies reported positive effects of topical analgesics on the severity of NP. However, none of the studies compared the use of topical analgesics to placebo or alternative treatment. All but one study and one case series used lidocaine and capsaicin. One study and one case series described a novel topical analgesic with 5% baclofen cream (case report) and 10% ketamine cream (case series).
Persons interviewed
Eight persons (four men) treated at the Institute for Neuropathic Pain were interviewed on their current or past experience of topical analgesics. Their age ranged from 49 to 71 years. Characteristics and their experienced pain relief are shown in Tables 3 and 4. All but one person were suffering from incomplete SCI. Six persons were experiencing below level NP and two were experiencing at level NP. Treatment included topical analgesics with the following APIs: phenytoin (anticonvulsant), baclofen (antispastic), ketamine (anesthetic), amitriptyline (antidepressant) or loperamide (opioid). Persons were informed to apply the topical analgesic when needed with a maximum of four times a day. Different topical APIs were tested on each person at the outpatient clinic. The next topical API was used if the previous did not suffice in effect on pain. Time to effect after administration of the topical analgesic ranged from instant effect to 30 min. Topical analgesics were used two times a day on average. All but one participant (participant 2) were current users of topical analgesics. Participant 2 stopped the treatment 1 year ago at the time of the interview, due to decreasing effect. All mentioned a decrease of at least 3 points on the NRS. All mentioned they were satisfied with the treatment and would recommend it to a friend or had recommended it already. One person (participant 2) reported getting a rash from previously used topical phenytoin 10%.
Discussion
Little is known about the use of topical analgesics in SCI-related NP. Solely case reports and case series on this treatment modality could be found in the literature. Case reports, case series and interviews with people with SCI treated at the Institute for Neuropathic Pain, describe a positive effect of topical analgesics on SCI-related NP.
Several theories on the mechanism of SCI-related NP exist. Its etiology is thought to be multifactorial. Altered firing of thalamic and cortical neurons, increased responsiveness to peripheral stimulation from the dorsal root and peripheral changes itself are believed to play a role in the pathophysiology of NP [14]. Current treatment of SCI-related NP is based on the central mechanism, as oral anticonvulsants and antidepressants are the most studied treatments [9]. To understand the rationale behind the use of topical analgesics, it is important to consider the changes and influence of peripheral nerves in SCI-related NP. SCI Rat models have shown the presence of peripheral sensitization in NP [15]. Sensitized nociceptors were found in the forelimbs of rats suffering from SCI. In vitro nociceptor responses of SCI rats showed a lowered mechanical and thermal threshold and increased spontaneous activity compared to naive and sham rats 35 days post injury. The increased background activity caused by this peripheral sensitization could explain the spontaneous pain in persons with SCI. Furthermore, there are reports that the degree of peripheral inflammation and neutrophil accumulation are modulated by the central nervous system [15, 23]. Low-grade peripheral inflammation is believed to be one of the causes of SCI-related NP shown in animal models [15]. The pathophysiology of this phenomenon has not been described in human studies.
The studies described in our results section suggest that peripheral influences play a role in SCI-related NP in humans. As described by Wasner, the effect of the lidocaine patch in a person with SCI was similar to the effect of a lidocaine patch in people with local peripheral NP syndromes [19]. NP in peripheral neuropathies is caused by an ectopic discharge by the damaged afferent neurons. In addition, intact afferents that share the territory innervated also show a spontaneous discharge of action potentials. These abnormalities found in intact afferents are likely to account for the fact that topical treatments are effective [24]. This phenomenon has also been observed in other types of central NP. In poststroke pain a pilot study of eight people suffering from a stroke showed a decrease in NP after a peripheral nerve block using lidocaine 2% was performed, showing that peripheral nerve blockage can influence pain in damage to the central nervous system [25].
Whether completeness of injury is related to the mechanism of NP in SCI is not studied to date. From people interviewed, all but one suffered from incomplete SCI. The pain described in the person interviewed suffering from a complete lesion was at the level of injury. Partial innervation at this level may play a role in the occurrence of NP. All but one of the case series describe the effect in persons with incomplete lesions. The case series on topical ketamine describes three out of five persons with complete SCI [22]. Pain phenotyping has been considered of importance in the treatment of NP [26, 27]. Adjusting treatment to the specific pain related symptoms can be a promising way to alter the treatment specific to the pain mechanism [28]. Completeness of injury can be an important parameter in different NP phenotypes. Further studies on the mechanism on NP and phenotypes in NP in SCI are needed to consider the role of completeness of injury in SCI-related NP.
None of the studies described compare the effect of the topical analgesics to placebo treatment. Placebo-effect can play an important role in topical analgesic treatment. A systematic review on knee-osteoarthritis pain shows an increased effect of topical placebo compared to oral placebo (Standardized Mean Difference: 0.20, 95% credible interval: 0.02–0.38) [29]. In addition, topical treatment with placebo can remain to have an analgesic effect, even after it has been revealed to the participant that it has no chemical compound [30]. The act of treating the skin, even without the chemical compound, seems to have a significant effect on pain in osteoarthritic pain. In contrast to this, another meta-analysis found that individuals with SCI and NP have no significant placebo response in clinical trials testing pharmacologic interventions lasting 4 weeks or longer [31]. Because no placebo-controlled studies on topical treatment in SCI exist, this meta-analysis did not include studies using topical treatments.
Using topical analgesics to treat NP has a definite advantage compared to systemic treatments when considering side effects [8, 10]. As shown in studies on peripheral NP, use of local treatments show little or no side effects, where systemic treatments with anticonvulsants and antidepressants cause considerably more [32]. This is also confirmed by reported side effects in the persons interviewed. Only one person reported a local side effect.
As described in the interviews and shown in the study on peripheral neuropathies, topical analgesics based on anticonvulsants, antidepressants, and antispastics might influence NP [13]. The effect of the topical analgesics compared to placebo can be evaluated in a double-blind placebo-controlled cross-over trial. In such a trial the participants will use both the cream containing an API and the placebo cream sequentially. Decrease in NP will be described during these treatments. This N-of-1 response test has been used in clinical practice to establish a personalized treatment [33, 34].
Limitations of this study include a low number of people interviewed and that the effect of topical analgesics to NP was asked as part of the interview and not longitudinally during treatment. Therefore, the NRS before and after applying the topical analgesic rely on a person’s memory of the NP. This might have given another result if the person was asked longitudinally during their treatment. In addition, someone could be more driven to consent to an interview, if the treatment was effective. A misrepresentation of the treatment effect should therefore be taken into account.
Conclusion
The body of evidence on topical analgesics on SCI-related NP is scarce. Case reports in the literature and interviews with persons suggest a beneficial effect of topical analgesics in SCI-related NP. Placebo-controlled studies on topical analgesics in SCI-related NP are required to confirm its pain reducing effect.
Data availability
The data can be provided by the authors on request.
References
Van Gorp S, Kessels AG, Joosten EA, Van Kleef M, Patijn J. Pain prevalence and its determinants after spinal cord injury: a systematic review. Eur J Pain. 2015;19:5–14.
Finnerup NB. Neuropathic pain and spasticity: intricate consequences of spinal cord injury. Spinal Cord. 2017;55:1046–50.
Sauri J, Chamarro A, Gilabert A, Gifre M, Rodriguez N, Lopez-Blazquez R, et al. Depression in individuals with traumatic and nontraumatic spinal cord injury living in the community. Arch Phys Med Rehabil. 2017;98:1165–73.
Burke D, Lennon O, Fullen BM. Quality of life after spinal cord injury: the impact of pain. Eur J Pain. 2018;22:1662–72.
Siddall PJ, McClelland JM, Rutkowski SB, Cousins MJ. A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain. 2003;103:249–57.
Widerstrom-Noga EG, Felipe-Cuervo E, Broton JG, Duncan RC, Yezierski RP. Perceived difficulty in dealing with consequences of spinal cord injury. Arch Phys Med Rehabil. 1999;80:580–6.
Van Middendorp JJ, Allison HC, Ahuja S, Bracher D, Dyson C, Fairbank J, et al. Top ten research priorities for spinal cord injury: the methodology and results of a British priority setting partnership. Spinal Cord. 2016;54:341–6.
Teasell RW, Mehta S, Aubut J-AL, Foulon B, Wolfe DL, Hsieh JTC, et al. A systematic review of pharmacologic treatments of pain after spinal cord injury. Arch Phys Med Rehabil. 2010;91:816–31.
Mehta S, McIntyre A, Janzen S, Loh E, Teasell R. Systematic review of pharmacologic treatments of pain after spinal cord injury: an update. Arch Phys Med Rehabil. 2016;97:1381–.e1.
Hatch MN, Cushing TR, Carlson GD, Chang EY. Neuropathic pain and SCI: identification and treatment strategies in the 21st century. J Neurol Sci. 2018;384:75–83.
Boldt I, Eriks-Hoogland I, Brinkhof MWG, de Bie R, Joggi D, von Elm E. Non-pharmacological interventions for chronic pain in people with spinal cord injury. Cochrane database Syst Rev. 2014;11:CD009177.
Guy SD, Mehta S, Casalino A, Côté I, Kras-Dupuis A, Moulin DE, et al. The CanPain SCI Clinical Practice Guidelines for rehabilitation management of neuropathic pain after spinal cord: recommendations for treatment. Spinal Cord. 2016;54:S14–23.
Kopsky DJ, Keppel Hesselink JM. Single-blind placebo-controlled response test with phenytoin 10% cream in neuropathic pain patients. Pharmaceuticals. 2018;11:122.
Siddall PJ. Management of neuropathic pain following spinal cord injury: now and in the future. Spinal Cord. 2009;47:352–9.
Carlton SM, Du J, Tan HY, Nesic O, Hargett GL, Bopp AC, et al. Peripheral and central sensitization in remote spinal cord regions contribute to central neuropathic pain after spinal cord injury. Pain 2009;147:265–76.
Kopsky DJ, Amelink GJ, Keppel Hesselink JM. Intractable neuropathic pain in spinal intramedullary cavernoma treated successfully with a novel combination cream. Pain Med. 2012;13:729–30.
Kopsky DJ, Keppel Hesselink JM, Casale R. Walking with neuropathic pain: paradoxical shift from burden to support? Case Rep Med. 2015;2015:764950.
Hans GH, Robert DN, Van Maldeghem KN. Treatment of an acute severe central neuropathic pain syndrome by topical application of lidocaine 5% patch: A case report. Spinal Cord. 2008;46:311–3.
Wasner G, Naleschinski D, Baron R. A role for peripheral afferents in the pathophysiology and treatment of at-level neuropathic pain in spinal cord injury? A case report. Pain. 2007;131:219–25.
Freo U, Ambrosio F, Furnari M, Ori C. Lidocaine 5% medicated plaster for spinal neuropathic pain. J Pain Palliat Care Pharmacother. 2016;30:111–3.
Trbovich M, Yang H. Capsaicin 8% patch for central and peripheral neuropathic pain of persons with incomplete spinal cord injury. Am J Phys Med Rehabil. 2015;94:e66–72.
Rabi J, Minori J, Abad H, Lee R. Spinal cord injury patients: an open label. Trial. 2016;20:517–20.
Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 2010;9:807–19.
Campbell JN, Meyer RA. Mechanisms of neuropathic pain. Neuron. 2006;52:77–92.
Haroutounian S, Ford AL, Frey K, Nikolajsen L, Finnerup NB, Neiner A, et al. How central is central poststroke pain? The role of afferent input in poststroke neuropathic pain: a prospective, open-label pilot study. Pain. 2018;159:1317–24.
Reimer M, Helfert SM, Baron R. Phenotyping neuropathic pain patients: implications for individual therapy and clinical trials. Curr Opin Support Palliat Care. 2014;8:124–9.
Vollert J, Magerl W, Baron R, Binder A, Enax-Krumova EK, Geisslingere G, et al. Pathophysiological mechanisms of neuropathic pain: comparison of sensory phenotypes in patients and human surrogate pain models. Pain. 2018;159:1090–102.
Forstenpointner J, Rehm S, Gierthmühlen J, Baron R. Stratification of neuropathic pain patients: the road to mechanism-based therapy? Curr Opin Anaesthesiol. 2018;31:562–8.
Bannuru RR, McAlindon TE, Sullivan MC, Wong JB, Kent DM, Schmid CH. Effectiveness and implications of alternative placebo treatments. Ann Intern Med. 2015;163:365.
Schafer SM, Colloca L, Wager TD. Conditioned placebo analgesia persists when subjects know they are receiving a placebo. J Pain. 2015;16:412–20.
Jutzeler CR, Warner FM, Cragg JJ, Haefeli J, Richards JS, Andresen SR, et al. Placebo response in neuropathic pain after spinal cord injury: a meta-analysis of individual participant data. J Pain Res. 2018;11:901–12.
Sommer C, Cruccu G. Topical treatment of peripheral neuropathic pain: applying the evidence. J Pain Symptom Manag. 2017;53:614–29.
Keppel Hesselink JM, Kopsky DJ, Bhaskar AK. Ethical justification of single-blind and double-blind placebo-controlled response tests in neuropathic pain and N-of-1 treatment paradigm in clinical settings. J Pain Res. 2019;12:345–52.
Kopsky DJ, Vrancken AFJE, Keppel Hesselink JM, van Eijk RPA, Notermans NC. Usefulness of a double-blind placebo-controlled response test to demonstrate rapid onset analgesia with phenytoin 10% cream in polyneuropathy. J Pain Res. 2020;13:877–82.
Acknowledgements
We are grateful for the people treated at the Institute for Neuropathic Pain for providing us with their information. We would also like to acknowledge O. Amjahdi for performing the interviews.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
DJK is holder of two patent applications: Topical phenytoin for use in the treatment of peripheral neuropathic pain (WO2018106107); and Topical pharmaceutical composition containing phenytoin and a (co-)analgesic for the treatment of chronic pain (WO2018106108).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Crul, T.C., Stolwijk-Swüste, J.M., Kopsky, D.J. et al. Neuropathic pain in spinal cord injury: topical analgesics as a possible treatment. Spinal Cord Ser Cases 6, 73 (2020). https://doi.org/10.1038/s41394-020-00321-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41394-020-00321-1