Abstract
Organ-specific metastasis is the primary cause of cancer patient death. The distant metastasis of tumor cells to specific organs depends on both the intrinsic characteristics of the tumor cells and extrinsic factors in their microenvironment. During an intermediate stage of metastasis, circulating tumor cells (CTCs) are released into the bloodstream from primary and metastatic tumors. CTCs harboring aggressive or metastatic features can extravasate to remote sites for continuous colonizing growth, leading to further lesions. In the past decade, numerous studies demonstrated that CTCs exhibited huge clinical value including predicting distant metastasis, assessing prognosis and monitoring treatment response et al. Furthermore, increasingly numerous experiments are dedicated to identifying the key molecules on or inside CTCs and exploring how they mediate CTC-related organ-specific metastasis. Based on the above molecules, more and more inhibitors are being developed to target CTCs and being utilized to completely clean CTCs, which should provide promising prospects to administer advanced tumor. Recently, the application of various nanomaterials and microfluidic technologies in CTCs enrichment technology has assisted to improve our deep insights into the phenotypic characteristics and biological functions of CTCs as a potential therapy target, which may pave the way for us to make practical clinical strategies. In the present review, we mainly focus on the role of CTCs being involved in targeted organ metastasis, especially the latest molecular mechanism research and clinical intervention strategies related to CTCs.
Similar content being viewed by others
Introduction
Metastasis is attracting increasing attention worldwide because it is a major cause of cancer-related mortality, with a high rate of over 90%.1 Statistical data reported by Patricia et al.2 states that the overall 5-year survival rates of patients with metastasis, especially patients with distant metastasis, were significantly lower than those of patients diagnosed with localized tumors. Metastasis is a complex systemic disease that is not only responsible for the decline or even total loss in function of target organs but also for the increases in the destructive power and impact brought by tumors in various organs, that is, more severe paraneoplastic syndromes.3 Moreover, it leaves “evil seeds” after treatment, resulting in a high risk of recurrence. Although current tumor treatments, including traditional surgery, chemoradiotherapy, immunotherapy and their flexible combinations, have obviously extended survival in cancer patients, the mortality rates of cancer patients with metastasis remain stagnant or are rising because of treatment failure caused by the inaccessibility of surgery, emerging drug resistance and dormancy of metastatic cancer cells.4,5 Accordingly, metastasis still poses a significant challenge, and exploring new strategies for the detection, prevention and effective treatment of metastasis is urgently needed.
Circulating tumor cells (CTCs) are tumor cells shed from the primary or metastatic foci into the blood or the lymphatic system.6 In 1869, Thomas Ashworth first reported the existence of CTCs.7 However, due to the limitations of the experimental conditions and techniques, it was not until 1976 that Nowell formally confirmed the definition of CTCs.8 With the advancement of molecular biology, immunolabeling and molecular biology technology, CTC isolation and enrichment technology has evolved from the initial method based on the physical properties of CTCs,9 to the immunomagnetic bead method that specifically binds to surface antigens (CK/EpCAM+, Nuclear+, CD45−) of CTCs,10 to the current chip technology.11 (Fig. 1) The development of CTC isolation technology has deepened the understanding of CTCs, including more comprehensive characterization of dynamically heterogeneous CTCs and more detailed exploration of CTC survival and distant metastasis mechanisms. In addition, the great application prospects of CTCs in early diagnosis,12,13 prognosis evaluation,14,15,16 precision treatment17,18 and drug efficacy19 have been ascertained. For example, CTCs have been officially included in the clinical guidelines for the diagnosis and treatment of breast cancer20 and prostate cancer.21
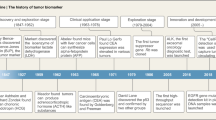
The timeline of progress in research related to the CTCs. In 1869, Thomas Ashworth first reported the tumor cells escaping from solid tumors and entering the blood. And in 1976 that Nowell formally confirmed the definition of CTCs. The first- and second-generation CTC isolation technologies are based on the physical properties and surface antigens of CTCs, respectively. A prospective study published in 2004 used Cell Search, an immunomagnetic bead method for positive binding of CTCs to the CTC surface antigen EpCAM, to isolate CTCs, which has become the first FDA-approved method for CTC isolation. With the progress of various experimental techniques, the isolation of CTCs has been developed to the third generation of chip technology. Nanomaterials have also been integrated to enhance the purity and efficiency of CTC isolation. High-throughput sequencing technology and single-cell sequencing technology are also used in the study of CTCs. In recent years, research on CTCs has indicated that they can serve as an indicator of tumor metastasis and may have potential for early tumor diagnosis. Furthermore, CTCs have shown promise as a positive prognostic factor for organ-specific metastasis. Notably, CTC was accepted as a tumor marker by the American Society of Clinical Oncology (ASCO) in 2007, and included in the 8th edition of AJCC breast cancer TNM staging system in 2017
In addition to the different biological characteristics that already exist in the primary cancer cells themselves, CTCs undergo a series of continuous evolutions, such as acquisition of mesenchymal or stem cell characteristics in order to adapt to the changing environment during the process from entering the blood to forming metastatic foci.22,23 Thus, CTCs, even parental populations, have obviously temporal and spatial heterogeneity in various aspects such as molecular phenotype, transcriptome and cytological characteristics. The “seed and soil” hypothesis proposed by Stephen Paget suggests that tumor metastasis occurs when CTCs, as one of the major sources of “seeds” of metastasis, can only colonize specific organ microenvironments with suitable growth environments which serve as the “soil” of metastasis, after being captured by capillaries through mechanical factors.24 This hypothesis reveals that CTC metastasis has obvious organotropism, and CTCs may have distinctive properties leading to the genesis of distant metastases. Moreover, to resist the environmental stress in the blood circulation and accelerate extravasation, some CTCs will aggregate with each other or recruit platelets, myeloid cells, and cancer-associated fibroblasts (CAFs) to form clusters, that greatly facilitates CTC dissemination and metastasis formation.25,26,27,28
Clinical data29,30,31,32 showed that the quantity and molecular phenotype of CTCs were closely associated with prognosis and resistance to therapy. The high detection rate of CTCs always portends a poor outcome for cancer patients. Their persistence throughout the treatment process has been proven to be critical for relapse and treatment failure in many types of cancer.33 Hence, clearing CTCs or blocking their metastatic process is of the utmost importance for patients with cancer who are at risk of metastasis or who have metastasized disease. However, CTCs, which are derived from subclones in the primary tumor with metastatic potential, are rarely able to colonize and successfully colonize the target organ by interacting with the specific microenvironment in the secondary loci.34,35 Thus, deeply understanding the biological characteristics of CTCs and the interaction between CTCs and the microenvironment in the circulation and target organs, which promotes organ-specific metastasis, will enable early prediction of metastasis and assist clinical treatment strategies.36
In this review, we systematically summarized the latest insights into the molecular mechanism of CTC-mediated target organ metastasis and its clinical application prospects, especially focusing on the mechanism of interaction between CTCs and target organs during their organ-specific metastasis and the potential clinical applications of targeted CTCs to suppress metastasis.
General metastasis mechanisms of CTCs
The formation of the premetastatic niche (PMN) and postmetastatic niche are key events in the process of tumors metastasizing to a specific target organ. The “seed and soil” hypothesis revealed that tumor cells colonize specific organ sites where the microenvironment is favorable for their chemotaxis and growth. Increasing evidence37 shows that primary tumors can release various factors, including tumor-derived secreted factors (TDSFs) and extracellular vesicles (EVs), into the blood circulation before CTCs arrive. These factors subsequently act on bone marrow-derived dendritic cells (BMDCs) or tissue-resident cells to reprogram the microenvironment of distant target organs to make it hospitable for CTC survival and colonization. The induction of primary tumors is purposeful rather than random, meaning that the CTCs have already confirmed their destination and prepared their “new home” before they leave the primary tumor. This supportive microenvironment induced by primary cancers or CTCs in the specific secondary organ site was defined as the PMN.38 Liu and Cao39 summarized six indispensable characteristics of PMNs that facilitate metastasis and make them suitable for colonization, namely, inflammation, immunosuppression, angiogenesis/vascular permeability, lymphangiogenesis, organotropism, and reprogramming.
Epithelial-mesenchymal transition (EMT) is a critical cellular program for malignant tumor progression and has been proposed as prerequisite for distant metastasis.40 Currently, the necessary of EMT for tumor metastasis is still controversial. In tumor mouse models and various autochthonous models based on EMT lineage tracing, suppression of one or more known EMT-inducing transcription factors (EMT-TFs) (notably Snail, Twist1, Zeb1, Slug, and Sox4) exerted no influence on tumor metastasis, and both early disseminated and metastatic tumor cells were found to be epithelial, that contrary to the conclusions of most studies about the necessity of EMT for tumor metastasis.41 However, given that different EMT-TFs have divergent effects on tumor metastasis in different cancers and EMT-TFs can compensate each other, suppression of one or more EMT-TFs does not completely block EMT.42 In addition, the abundance of tumor cells that undergo partial EMT distinctly affects cell enrichment and identification, which may lead to false results. Thus, ascertaining the necessity or dispensability of EMT still has much work ahead, such as improving understanding of partial EMT, general exploration of EMT mechanisms in various malignancies and developing more sensitive capture techniques. Triggered by extracellular molecules (such as TGF-β, hepatocyte growth factor and insulin-like growth factor) and tumor microenvironment stimuli (such as hypoxia), EMT transforms tumor cells from an epithelial state to a mesenchymal state and endows tumor cells with stronger invasion ability and higher metastasis potential, thereby inducing intravasation and shedding of tumor cells.6,43 EMT is directly connected to the gain of mesenchymal and stem-cell properties which enhanced self-renewal and tumor-initiating capabilities of cancer cells.44 A subtype of CTCs with unique stem cell-like markers called circulating cancer stem cells (CCSCs) which derive from the cancer stem cells (CSCs) intravasating into the bloodstream45 or CTCs acquiring stem cell characteristics in the process of EMT.46 CCSCs with the potential of self-renewal and proliferation have the distinct advantages of survival and motility for metastatic dissemination.47 Currently, CTCs with different stem-like phenotype, such as CD44+CD24−/low, ALDH1highCD24−/low, ALDH1+MRP+, CD133+ and CD45−ICAM-1+, have been identified in cancer patients and significantly correlate to high risk of metastases, shorter progression-free survival (PFS), malignant tumor stages and intrinsic drug resistance.48,49,50 Moreover, the EMT markers (such as CD47, MET, vimentin, fibronectin, Twist1) and stem-cell markers (such as CD44, ALDH1, CD133) are sometime co-expressed in CTCs of metastatic patient and the expression of the two types of markers is closely related, that further promotes successful establishment of distant metastases.47 The incidence of EMT is also linked to the metabolic condition of CTCs. CTCs can exhibit glucose metabolism reprogramming characterized by activated glycolysis and an increase in the pentose phosphate pathway.51 PGK1 and G6PD, the vital enzymes engaged in glycolysis and pentose phosphate pathway metabolism respectively, are critical indicators that mirror the metabolic features of CTCs.52 CTCs exhibiting high expression levels of PGK1 and G6PD possess active glucose metabolism, which is correlated with the incidence of EMT and an increased invasiveness.53 Additionally, the process of EMT may be accompanied by the up-regulation of asparagine synthetase (Asns) in CTC, leading to increased utilization of asparagine.54 These CTCs exhibit high invasiveness and metastatic potential. An interesting discovery is that the generation of CTCs is not due to continuous shedding from the primary tumor, but instead, they are more likely to be produced while the patient is in a state of rest.55 The CTCs that are generated during the resting phase of the patient are more invasive and more likely to metastasize than the CTCs that are generated when the patient is active. This phenomenon is related to key circadian hormones, such as melatonin, testosterone and glucocorticoids, which regulate the generation and invasiveness of CTCs in a time-dependent manner.55
After shedding from the primary tumor, the invasive and metastasis-competent tumor cell clones enter the bloodstream as individual CTCs or CTC clusters (Fig. 2). In circulation, various environmental pressures, including immune killing, anoikis, oxidative stress, blood fluid shear forces and oxygen/nutrient deprivation, contribute to apoptosis of most CTCs, with very few surviving to successful metastasis.56 Several studies have shown that CTC clusters had greater viability compared with individual CTCs when faced various death threats.57 Firstly, with structural properties of larger volume, CTC clusters run with slow speed and are apt to be marginalized and attached to the walls of blood vessels which would greatly reduce the residence time in the circulation and increase the chance of tumor cells colonizing distant organs.58 Secondly, the clustered structure provides a special hypoxic microenvironment for CTCs that is conducive to the survival.59 The CTC clusters are not simply a collection of CTCs, rather, they display apparent variances in the DNA methylation landscape of individual CTCs and CTC clusters. Compared with individual CTCs, CTC clusters exhibit hypomethylation of the binding sites of stemness - and proliferation-related TFs, such as OCT4, NANOG, SOX2, and SIN3A, and hypermethylation of Polycomb target genes.60 This difference in DNA methylation was found to correlate with increased proliferative potential and a poorer prognosis.60 Apart from CTCs themselves, CTC clusters sometimes gather immune cells, platelet and CAFs which are conducive to survival because of enhancing stemness, proliferation ability and immune evasion of CTCs.61 For example, regulatory T cells (Tregs) and neutrophils recruited by CTCs-released inflammatory factors can induce an immunosuppressive environment by direct or indirect ways, including disrupting CD8+ T cell activation and inhibiting the activity of natural killer cells (NKs). Simultaneously, CTCs can bind to platelet-derived RGS18 to upregulate the immune checkpoint molecule HLA-E, which enables CTCs to evade immune surveillance by NK cells.62 Furthermore, a study revealed that the bacteria residing in CTCs could modulate the host-cell actin network that would protect CTCs from fluid shear stress and enhance survival of CTCs in the bloodstream.63 Melanoma and epithelial cancer cells often migrated through lymphatics before invading into bloodstream, that can also promote CTC survival against ferroptosis and oxidative stress during subsequent dissemination through the bloodstream.64
The process by which CTCs detach from the primary tumor and survive in the circulation. a Tumor cells detach from the primary tumor. In the primary tumor, tumor cells will undergo epithelial-mesenchymal transition (EMT) triggered by some factors such as TGF-β, making them transition from the epithelial state to the mesenchymal state and acquire stronger invasion ability and higher metastatic potential. b Survival in the circulation. Tumor cells that escape from the primary tumor can enter the bloodstream as individual circulating tumor cells (CTCs) or as multicellular CTC clusters while the individual CTCs are likely to be exposed to physical stress or rapid natural killer (NK) cell clearance. Moreover, CTC clusters could bind to platelets or neutrophils. CTCs can activate platelets to secrete high levels of TGF-β, and activated platelets assist CTCs in evading immune surveillance and tumor-endothelial interactions. Tumor-derived inflammatory factors can stimulate neutrophil formation of “neutrophil extracellular traps” (NETs) that facilitate the attachment of CTCs to endothelial cells and promote metastatic dissemination. This figure was created with biorender.com
Surviving CTCs that arrest the target organ vasculature can resume growth, remain solitary dormancy, or form dormant micrometastatic lesion which may be caused by the mechanisms that immunosurveillance and limited blood supply restrict the extent of CTC proliferation and proliferative expansion of micrometastasis.65 Overwhelming evidence support a view that the interaction of CTCs and organ microenvironment decides whether CTCs become dormant or metastatic and regulate the switch between dormancy and metastasis of CTCs.66,67 The most essential mechanism of dormancy is that no genetical progression make CTC unable to grow autonomously or transduce growth signals from organ microenvironment.65 Aguirre et al.68 found that loss of surface receptor, such as urokinase plasminogen activator receptor (uPAR), α5β1 integrin or epidermal growth factor receptor (EGFR), showed a weak response for stimulation of growth signals from the organ microenvironment and induced protracted dormancy of CTCs with G0/G1 arrest in vivo, that might be mediated by diminishing uPA/uPAR/α5β1-dependent signal or FAK-dependent signal transduction.69,70 uPAR and α5β1 integrin are also able to regulate ERK/p38 activity ratio, that high rate facilitates the proliferative state with activated α5β1 and EGFR, whereas low ratio induces tumor growth arrest.71 And the ability of CTCs to correct the imbalance of ERK-to-p38 activity ratio affects preference for dormancy or growth.72 Cells lodged in metastatic sites were found to retain the ability to maintain the proliferative ERK/p38 balance, that they can rapidly silence p38 signaling by activating Ras-ERK signaling and uPA/uPAR/α5β1-dependent signaling.68,71,73 In addition, stress from dissemination, microenvironment signals and/or the cells within PMNs, including bone-forming cells or osteoblasts,74 hepatic NK cells,75 and brain astrocytes,76 might contribute to growth suppression.
However, cellular plasticity of CTCs offers the possibility to control the switch between dormancy and proliferation.77,78 Diverse epigenetic, transcriptional, and translational regulatory processes, as well as complex cell-cell interactions, regulate genetical progression of dormant cell to coordinate cell states.77 When receiving instructive signals from microenvironment of metastatic sites and acquire new mutations of self-perpetuate cell-cycle progression that re-activate uPAR and mitogenic signaling (ERBB2 or EGFR) to induces ERK activation and p38 inactivation,65 dormant CTCs which arrival at the secondary site determined by primary tumors79,80 will quickly exit dormant stage and prepare for metastasis. Moreover, the expansion and colonization of proliferating tumor cell populations might require other programmes to induce angiogenesis81 and immune escape.82,83 During extravasation, CTCs revert to an epithelial phenotype via mesenchymal-epithelial transition (MET) or reside as disseminated tumor cells (DTCs).3,84 DTCs regain their adhesion ability and stem cell properties, which are conducive to “homing” into a new metastatic site and thriving there.
In general, tumor metastasis incorporates the following processes. First, before the formation of metastases, primary tumors or CTCs induce PMNs that provide support for the growth and colonization of tumor cells in distant organs. Second, CTCs undergo changes such as EMT to increase invasiveness and respond to different environmental stresses to survive in the circulation. Additionally, CTCs that metastasize to distant organs may not immediately form metastases. Instead, certain CTCs may enter a state of dormancy before later proliferating under favorable circumstances, ultimately leading to the formation of metastases or tumor recurrence.
The organotropism of CTCs
In recent years, there have been number of interesting results in the study of CTCs involved in organ-specific metastasis, which have not only provided fundamental data to explore the mechanism, but also offered new ideas and methods for subsequent researchers. Metmap is a representative strategy and online resource, that contains the metastatic profiles of more than 500 cell lines from 21 solid tumor types, detailing the metastatic potential of various cancer cell lines and providing a model for exploring the mechanism of metastasis.85 Metmap shows that the intrinsic characteristics of tumor cells are important factors in determining the metastatic organ, providing evidence for the occurrence of CTCs and organ-specific metastasis. This DNA barcoding technique was also used in another study that revealed the change of the clonal types between the primary tumor and the corresponding metastatic tumor using a patient-derived xenograft model86 These results provide a possibility to predict the occurrence of metastasis according to the characteristics of CTCs. Compared to traditional transcriptome sequencing, Flura-seq, an in-situ sequencing technology, shows greater potential for application in studying the organotropism of metastasis because of its high sensitivity and efficiency in detecting changes in typical molecules that are dependent on changes in the microenvironment.87 According to Flura-seq data, the development of early-stage lung metastasis in breast cancer is linked to heightened oxidative stress and increased anti-apoptosis activity in CTCs.87 In addition, a high-throughput study utilizing single-cell sequencing revealed the differentiation and specific gene expression characteristics of CTCs that pertain to metastasis, and demonstrated a hierarchical model for metastasis.88 In terms of animal experiments, a high-throughput in vivo screening method in mouse identified the regulators in CTCs associated with metastasis.89 Together, these advanced research tools offer more chances for fully comprehending the organotropism of CTCs during metastasis and designing more effective cancer therapies.
It is obvious that metastasis has organ-specific patterns, for example, bone metastasis in prostate cancer90 and liver metastasis in pancreatic cancer91 and uveal melanoma.92 Different subtypes of the same tumor show different metastatic site preferences. For example, breast invasive ductal carcinomas have a higher risk of lung, liver and bone metastasis, while invasive lobular carcinoma shows a tendency to metastasize to enterocoelia.93 Besides luminal breast cancer with ER and PR positivity has a higher rate of bone metastasis, and luminal breast cancer with HER2 positivity has a preference of brain metastasis.94 The anatomical features of the primary tumor-located tissues and distant metastatic organs can to some extent explain the organotropism of some tumors, but recent studies on the intricate interactions between CTCs and their corresponding metastatic target organs prefer to show that some important signaling molecules might play a vital role in the process of organotropism.95,96 (Fig. 3) (Table 1).
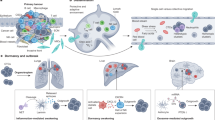
Overall view of CTCs in organ-specific metastasis. The heterogeneity of the circulating tumor cell (CTC) repertoire in the blood reflects the diversity of molecular mechanisms leading to tumor dissemination. Different CTCs differ in their ability to extravasate to distant organs such as bone, lung, brain, liver, or lymph nodes and in their ability to colonize by forming overt metastases. The occurrence of distant metastasis in different organs is related to the characteristics of CTCs, the specific molecular mechanism, and the microenvironment of distant organs. The cells in this figure were created with biorender.com
Distant organ metastasis
Brain metastasis
The brain, which has an adequate blood supply, is a common site for tumor metastasis, with the top five primary tumors being lung cancer, breast carcinoma, melanoma, renal cancer, and colorectal adenocarcinoma.97 The brain microenvironment imposes more stringent and distinct requirements for invasive tumor cells because of its unique cell types, anatomical structures, metabolic constraints and immune environment.98 CTCs involved in brain metastasis always acquire some unique molecular properties through continuous evolution under environment pressure. Firstly, CCSCs, with stronger tumorigenic and metastatic potential,99 possess a special superiority in terms of brain metastasis. Sihto et al. confirmed that breast tumors whose first metastatic location was the brain typically shared features of neural stem cells that showed expression of nestin and CD133 and might be more adapted to the brain microenvironment to initiate brain metastases.100 Similarly, a subset of the triple-negative breast cancer (TNBC) cell line GI-101 with high expression of CD133 and CD44 was found to have greater potential to form brain metastases.101 The overexpression of the CD44 variant isoform, CD44v6, in CTCs is associated with brain metastasis of small cell lung cancer, which may enhance the invasiveness of the cells by activating the EMT process and thereby promoting metastasis.102 Furthermore, Zhang et al.103 identified a potential feature of breast cancer brain metastases in which EpCAM-CTCs overexpress brain metastasis-selected markers (HER2, EGFR, HPSE and Notch1) and demonstrated that these CTCs were indeed highly invasive and able to generate brain metastases in nude mice. In addition, RAC1 is also highly associated with brain metastasis of lung adenocarcinoma (LUAD), mainly through facilitating invadopodia-mediated ECM degradation and affecting the reorganization of the actin cytoskeleton, which can regulate the motility of CTCs.104,105 To successfully survive in the circulation or adapt to the brain microenvironment, CTCs also undergo a series of adaptive cytoprotective gene mutations and adaptive metabolic reprogram. CTCs in the microenvironment of brain metastases exhibit elevated levels of glycolysis, enhanced fatty acid oxidation, and increased pentose phosphate pathway.106 These changes are necessary to fulfill the high energy demand required to support the CTCs’ sustained growth and development in the brain metastasis microenvironment.107,108 Simultaneously, upregulating AMP-activated protein kinase (AMPK) enhances the mitochondrial respiratory chain pathway to produce energy and activate antioxidant defense mechanisms, thereby sustaining a high intracellular ATP concentration.109 Because of the upgraded pentose phosphate pathway resulting in a higher NADPH production, along with increased glutathione reductase functionality, CTCs possessing a propensity for brain metastasis maintain elevated levels of glutathione.106 This elevation effectively enhances their antioxidant defenses, preventing oxidative stress. In addition, Nrf2 mutation and activation were found in CTCs with brain metastasis of lung cancer, which was closely related to the poor prognosis of patients.110 Nrf2 is a transcription factor that translocates to the nucleus during stress, binds to antioxidant response elements (ARE), and drives the expression of antioxidant genes.111 Moreover, the RPL/RPS gene signature in CTCs is associated with melanoma brain metastasis.112 This gene signature is associated with ribosome production, translation and metabolism of CTCs, cell proliferation of CTCs and tumor progression.113
Penetration of the blood‒brain barrier (BBB) is a significant step for CTC dissemination into the brain that involves mediators of extravasation through nonfenestrated capillaries complemented with specific enhancers of BBB crossing114 (Fig. 4a). Before the arrival of CTCs, the EVs derived from the cancer sublines that would metastasize to the brain could travel exclusively to the brain and be taken up by vascular endothelial cells, thereby facilitating a favorable milieu for CTCs to effectively cross the BBB.115 These tumor-derived EVs were confirmed to awaken dormant DTCs or to facilitate microvascular hyperpermeability. For example, after transfer into endothelial cells, miR-105 in EVs from metastatic breast cancer cells diminishes tight junctions between cells and weakens the barrier function of endothelial monolayers by targeting zonula occludens 1 (ZO-1), which increases vascular permeability and facilitates the penetration of the BBB by CTCs.116 Bos et al. identified several possible mediators that can drive CTCs to cross the BBB and colonize the brain: cyclooxygenase COX2 (also known as PTGS2), the EGFR ligand HBEGF, and the 2,6-aldoltransferase ST6GALNAC5. COX2 and HBEGF are involved in brain infiltration, affecting the accessibility of nonfenestrated capillaries and promoting CTC extravasation into the brain.114 Additionally, increased COX2 in CTCs of TNBC activates metalloproteinase 1 (MMP-1), breaking down endothelial cell connections and aiding CTCs in crossing the BBB.117 In breast cancer, ST6GALNAC5 appears to be a specific mediator of CTC transmigration into the brain, and high ST6GALNAC5 expression enhances the adhesion of CTCs to brain endothelial cells and increases the permeability of the BBB.114,118 Klotz et al.15 showed that semaphorin 4D (SEMA4D) was also related to the occurrence of brain metastasis, likely because it mediates the capacity of CTCs to migrate through the BBB. The interaction of SEMA4D and PlexinB1 activates the Rho pathway to promote tumor cell transition into a proangiogenic phenotype.119
The molecular features and mechanisms of CTCs mediating brain, lung, liver and bone metastasis. a For brain metastasis, cancer stem cell-like CTCs or CTCs with specific phenotypes (e.g., HER2, EGFR, HPSE and Notch1) might be more adapted to the brain microenvironment. CTCs can cross the BBB with the help of mediators (e.g., COX2 and ST6GALNAC5) and tumor-derived EVs. CTCs express high levels of anti-PA serpins, which can inhibit the pro-apoptotic effects of reactive astrocytes. In addition, CTCs can induce astrocytes to secrete cytokines suitable for survival. b For lung metastasis, CTCs expressed FADS3 and ICAM can extravasate with the help of miRNAs and integrins. And miR-122 ensures sufficient glucose uptake by CTCs. The periostin (POSTN) and tenascin C (TNC) derived from CTCs and myofibroblasts stimulate CTC survival by amplifying Wnt and Notch signaling. Integrins α6β4 and α6β1 are preferentially taken up by lung-resident cells, creating a microenvironment suitable for CTCs. CTCs can express high levels of B7x to induce immune escape and avoid elimination by immune cells. c As for liver metastasis, CTCs can release exosomes and secrete TGF-β to activate stellate cells, thereby recruiting bone marrow-derived cells (BMDCs). CTCs also activate fibroblasts to produce IL-11 through the secretion of TGF-β, which support CTC survival in the liver. In the circulation, TPO binds to CTCs to regulate metabolism and activate Wnt signaling. High Src signaling protects CTCs from TRAIL-mediated apoptosis. d For bone metastasis, the activation of osteoclasts is facilitated by CTC-derived mediators, including PTHrP, IL-1. CTCs induce IL-6 secretion from osteoblasts, which in turn induces osteoclast differentiation. Activated osteoclasts undergo bone resorption and release other growth factors, such as TGF-β, which further stimulates the expression of osteolytic factors in CTCs, resulting in a vicious cycle. Some parts of the figure were created with biorender.com
Once CTCs enter the brain, resident cells in the brain metastatic microenvironment are activated to resist the dissemination of CTCs. For instance, astrocytes change the brain microenvironment by secreting serine protease plasminogen activators (PAs) (Fig. 4a). PAs process the zymogen plasminogen into plasmin, which limits the survival of tumor cells by promoting Fas-mediated cancer cell killing and suppressing inactivation of axon pathfinding molecule (L1CAM)-mediated vascular cooption.120 To survive in the brain, CTCs produce high levels of anti-PA serpins, including neuroserpin and serpin B2, to protect against the effects of PAs.121 Furthermore, MYC, a cofactor for SEMA4D, is a crucial regulator of DTC adaptation to the activated brain microenvironment.122 Via direct upregulation of glutathione peroxidase 1 (GPX1) expression, MYC mitigates oxidative stress and assists colonizing CTCs in escaping being killed by activated microglia in the brain microenvironment.15 Interestingly, some findings have suggested that astrocytes are always hijacked by tumor cells to support metastatic growth.123 For example, brain metastatic cancer cells can express protocadherin 7 (PCDH7) to promote the assembly of carcinoma-astrocyte gap channels and transfer cGAMP to astrocytes through these channels.124 The cGAMP excites astrocytes to express and secrete inflammatory cytokines such as interferon-α (IFN-α) and tumor necrosis factor (TNF), which activate the STAT1 and NF-κB pathways in brain metastatic cancer cells as paracrine signals to support tumor growth. Intriguingly, stroma-derived EVs can also be integrated by brain metastatic CTCs and regulate their gene expression to promote brain metastasis. For instance, Zhang et al.125 discovered that PTEN-targeting miR-19a in astrocyte-derived EVs was taken up by brain metastatic CTCs and downregulated PTEN expression in the cells, which contributed to the recruitment of myeloid-derived suppressor cells (MDSCs) via nuclear factor-κB (NF-κB) activation and upregulation of CC-chemokine ligand 2 (CCL2).
Lung metastasis
The lung is another preferred site for metastasis of multiple malignant tumors, such as breast and colon cancer and melanoma. Except for the substantial blood supply that runs to the lungs allowing CTCs to easily metastasize through the bloodstream to the lungs, there are also a variety of different molecular mechanisms involved in the generation of secondary lung tumors (Fig. 4b). Primary tumors systemically reprogram the lung microenvironment by early secretion of various TDSFs, and shed EVs were shown to be necessary for the colonization and outgrowth of DTCs in the lung.126,127 After mobilization and recruitment to premetastatic lungs by TDSFs, BMDCs expressing vascular endothelial growth factor receptor 1 (VEGFR1) and VLA-4 (integrin α4β1) interact with highly expressed fibronectin to mediate the adhesion of BMDCs as a premetastatic cluster and to enhance MMP-9 expression.128 Then, MMP-9 alters the microenvironment and enhances the expression of SDF-1, which creates a chemokine gradient to attract CTCs expressing CXCR4 for incorporation into the niche. Gradients of the chemokine CCL2 existing in the PMN can recruit CC-chemokine receptor 2 (CCR2)-positive inflammatory monocytes to assist tumor cell survival by producing vascular endothelial growth factor (VEGF).129 In addition to directly acting on BMDCs, some TDSFs can also stimulate the expression of chemoattractants (such as S100A8 and S100A9), which elicit a large number of myeloid cells to accumulate in the premetastatic lung by inducing the serum amyloid A3-TLR4- NF-κB signaling cascade.130 Erler JT et al.131 found that hypoxic primary breast tumor cells secreted lysyl oxidase, which crosslinked collagen IV in the lungs, recruiting myeloid cells to support metastatic colonization. Besides tumor-derived factors, other factors also participate in the reprogramming of the lung microenvironment and promote metastatic outgrowth.132 For example, lipopolysaccharide (LPS)-mediated lung inflammation allows the recruitment of bone marrow-derived neutrophils that release the Ser proteases elastase and cathepsin G to proteolytically destroy the antitumorigenic factor thrombospondin-1 (Tsp-1). Inflammation in the lung can also awaken dormant DTCs through the activation of the EMT program induced by ZEB1.133 Active transforming growth factor-β1 (TGF-β1) and periostin derived from endothelial cells are the tumor-promoting factors that enable DTCs to escape cancer dormancy and spark micrometastatic outgrowth.134
Emerging evidence also implicates the contribution of exosomes that can directly regulate or deliver molecules as vesicles to facilitate organ-specific metastasis. For instance, miR-105 delivered by breast cancer exosomes breaks down vascular endothelial barriers in endothelial monolayers, facilitating the ability of CTCs to breach vascular barriers into the lung parenchyma by degrading the tight junction protein ZO-1.116 In laminin-rich lung microenvironments, integrins α6β4 and α6β1 in cancer-derived exosomes are preferentially taken up by lung-resident fibroblasts and epithelial cells and upregulate the expression of a metastasis-promoting factor, S100A4, via a mechanism involving Src, Akt, and NFAT.93,135,136 It has been reported that cancer cell-derived miR-122 increases nutrient availability in the lung PMN by downregulating the glycolytic enzyme pyruvate kinase to suppress glucose uptake by niche cells137.
The specific molecular profile of CTCs determines cell plasticity and adaptation, which reflect the tumorigenic potential of CTCs in the lung. Fatty acid desaturase (FADS3) upregulation in CTCs of breast cancer can enhance cell membrane fluidity that promotes individual CTC or CTC clusters spreading through blood vessels and colonization of the lungs.138 CTCs expressing LT receptors (BLT2 and CysLT2) possess intrinsically higher tumorigenicity that is enhanced by CD11b+Ly6G+ neutrophil-secreted leukotrienes in the lung PMN.139 In a study of TNBC, the expression of intercellular cell adhesion molecule-1 (ICAM-1), which contributes to mediating homophilic interactions, was found to drive CTC cluster formation and lead to lung metastasis.140 Aberrant expression of vascular cell adhesion molecule-1 (VCAM-1) in DTCs of breast cancer activates the VCAM-1-Ezrin-PI3K/Akt pathway, which provides a survival advantage for DTCs in leukocyte-rich lung microenvironments.141 DTCs might also induce lung stromal expression of periostin and tenascin C, which can activate Wnt and Notch signaling, which are required for cancer stem cell maintenance.142,143
Liver metastasis
Because of the accessibility of liver capillary sinusoids and the unique characteristics of the mesenteric circulation, the liver is considered the most common metastatic site for colorectal cancer (CRC) and a common metastatic site for lung cancer, gastric cancer and pancreatic cancer144,145,146,147 (Fig. 4c). The types of CTCs determine whether intrahepatic or extrahepatic metastasis will occur.148 Hybrid CTCs with both epithelial and mesenchymal markers mainly mediate the occurrence of intrahepatic metastasis, while mesenchymal CTCs are more likely to lead to the occurrence of extrahepatic metastasis.149 In addition, the molecular properties of the CTCs could also imply their association with liver metastasis. Zhang et al. demonstrated that the lung cancer cell line A549 had an obvious preference for liver metastasis when it expressed a high level of CD133.150 Wu et al. showed that CRC CTCs expressing CD110, the thrombopoietin (TPO)-binding receptor, were modulated by TPO-mediated lysine catabolism and TPO reprogramming and therefore exhibited a significant preference for the liver.151 Lysine catabolism contributes to the self-renewal of CTCs and enhances the antioxidant capacity of CTCs, which assists in the survival and successful colonization of CTCs in the liver. In colorectal cancer liver metastases, CTCs express metabolites that differ from those of the parent tumor cells. These metabolites are associated with carbon pool pathways, including folate, folate biosynthesis, and histidine metabolism.152 Notably, folate biosynthesis plays a pivotal role in single carbon transport, with increased expression of MTHFD2, contributing to intracellular reactive oxygen species (ROS) removal.153 Additionally, CTCs have the ability to produce serine through the up-regulation of three crucial enzymes in the serine synthesis pathway (SSP): PHGDH, PSAT1, and PSPH.154,155 This increase in single carbon unit supply facilitates rapid proliferation.152
Before the arrival of CTCs, tumor-derived EVs released into the circulation act on liver-resident cells, such as Kupffer cells and hepatic stellate cells, and regulate their gene expression to induce liver PMN formation.156 Macrophage migration inhibitory factor (MIF) is highly expressed in exosomes derived from pancreatic ductal adenocarcinoma cells and is selectively taken up by Kupffer cells to induce the cells to secrete TGF-β, which causes hepatic stellate cells to produce excess fibronectin.157 Then, fibronectin deposits promote bone marrow-derived macrophage and neutrophil aggregation in the liver, which is a crucial part of liver PMN formation. In fibronectin-rich liver microenvironments, integrin αvβ5 in liver-tropic exosomes derived from pancreatic cancer can also stimulate Kupffer cells to express proinflammatory S100A8 and S100P, which initiate PMN formation in the liver by recruiting MDSCs.136,158 Another study159 showed that exosomal miR-135a-5p was released into the blood circulation from primary CRC lesions under the induction of a hypoxic microenvironment and was preferentially phagocytosed by Kupffer cells. Subsequently, miR-135a-5p initiated activation of the LATS2-YAP1/TEAD1-MMP-7 axis to promote liver metastasis of CRC by inhibiting the CD30-mediated activation of CD4+T cells and enhancing CRC CTC adhesion. According to Xie et al.,146 pancreatic cancer exosome-delivered CD44v6/C1QBP complex activated insulin-like growth factor 1 (IGF-1) after incorporation by hepatic stellate cells, which initiated the activation of hepatic stellate cells and facilitated liver fibrosis. Besides tumor-derived exosome-mediated liver PMN formation, lipopolysaccharide-induced systemic inflammation also enhances the adhesion of neutrophils and CTCs, which is mediated by selectin-selectin ligand interactions, increasing the retention of lung CTCs in hepatic sinusoids.160
In addition to the formation of a proinflammatory microenvironment, EVs also function in promoting EMT and inducing vascular remodeling. Because of the dual blood supply and much lower sinusoid blood pressure gradient, hematogenous transmission is a major route of hepatic metastasis.79 When flowing in the circulation, some CTCs will be entrapped in the liver microvasculature and extravasate into the liver parenchyma through the participation of multiple mechanisms.161 For instance, miR-122-5p selectively enriched in EVs from lung cancer with extremely low expression in other tissues and tumors was found to be specifically internalized by liver epithelial cells and to facilitate the migration and EMT of liver epithelial cells to benefit the extravasation of CTCs into the liver.162 Zeng et al.163 revealed that CRC-derived miR-25-3p uptake by liver sinusoidal endothelial cells downregulated ZO-1, occludin, and claudin-5 and upregulated VEGFR2 by suppressing the transcription factors Krüppel-like factor 4 (KLF4) and KLF2 in endothelial cells, consequently enhancing vascular permeability and angiogenesis. Similarly, miR-638, miR-663a, miR-3648, miR-4258 and miR-103 in highly intrahepatic metastatic hepatocellular carcinoma (HCC)-cell-derived exosomes can also downregulate the endothelial expression of endothelial junction proteins, such as ZO-1, VE-cadherin and p120-catenin, which enhance vascular permeability.164,165 Takano et al. showed that CRC-derived exosomes delivered miR-203 into monocytes and that miR-203 could induce the polarization of macrophages to M2-tumor-associated macrophages (TAMs), which exert prometastatic functions.166 They also observed the tendency of miR-203-transfected CRC CTCs to metastasize to the liver in a xenograft model. Tissue-resident iNKT17 cells produce IL-22, which induces endothelial expression of aminopeptidase N to facilitate endothelial permeability and thereby cancer cell extravasation in liver metastasis.167
Bone metastasis
Bone is one of the most common target organs for metastasis of cancer, such as breast, prostate and thyroid cancers, which possess a propensity to spread to bone. The environment in the bone marrow sinusoids is probably more amenable to CTC colonization than that in any other kind of capillaries.121 Cancer cells disseminating to bone can stimulate local bone cell activity and disrupt normal bone homeostasis maintained by osteoclasts and osteoblasts, which is conducive to driving bone destruction and metastatic growth.168 The ability to ultimately stimulate bone resorption induced by monocyte/macrophage-derived osteoclasts and increase bone formation mediated by osteoblasts is essential for tumor progression and the most critical step of bone metastasis169 (Fig. 4d). The bone metastasis of breast cancer is always lytic with substantial bone loss, and approximately 25% of cases involve osteoblastic lesions.170 The bone metastasis of prostate cancer is generally accompanied by osteocyte recruitment and an increase in alkaline phosphatase and osteocalcin, in which osteoblast activity stimulates bone formation adjacent to the metastatic tumor.169 Once overt metastasis occurs, CTCs alter not only their molecular expression profile but also the target organ microenvironment in favor of their colonization and survival. The process by which prostate CTCs invade bone supports this perspective.171 Metastasis in bone upsets the balance of osteogenesis and osteoclasis, enhancing osteoblastic activity. CTCs show osteomimicry, acquiring characteristics of bone cells after crossing the vessel barrier and entering the bone marrow.93,172 Subsequently, several proteins, including osteopontin (OPN), parathyroid hormone-related peptide (PTHrP) and HPSE, and cytokines, such as IL-1, IL-6 and prostaglandin E2 (PGE2), derived from CTCs are released to increase the production of osteoclasts and encourage bone renewal.173,174 Furthermore, bone degradation and resorption induced by osteoclasts cause the release of tumor-related growth factors, including IGF-1, platelet-derived growth factor (PDGF), and TGF-β.175 These effects provide space and fertile soil for tumor growth.93,176 As the tumor grows, more tumor-derived factors destroy the bone, forming a vicious cycle93,173 (Fig. 4d).
This vicious cycle involves the joint action of CTCs, the resident cells in bone PMN, and their secreted factors. Tumor-derived factors, such as PTHrP, Dickkopf-1 (DKK-1), IL-8, TNF, TGF-β, and HPSE, can activate osteoclast maturation and promote bone resorption by receptor activator for NF-κB ligand (RANKL)-dependent and RANKL-independent mechanisms. DKK-1, a Wnt signaling inhibitor secreted by breast cancer cells, causes CTCs to preferentially metastasize to the bone rather than the lung. Yue et al.177 revealed that RSPO2 and RANKL upregulated the expression of the secretory protein DKK-1 by binding to LGR4 receptors on the surface of breast cancer cells and activating the β-Catenin/Gαq signaling pathway. After entering the bone microenvironment, DKK-1 recruits osteoclast precursor cells and forms a PMN suitable for the survival of CTCs to promote breast cancer bone metastasis by restraining nonclassical Wnt signaling that is involved in the Wnt/PCP-RAC1-JNK and Wnt/Ca2C-CaMKII-NF-κB signaling pathways.178 Bone resorption leads to the secretion of various tumor-related factors, including FGFs, IGFs, VEGFs, endothelin 1, Wnt signaling pathway factors and bone morphogenetic proteins (BMPs), which accelerate the renewal of the bone matrix and eventually cause replacement of the bone marrow121 (Fig. 4d). Zhang et al.179 showed in a bone metastasis model of breast cancer that TGF-β acts as a cell-survival factor to promote CTC colonization in the bone microenvironment in a manner mediated by the Src pathway. Yoneda et al.180 proved that TGF-β specifically inhibits the growth of cells separated from brain metastatic tumors in vitro, but it did not inhibit the growth of cells taken from bone metastatic tumors. IGF-1 was found to have a growth-promoting effect only on CTCs that preferentially metastasized to bone and not on cells from brain metastasis or primary tumors. IGF-1 receptor (IGF-1R) is more phosphorylated in CTCs from bone metastasis in response to IGF-1 stimulation than in CTCs from brain metastasis.180
Gay and Felding-Habermann181 described each stage of bone metastasis formation in which platelets participate, including survival in the blood, passage through the vessel barrier and adaptation to the metastatic microenvironment. Platelets release all kinds of cytokines or interact with the surface of CTCs to protect CTCs from immune response and help them adhere to vessel endothelial cells and invade vessels to ultimately colonize secondary metastatic sites.181 After entering the bloodstream, platelets can quickly coat CTCs and impair the function of NK cells to prevent NK cells from recognizing and lysing CTCs by releasing TGF-β and PDGF.182 Carvalho et al.183 found that overexpression of PDGFR-α, a PDGF ligand subtype, caused breast cancer CTCs to transform into a more aggressive phenotype and gain the potential to metastasize, specifically when it was coexpressed with Bcl-2. Leblanc et al.184 confirmed that CTCs induce platelet activation and aggregation, leading to the secretion of autotaxin (ATX). ATX directly interacts with integrin αvβ3 to promote early colonization of bone in breast cancer (Fig. 4d). This process is lysophosphatidic acid dependent.184 Megakaryocytes, from which platelets originate, have been identified to be increased in bone marrow when bone metastasis occurs in breast cancer. Further research found that megakaryocytes might also affect the extravasation of CTCs.185
Moreover, Nguyen et al.186 suggested that the organ specificity of metastases formed by CTCs might depend on selective pressures from the corresponding organ microenvironment. These pressures may lead captured CTCs to develop the capability to metastasize. Tumor recurrence after an extended latency period is more likely to occur in the organ where metastasis last occurred, specifically in breast and prostate cancer, supporting the above idea.186 Sun et al.187 (2005) demonstrated that SDF-1/CXCR4 is involved in the localization of tumors in the bone marrow in prostate cancer and that the activation of SDF-1/CXCR4 promotes the establishment of bone metastases. SDF-1 has been reported to mediate the adhesion between bone marrow endothelial cells and CTCs in prostate cancer. Produced by CTCs, SDF-1 may help establish a migratory system that causes CTCs to localize among endothelial cells and osteoblasts that produce SDF-1 in the bone marrow, and chemokines may directly stimulate the proliferation of CTCs.188 CXCR4 may not only be responsible for invasion but may also be critical for the growth of micrometastases in some cancers.187 Wu et al.189 revealed that ER-regulated secretory protein (SCUBE2) contributes to the bone tropism of luminal breast cancer by modulating osteoblast differentiation and immune-suppressive osteoblastic niches. Autocrine SCUBE2 induces tumor cells to release membrane-anchored SHH, which results in Hedgehog signaling activation and the differentiation of osteogenic cells. It has also been found that CTCs tending toward bone metastases express a higher level of trefoil factor 3 (TFF3) than either lymph node metastases or primary tumors.190
Lymph node metastasis
Lymph node metastasis is an important factor in the staging of malignant tumors, and in many cases, lymph nodes (LNs) are the first organs to metastasize. It has been demonstrated in several studies191 that most types of carcinomas, especially breast cancer, colorectal cancer and melanoma, almost invariably metastasize to regional LNs. Although rich in various kinds of immune cells that are intrinsically hostile to extrinsic cells, the endogenous LN microenvironment is capable of supporting the survival and even metastatic outgrowth of tumors, except some tumors that can elicit immunological responses, such as melanoma and renal cell carcinoma.191,192 Melanoma were found to induce tumor-specific cytotoxic CD8+ T-cell responses when injecting directly into LNs that resulted in tumor rejection.193 Prior to LN metastasis, CTCs, in addition to preparing suitable PMNs, induce an immunosuppressive microenvironment in lymph nodes that plays a critical role in sustaining tumor growth and metastasis. Primary extra-lymphoid tumor-induced draining lymph nodes (dLNs) are in a state of immunosuppression. For example, tumor-derived VEGF induces a Th2-mediated chronic inflammatory milieu in patients with metastatic melanoma.194 Furthermore, VEGF-C protects melanomas expressing a foreign antigen ovalbumin (OVA) against preexisting antitumor immunity and helps to increase apoptosis rates and lower the cytotoxic activity of OVA-specific CD8-positive T cells.195 In the murine ovarian tumor microenvironment, tumor-derived PGE2 and TGF-β restrain T-cell priming in dLNs by inducing the immunosuppression of dendritic cells (DCs).196 For example, T regulatory cells accumulating in tumor-draining lymph nodes (TDLNs) were found in an inducible murine model of melanogenesis and breast cancer patients.197,198 Finally, the function of B cells undergoes a tumor-dependent shift in TDLNs to promote LN metastasis. The shift may be initiated by lymph-borne EVs and may let B cells exhibit a regulatory phenotype that can generate immunosuppressive cytokines (IL-10 and TGF-β) and convert helper T cells to regulatory T cells.199
In breast cancer, the secretion of VEGF-A/C can activate LN lymphangiogenesis and induce lymphatic network expansion.200,201,202 It has also been shown that VEGF-C enhances interstitial flow in the tumor stroma, which contributes to fibroblast activation, matrix stiffening, and the bias of chemokine gradients, creating conditions favorable for CTC survival and thus promoting metastasis.195,203 Lymphangiogenesis is also associated with tumor-associated neutrophils (TANs). Tumor cells secrete CXCL1 and CXCL8 to recruit neutrophils, activate the ERK and JNK pathways in neutrophils, and express VEGF-A and MMP-9, leading to LN metastasis.204
The overexpression of specific genes and factors expressed or secreted by CTCs can control LN metastasis and has a pivotal role in the organotropism of tumor metastasis. In cervical205 and breast206 cancer, CTCs with a mesenchymal phenotype have a tendency to metastasize to lymph nodes, especially those expressing VIM, uPAR and CXCR4. (Fig. 5) This is because CTCs with a mesenchymal phenotype are more aggressive, giving the CTCs a particularly malignant phenotype. Furthermore, CTCs with the stem cell markers CD44207,208 and CD24209 are also associated with lymph node metastasis. FR+ CTCs210,211 in LUAD also show a propensity for lymph node metastasis, which can be used as a predictor of lymph node metastasis. KRT19, the gene encoding cytokeratin 19, is expressed in normal epithelium, epithelial primary tumors, and metastatic tumors but is not expressed in normal peripheral blood and lymphoid tissues.212 Moreover, KRT-19-upregulated CTCs are closely related to the occurrence of lymph node metastasis.213 Upregulation of other tumor markers, such as mammaglobin (hMAM),214 Survivin,215 and human telomerase reverse transcriptase (hTERT),216 was also associated with lymph node metastasis.217 And the overexpression of Bcl-xL on breast cancer cells was proven to have enhanced organ-specific metastatic activity that guided cancer cells to preferentially metastasize to LNs.218
The molecular features and mechanisms of CTCs mediating lymph node metastasis. For lymph node metastasis, CTCs expressing VIM, uPAR and CXCR4 tended to develop lymph node metastasis. Various factors secreted by CTCs can induce the formation of a pre-metastatic niche (PMN) and an immunosuppressive environment in lymph nodes suitable for tumor colonization and growth. CXCL1 and CXCL8 secreted by CTCs can recruit neutrophils, promote the activation of the ERK and JNK signaling pathways and the expression of VEGF-A and MMP9 in neutrophils, induce tumor lymphangiogenesis, and promote lymph node metastasis. Tumor-derived PGE2 and TGF-β induce immune suppression of dendritic cells (DCs) and T cells. Some parts of the figure were created with biorender.com
Distant lymph node metastasis is strongly associated with regional lymph node metastasis. When regional LNs are involved, CTCs are likely to spread to the next level of lymph nodes through the lymphatic vessels, resulting in distant lymph node metastasis. Lymphatic vessel formation plays a crucial role in the occurrence of distant lymph node metastasis. Primary cancer cells release VEGF-A/C, which triggers LN lymphangiogenesis and induces lymphatic network expansion in regional LNs.200,201,202 The characteristics of CTCs influence the incidence of distant lymph node metastasis as well. The aggressiveness of CTCs correlates with the likelihood of distant lymph node metastasis. Additionally, the level of FR+ CTCs shows a positive correlation with the extent of lymph node involvement, meaning a higher number of FR+ CTCs increases the probability of developing distant lymph node metastasis.210,211
Clinical applications targeting CTCs
Generally, the gold standard for solid tumor diagnosis is still assessment based on histological characteristics of tumor tissues that are obtained by invasive means such as surgical resection or biopsy. However, for patients who can’t surgery because of anatomically inaccessible cancer or a high risk of post-biopsy complications, noninvasive liquid biopsy to detect CTCs in the blood gives more useful information for tumor diagnosis and prognostic evaluation and especially for monitoring the status of the tumor in real time.219 Current research on liquid biopsy of CTCs is focused on three main aims: the capture of CTCs to determine quantities, the identification of CTC phenotypes to assess the tumor status, and the analysis of CTC gene variants to reveal tumor heterogeneity.220
The detection of the number and molecular characteristics of CTCs in blood can be used to predict the risk of metastasis and determine clinical prognosis.221 CTC detection also enables dynamic assessment of tumor status and therapeutic prognosis at any point in the preoperative and postoperative period.222 Moreover, CTC features may be related to sensitivity and resistance to antitumor drugs, and this information can provide guidance for precision medicine.36 Numerous studies and clinical trials have demonstrated that higher CTC counts have significant correlation with poor prognosis and poor therapeutic prognosis, including shorter progression-free survival (PFS) and overall survival (OS).223,224 For example, the average number of 11 CTCs per 7.5 mL in lung cancer has been shown to be related to poor prognosis of patients.224 The CTC count is the clinical factors significantly associated with survival of stage IB LUAD patients, which more than 4 predicts worse prognosis and therapeutic effect of AP-chemotherapy regimens and gefitinib after radical surgery.222 Zhang et al. revealed that baseline CTC count was negatively correlated with survival of NSCLC patients by multivariate analysis and the CTC count of more than eight before chemotherapy predicted significantly decreased PFS and OS brought by chemotherapy.223 The American Joint Committee on Cancer (AJCC) and Chinese Society of Clinical Oncology (CSCO) listed CTC as a prognostic evaluation tool for breast cancer in guidelines, believing that the presence of CTCs in peripheral blood of breast patients suggests poor prognosis. The National Comprehensive Cancer Network (NCCN) Guidelines for Prostate Cancer version 2.2019 pointed out that the expression status of AR-V7 in CTC can guide the treatment of prostate cancer.
The isolation and enrichment technology of CTCs is crucial in the study of CTCs, and obtaining accurate samples is the prerequisite for ensuring the accuracy of subsequent research. The technology used to isolate CTCs has also evolved significantly over the years. The first generation was crude separation technology based on CTC biophysical properties, such as size and surface charge. Since CTCs are usually larger than other cells in the blood, a microfiltration membrane with a certain pore size can be used to filter out other blood cells and leave CTCs, such as the CelSee System.225 However, this technology is difficult to obtain high purity of CTCs with the high heterogeneity. There is another method to exploit the unique metabolic behavior of cancer cells based on high glycolysis to develop unique magnetic nanoprobes (MNPs).226 This high glycolytic capacity, the Warburg effect, results in a negative surface charge of CTCs, which disrupts the balance of membrane potential and thus distinguishes them from other cells in the blood. The second-generation technology, immunomagnetic bead technology, is based on the immuno-affinity strategy, the other is based on biophysical factors of CTCs, such as size and surface charge, and its most representative platform is the CellSearch system, an immunomagnetic bead method for positive binding to the surface antigen EpCAM to isolate CTCs, which has been approved by the U.S. Food and Drug Administration (FDA).14 In addition, AdnaTest system227 which combined immunomagnetic bead separation technique with RT-PCR technique, and the Epic platform228 and AccuCyte CyteFinde system229 using high-throughput imaging technology to detect fluorescein labeled CTCs, are also the efficient CTC enrichment platform. These two generations of technology, which are not fully segmented, can also be combined to increase the specificity of isolating CTCs. For example, the CanPatrol system uses both immunonegative selection and size principles for CTC isolation by first separating leukocytes from a blood sample with a CD45 antibody and then passing them through a nanofiltration membrane to remove smaller cells.149 In addition, the use of nanoparticles increases the contact area between the antibody and the sample, thereby improving the performance of the CTC capture system.11 The latest generation of CTC enrichment technology is microfluidic chip technology, which sets up multiple microcolumn arrays coated with EpCAM antibodies in the chip, and facilitates the maximum adhesion of CTCs to the chip by antigen-antibody binding reaction.230 The technology is able to gently and sensitively capture alive CTCs, which is particularly crucial for culture and further analysis. With the continuous optimization of microfluidic chip technology, from CTC-Chip, HB-Chip to CTC-iChip, the enrichment efficiency of CTC is continuously improved. The difficulty to capture alive CTCs fixed to the surface of the device makes the development of pure chip technology face great challenges. CTC-iChip uses continuous deterministic lateral displacement (DLD) to capture larger WBCs and CTCs, inertial focusing to line up the larger cells, and then integrates microfluidic magnetophoresis to negatively or positively enrich CTCs.231,232,233 Moreover, IsoFluxTM system integrates immunomagnetic beads technique and DEPArrayTM system combines dielectrophoresis to address the limitations of surface capture devices.234
With the advancement of CTC enrichment detection technology, a better understanding of the correlation between CTCs and metastasis has been achieved. The cause of death in most oncology patients is metastatic cancer in vital organs other than the primary tumor itself. Current clinical strategies for the treatment of metastatic cancer mainly aim to inhibit growth and tumorigenesis instead of the metastatic process itself. However, they usually fail to carry out acceptable effects once CTCs colonize vital organs successfully. Surgical tumor resection or systemic treatment are common methods to prevent tumor progression. Nevertheless, recent studies discovered instances wherein surgery speeds up the spread of tumor cells into the blood circulation, ultimately generating metastases.235,236 Moreover, chemotherapy and radiotherapy also have the tendency to induce new aggression and metastasis.237 Thus, as the key link in the distant metastasis of malignant tumors, CTCs have great prospects in precision medicine applications for the treatment of tumor metastasis.238 Several therapeutic strategies targeting the tumor microenvironment or CTCs themselves are used to limit CTC survival and thus decrease metastasis or inhibit disease progression to a more aggressive phenotype, including inhibiting the EMT process, reversing the MET process, and clearing CTCs in the blood circulation.239 (Table 2) Researches on drugs targeting CTCs are mainly in the experimental stage, while certain drugs have undergone clinical trials in recent years (Table 3). For instance, an ongoing phase 1 clinical trial in breast cancer incorporates Digoxin to target and inhibit the EMT process, resulting in a reduction in CTCs (NCT03928210). Nevertheless, early clinical trials were terminated due to low accrual or challenges in detecting CTCs, indicating the significance of technological improvements in CTC isolation and enrichment for future clinical trials.
Targeting the EMT/MET process
In the primary tumor site, most noninvasive CTCs undergo the process of EMT and acquire the ability to migrate and invade (Fig. 6a). During the progression of tumors, the EMT process of tumor cells is highly dynamic (transient and reversible), resulting in the existence of hybrid epithelial/mesenchymal (E/M) phenotypes.240 The plasticity between epithelial and mesenchymal states is the basis of the dissemination and metastatic potential of tumor cells.241 The EMT process also occurs in circulating tumor cells, and accumulated evidence has supported the importance of mesenchymal (M+) phenotype CTCs in the formation of CTC clusters, drug resistance, progressive disease and metastasis.242,243 This process depends on the involvement of multiple molecules, such as the interaction of platelet adhesion and the activation of the TGF-β pathway and Forkhead box protein C1 (Foxc1).244 However, at present, the development of specific novel drugs against EMT or EMT‐related signaling pathways is still in the early stages. Blocking or reversing the EMT process in CTCs to decrease the proportion of M+ CTCs in the blood by targeting the above molecules is a potential treatment means to intercept the establishment of metastasis sites.40
Strategies targeting CTCs. a EMT process. TGF-β promotes EMT in concert with other signaling pathways through a variety of pathway, which in turn activates the expression of transcription factors and regulates apoptosis and cell adhesion gene and avoid anoikis. The development of inhibitors that target pathways in the EMT process can inhibit EMT, allowing CTCs to upregulate epithelial markers and downregulate mesenchymal makers, thereby reducing the invasiveness of CTCs. b Tumor microenvironment. Cancer-associated fibroblasts (CAFs) and bone marrow-derived dendritic cells (BMDCs) are important supportive cells during metastasis, helping to maintain the invasive tumor microenvironment and the metastatic phenotype of CTCs. Targeting CAFs can reduce CTC leakage, and targeting BMDCs can eliminate premetastatic niche (PMN) formation so that the target organ cannot provide a suitable microenvironment for CTCs to colonize. c CTC clusters. CTC clusters can be decomposed in three ways. First, it can specifically capture CTCs and induce apoptosis. Second, antibodies or DNase can be used to destroy the binding between CTCs and neutrophils. Finally, antithrombotic drugs can be used to degrade platelets in CTC clusters to decompose CTC clusters. d Pan-CTC. Rg3-LP/DTX can accurately capture CTCs via the Glut1-Rg3 interaction. In addition, drugs such as mifepristone can be used to directly interact with Bcl-2, a member of the antiapoptotic protein family, and activate the p38 MAPK pathway to induce apoptosis of CTCs. Moreover, some CTCs have cell markers of CSCs; therefore, it is possible to target stem cell markers to achieve the goal of eliminating CTCs. This figure was created with biorender.com
Foxc1 acts as a DNA-binding transcription factor245,246 involved in EMT induction, mediating the metastasis and transformation of invasive CTCs.247,248 Single-cell profiling of CTCs in breast cancer shows that metastasis-related gene expression synergistically elevates VIM, TGF-β, and Foxc1, which maintain EMT induction.249 In Bin Xiong’s study,250 CD163+ TAMs infiltrated in a premetastatic microenvironment were positively associated with the mesenchymal CTC ratio. CD163+ TAMs drive the EMT process in primary CRC tumor cells through the IL-6/STAT3/miR-506-3p/Foxc1 axis, prompting CRC cell invasion to generate mesenchymal CTCs. The cells accomplished the EMT process in turn, leading to the secretion of CCL2, which enhanced the recruitment of macrophages in the premetastatic microenvironment to complete the positive feedback loop. Knockdown of IL-6, STAT3, and CCL2 or treatment with miR-506-3p mimics markedly reduces metastasis mediated by mesenchymal CTCs.250 In addition, gene expression profile analysis between patients with different percentages of M+ CTCs indicated that BCAT1 is an essential molecule mediating the transformation of M- CTCs, with an over 90% positive rate. Suppression of BCAT1 has the potential to impair the invasion ability and apoptosis resistance of M + CTCs.149 Another study provides evidence that BCAT1 is a positive upstream regulator of FoxC1. Treatment with BCAT1- or FoxC1-specific inhibitors reduces cell migration and invasion.251 However, whether the BCAT1/FoxC1 axis is involved in the EMT process of CTCs still needs to be further studied.
For the immune and EMT-related TGF-β/TGF-βR/Smad pathway, specific or nonspecific agents, including small molecules, antisense oligonucleotides, vaccines, neutralizing antibodies, and receptor IgG-Fc fusion proteins, have been developed for oncology therapy by targeting and blocking TGF-β receptors. TGF-β derived from platelets is an important source of the EMT process that prompts CTCs to transform into a more invasive phenotype.252,253,254 Richard O. Hynes et al. demonstrated that the NF-kB/MCP-1 pathway activated by direct adhesion of platelets cooperates with platelet-derived TGF-β, facilitating the extravasation of tumor cells injected into the blood circulation out of vessels and promoting lung colonization. In this process, disseminated cells undergo EMT activation and adopt a more mesenchymal morphology, with increased gene expression related to EMT, ECM remodeling and premetastasis, such as VIM, VEGF, MMP-9 and Serpine1.61 Pharmacological inhibitors of TGF-β and NF-κB specifically abrogate EMT induction and metastasis formation. Aspirin specifically decreases M+ CTCs in metastatic colorectal cancer with an increase in E+ CTCs, potentially mediated by the depolymerization of platelets.255 Curcumin and flavonoids are potential agents to target CTC proliferation and metastasis,237,256 and they have been shown to inhibit the process of EMT by affecting the NF-κB/MPP pathway.257 Another study confirmed that genome editing of TGFβRII led to the reversion of the EMT process and an increase in EpCAM+ CTCs. However, inactivation of the TGF-β pathway triggers the ERK feedback response, promoting the high aggressiveness of CTCs. A combination therapy of anti-TGFβ and ERK inhibitors is essential for targeting the EMT/MET process in CTCs.258 In addition, eribulin, a microtubule-depolymerizing agent, has been reported to suppress EMT, which might be the mechanism for metastatic breast cancer therapy. E + CTC and M + CTC numbers are reliable prognostic markers for patients receiving eribulin treatment.259
Destroying the tumor microenvironment of CTCs
Recent studies in CTCs have revealed the critical role of the tumor microenvironment in the initiation and progression of metastatic disease. Experimental data have uncovered a reciprocal relationship between the cells in the microenvironment.260 Specifically, tumor-associated cells, such as TAM2, CAFs, and BMDCs, help sustain the aggressive tumor microenvironment and metastatic phenotype of CTCs. In return, the direct interaction with or secretion factors of CTCs will accelerate the transformation of tumor-related cells into pro-tumor phenotypes.250,261 Thus, the cellular components and factors in the tumor microenvironment are attractive therapeutic targets to inhibit metastasis.262
CAFs play an important role in the formation of PMNs. CAFs maintain a proinflammatory, immunosuppressive, and oxygen-rich TME phenotype by ECM remodeling, metabolism regulation, angiogenesis, and growth factor secretion. Fibroblasts, marrow mesenchymal cells, and pericytes that CAFs originate from implement transdifferentiation into CAFs under the interaction of primary tumor cells. This positive feedback regulation enables vascular extravasation and tissue infiltration to release CTCs and establish metastasis sites. Tumor-derived PDGF-BB induces pericytes to separate from tissue vessels and further differentiate into CAFs, with a decrease in the pericyte marker NG2 and an increase in the CAF marker αSMA. Transformational CAFs display elevated expression of EMT-related genes and PDGFRα, which is activated by PDGF-BB and helps primary tumor cells to invade vessels and release CTCs.263 The anti-PDGF drug imatinib sustains pericyte coverage on blood vessels and blocks CAF transdifferentiation in a high PDGF-BB secretion microenvironment, contributing to a decrease in CTC leakage.264 Curcumin can be a potential drug to target CTC proliferation and metastasis by influencing the microenvironment.237,256 The mechanism of the antitumor effects might be that curcumin specifically downregulates HMOX1, an NF-κB pathway gene, in the MCF-7/CAF coculture model. This change inhibits EMT induced by the interaction of CAFs.265 Other protein alterations, such as AKR1C2 and RRAGA, were also observed in the coculture model with curcumin treatment.265 Moreover, Dorraya El-Ashry et al. confirmed the existence of circulating CAFs (cCAFs) in peripheral blood and the higher population of cCAFs in metastatic breast cancer than in nonmetastatic breast cancer.261 High serum exosomal miR-1247-3p levels might activate the B4GALT3/β1 integrin/NF-κB axis of CAFs in CTC clusters, promoting the secretion of the proinflammatory factors IL-6 and IL-8 and the establishment of lung metastasis266 (Fig. 6b).
TAMs are the most abundant innate immune population in the tumor microenvironment (TME), with heterogeneity and differentiable plasticity from antitumor to protumor. Under the influence of the tumor microenvironment, TAMs can differentiate into M1 or M2 macrophages. M1 macrophages are generally considered to be tumor killing macrophages, meanwhile M2 macrophages carry out protumor effects through immunosuppressive action.267 This crosstalk provides potential targets for metastasis prevention. PDGF-BB-PDGFRβ signaling mediates the phosphorylation of Akt/MAPK and the enablement of SOX7 transcription in perivascular cells and the stroma, which increases IL-33 release. IL-33 signaling recruits and stimulates TAMs to transform into the M2 phenotype, contributing to primary tumor cell dissemination into the peripheral blood. Pharmacological inhibition of PDGF-BB and IL-33 acceptably impairs the transformation of TAMs and the dissemination of CTCs.264,268 VCAM1 expressed on the membrane of CTCs mediates the recruitment of macrophages in the lung leukocyte-rich microenvironment, which is dependent on α4 integrin. Surrounding macrophages provide Akt-activated pro-survival signaling and assist in CTC seeding in lung metastatic sites. Knockdown of VCAM1 and anti-α4 integrin does not influence CTC migration through human pulmonary microvascular endothelial cell monolayers, instead impairing the survival of cancer cells.141 Chi-Hung Lin et al. discovered that the population of mesenteric CTCs was accompanied by a positive correlation with IL-17A and a negative correlation with GM-CSF in the microenvironment. Treatment with recombinant GM-CSF affects the polarization of macrophages toward the M1 phenotype and chemotaxis of CD8 cells, establishing an adverse environment for CTCs to seed in the metastatic site.269 This immune-related alteration of the tumor microenvironment prevents CTCs from evading cytotoxic T lymphocyte-mediated killing and metastasizing to secondary organs.270
BMDCs mobilized by factors released by the primary tumor and expressing VEGFR1 create a suitable microenvironment for metastatic colonization before CTCs arrive.128 Various specific inhibitors of VEGFR1, including ribozyme,271 mAb,272 peptides,273 and DNAzyme,274 have been found to inhibit tumor metastasis formation. For example, the selective blockade of VEGFR1 by a novel peptide antagonist called iVR1 completely inhibited lung metastasis in a mouse model of colorectal cancer by abrogating the formation of premetastatic clusters. PMN formation is also associated with the activation of P2Y purinergic receptor 2 (P2RY2) and the subsequent HIF1-LOX axis by ATP released from tumor cells, inducing collagen cross-linking and BMDC recruitment. In preclinical models of breast cancer, PMN formation can be prevented by inhibition of P2RY2 signaling or modulation of LOX with β-aminopropionitrile (β-APN), function-blocking antibodies, or LOX-specific RNA interference. In addition, expression of protein tyrosine phosphatase receptor type O (PTPRO), a multitargeted negative regulator of VEGF-A, PDGF and FGF receptor 1 (FGFR1), also prevented PMN formation and lung metastasis in the mouse breast cancer cell line Py8119275 by attenuating tumor-associated angiogenesis, inducing the apoptosis and necrosis of tumor cells, and mediating M1-like macrophage polarization.276
Decomposing CTC clusters and clearing single pan-CTCs
CTCs that enter circulation can not only exist in the blood alone, but can also interact directly or with other cells in the blood to form CTC clusters. The structure of the CTC cluster is more conducive to the survival and metastasis of CTC in circulation. Therefore, the direct targeting of single pan-CTCs or breaking down the CTC clusters can be employed for metastasis inhibition (Fig. 6c).
Several agents have been developed to directly capture CTCs. For example, Li et al.277 created a cancer-specific calcium nanomodulator (named CPDD). They used PLGA nanoparticles loaded with doxorubicin (DOX) and digoxin (DIG) in a complex coated with cancer cell membranes originating from the 4T1 cell line. Digoxin is an anticancer agent that specifically depolymerizes CTC clusters by inhibiting cell‒cell interactions. CPDD shares homology with CTC clusters and can specifically target CTC clusters in the lymphatic system and blood vessels. When CPDD comes into contact with CTCs, it can be internalized by CTCs and induce mitochondria-mediated apoptosis by increasing the levels of intracellular Ca2+, impairing CTC aggregation, and ultimately inhibiting tumor metastasis.277 Michael R. King et al. constructed a kind of liposomal-coated TRAIL and E-selectin that specifically attach to leukocytes. TRAIL/E-selectin-based liposomes targeted CTC clusters and significantly killed over 75% of the CTC clusters and single CTCs according to the test results.278
Astragalus mongholicus Bunge-Curcuma aromatica Salisb (AC) is a traditional Chinese medicine utilized to treat colorectal cancer. AC effectively regulates amino acid metabolism in CTCs through the down-regulation of serine and glycine, thus inhibiting the proliferation of CTCs.279 Furthermore, AC suppresses the gene expression of TGF-β1, IL-6, IL-10, and TGF-βR2, which inhibits the tumor-promoting M2 macrophages and Tregs, and activates the tumor-inhibiting M1 macrophages, creating a tumor-inhibiting environment.280
Dissociating CTC clusters into individual CTCs is an effective method to inhibit metastasis. For instance, Na + /K + -ATPase inhibitors like ouabain and digitoxin reduce the formation of cell-cell junctions by increasing the Ca2+ concentration in CTCs.281 This leads to the dissociation of CTCs into individual CTCs or the failure of individual CTCs to form CTC clusters.282,283 This process alters DNA methylation at stemness- and proliferation-associated binding sites within CTCs, reducing the proliferative capacity of individual CTCs and ultimately inhibiting metastasis.60 Focusing on other components may also play a role in dissociating CTC clusters. Neutrophils and platelets were the most common components of CTC clusters, except for CTCs. The neutrophils in CTC clusters are similar to “guardsmen” that protect CTCs from immune clearance (Fig. 6c). Therefore, it is possible to restore immune function and obliterate CTCs by eliminating neutrophils in the CTC cluster. For example, in mouse models of breast cancer, neutralizing antibodies against Ly-6G (lymphocyte antigen 6 complex site G6D) destroy CTC-neutrophil clusters and delay their shedding of VCAM-1, functionally mediating the interaction between CTCs and neutrophils.25 Moreover, knockdown or treatment by the specific inhibitor resveratrol of VCAM-1 significantly blocks the emergence of CTC-neutrophil clusters and inhibits metastatic growth of melanoma cells in vivo.284 Platelets mediate the formation of CTC-platelet clusters and distant metastasis by relying on the adhesion of P-selectin, a main target of antithrombotic drugs such as aspirin and warfarin.285,286 The metabolic pathway of individual CTCs usually switches to reductive metabolism and glycolysis after dissociation, which leads to an increase in ROS that cannot be eliminated in time. Therefore, individual CTCs are less likely to survive in the circulation.287
Some CTCs that exhibit cell markers of CSCs, such as CD133 and CD44, display a powerful capacity to adapt to the changing environment and are the main cause of metastasis. Targeting stem cell markers to eliminate stem cell phenotypic CTCs might be a feasible treatment18,288 (Fig. 6d). For example, Kakar et al.289 found that treatment of primary ovarian cancer sites with withaferin A alone or in combination with the first-line antineoplastic agent cisplatin specifically reduced the expression of CD24 and CD44. Therefore, withaferin A may specifically target CTCs with the characteristics of cancer stem cells and inhibit tumor growth and metastasis.289 Niclosamide (NCS), an anthelmintic drug, displays antimetastatic effects by acting against cancer stem cells. Simo´ Schwartz Jr. et al. described CD44v6 as a robust stemness biomarker positively related to the expression of other defined stemness markers, such as ALDH1A1, CD44v3 and CXCR4, which function in tumorigenesis and cell invasion. Based on this information, CD44v6-targeted polymeric micelle (PM)-encapsulated NCS was developed for anti-CSC treatment and was astonishingly accompanied by a decrease in CTC quantity and distant metastasis. The mechanism might involve a reduction in CTCs with high CD44 expression that can aggregate in the blood and form CTC clusters to promote survival and metastasis.290 Chengfeng Yang et al. created a nanoparticle-delivered drug named uPtD NPs that consists of a novel ultrasmall Pt(II) dot separated from miriplatin and loaded in nanoparticles modified by an active integrin α5 antibody. Cell cycle arrest induced by uPtD NPs leading to DNA damage has a unique pertinence to CSC-like cells in CTC clusters and ultimately suppresses lung metastasis in TNBC.291 ICAM1 has been documented as a stemness marker, and the discovery of CD45-ICAM+ CTCs provides evidence for the existence of CTCs possessing CSC properties, suggesting that ICAM1 is an effective target against metastasis.292
The mechanisms mediating apoptosis of CTCs also play a role in the inhibition of micrometastasis establishment in distant organs. In HCC CTCs, USP1 can reduce the degradation of transducin beta-like 1 X-linked receptor 1 (TBLR1) by deubiquitination, activate the Wnt signaling pathway, and prevent CTCs from undergoing apoptosis.293 Therefore, USP1 inhibitors can target CTCs to inhibit the occurrence of metastasis. Xia et al.270 developed the drug Rg3-Lp/DTX by loading docetaxel (DTX) on Rg3-based liposomes, which effectively inhibited the metastasis of TNBC by directly targeting and destroying disseminated CTCs in the bloodstream. DTX is a typical first-line anti-metastatic agent for TNBC,294 and Rg3 combined with DTX can not only exert its synergistic anticancer effects but can also maximize the cytotoxicity of DTX on CTCs by inhibiting the activation of NF-κB and thereby increasing the expression of the pro-apoptotic protein Bax.295
Future perspectives and conclusions
The role of CTCs is well established and implies that phenotypic characteristics and their microenvironment are pivotal factors inhibiting organ-specific metastasis. The current technology for assessing CTCs is not sufficiently mature, and CTCs are difficult to isolate from collected specimens. Although there are FDA-approved standards, CTCs are highly heterogeneous, and the surface markers and numbers of CTCs vary greatly among patients and even within the same patient, which directly affects the efficiency of capturing CTCs and the accuracy of subsequent quantitative and molecular analyses. Currently, most potential target molecules on CTCs, such as SEMA4D, VCAM-1, and CXCR4, are still at the experimental study stage, and further exploration is needed to validate their function as therapeutic targets for patients. Based on this phenomenon, we propose three possible directions for future CTC-related clinical and translational research. The first is the development of ultrasensitive capture techniques to enrich as many CTC subpopulations as possible to reflect the true abundance of CTCs in the peripheral blood in vitro. This will pave the way not only for dynamic and real-time evaluation of the therapeutic effect or disease progression but also for developing new drug targets against CTCs themselves. While much progress has been made in capture and enrichment technology as mentioned above, the Cell Search system is the most common in vitro diagnostic device approved by the FDA for clinical use, so the clinical application of CTCs is still limited. Because the technical principle of Cell Search has some limitations, such as the lack of tumor-specific antigens, insensitivity to tumor cells of non-epithelial origin and tumor cells that lose EpCAM in EMT, and the fact that some white blood cells may express EpCAM, leading to false-positive results. Therefore, it is necessary to establish new methods with high sensitivity and specificity, which have technical and commercial viability and can be used for isolation and enrichment of CTCs. The second is to perform cluster analysis according to the phenotypic similarity of different CTC subpopulations and screen out some regular and representative biomarkers whose abnormality may indicate the tendency toward organ-specific metastasis. The third is to further reveal the detailed function of the signaling pathways in mediating CTC biological behavior and the interaction among the cellular components in the microenvironment to illustrate how CTCs are involved in the process of organ-specific metastasis.
CTC-related applications are mainly in the experimental stage, and further research is needed. As summarized here, future directions of studies on CTCs could include CTC use in the early screening and diagnosis of cancer, assessment of patient prognosis based on the numbers and characteristics of CTCs, and CTC detection to determine the risk of tumor metastasis and ideal drug candidates and predict treatment response.296,297 Because peripheral blood is extremely easy to obtain, assessing CTCs during treatment allows for real-time monitoring of patient response to drugs. As they can be extracted via liquid biopsy, ctDNAs are also easy to assess, but the ctDNAs source is more difficult to determine than the CTC source, and there is no guarantee that all DNA detected is from the primary tumor.298 CTCs originating from the patient’s primary tumor site more accurately reflect the situation of the primary tumor and are more informative for subsequent treatment.299 Furthermore, during the process of metastasis, the gene expression profile and molecular characteristics of CTCs will change to adapt to the specific organ microenvironment, and thus, we could identify targets that have the potential to allow detection and inhibition of metastasis. Therefore, the significance of specific tumor markers expressed on CTCs needs to be studied, strategies to make more accurate decisions in precision medicine are needed, and relatively unified, referenceable and more detailed standards for CTC analyses need to be developed. However, there is still a long way to go to determine the mechanisms underlying the organ-specific metastasis of various types of CTCs. To achieve this, more research is needed, the relevant methods need to be improved, and more applications related CTCs need to be brought into clinical practice as soon as possible.
Metastasis is the most unpredictable and serious secondary lesion of cancer. On the basis of conventional imageological examination, we can only identify metastatic sites after its occurrence. Hysteretic detection may cause patients to miss the optimal treatment time points. Thus, it is essential to develop a precise method in vitro to dynamically monitor or evaluate the biological status of tumors. As the seeds of metastasis, CTCs have been proven to be a bridge between the primary lesion and targeted metastatic organs. Furthermore, targeted drugs against the specific antigen on CTCs have been found to be effective in clearing CTCs themselves, providing a promising avenue for clinically inhibiting metastasis. Because CTCs can represent some of the biological characteristics of primary tumors and simultaneously reflect partial biological behaviors of metastatic foci, an extensive understanding of CTC-specific features, such as stemness, hybrid EMT state, escape from immune surveillance, and drug tolerance, will be of great potential to reveal the detailed molecular mechanism by which tumors develop distant organ-specific metastasis.
References
Chaffer, C. L. & Weinberg, R. A. A perspective on cancer cell metastasis. Science 331, 1559–1564 (2011).
Steeg, P. S. Targeting metastasis. Nat. Rev. Cancer 16, 201–218 (2016).
Aleckovic, M., McAllister, S. S. & Polyak, K. Metastasis as a systemic disease: molecular insights and clinical implications. Biochim. Biophys. Acta Rev. Cancer 1872, 89–102 (2019).
Ganesh, K. & Massague, J. Targeting metastatic cancer. Nat. Med. 27, 34–44 (2021).
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 70, 7–30 (2020).
Ring, A., Nguyen-Strauli, B. D., Wicki, A. & Aceto, N. Biology, vulnerabilities and clinical applications of circulating tumour cells. Nat. Rev. Cancer 23, 95–111 (2023).
R, A. T. A case of cancer in which cells similar to those in the tumors were seen in the blood after death. Aus. Med. J. 14, 146–149 (1869).
PC, N. The clonal evolution of tumor cell populations. Science 194, 23–28 (1976).
Seal, S. H. Silicone flotation: a simple quantitative method for the isolation of free-floating cancer cells from the blood. Cancer 12, 590–595 (1959).
Racila, E. et al. Detection and characterization of carcinoma cells in the blood. Proc. Natl Acad. Sci. USA 95, 4589–4594 (1998).
Yoon, H. J., Kozminsky, M. & Nagrath, S. Emerging role of nanomaterials in circulating tumor cell isolation and analysis. ACS Nano. 8, 1995–2017 (2014).
Eyles, J. et al. Tumor cells disseminate early, but immunosurveillance limits metastatic outgrowth, in a mouse model of melanoma. J. Clin. Invest. 120, 2030–2039 (2010).
Ilie, M. et al. “Sentinel” circulating tumor cells allow early diagnosis of lung cancer in patients with chronic obstructive pulmonary disease. PLoS ONE 9, e111597 (2014).
Cristofanilli, M. et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N. Engl. J. Med. 351, 781–791 (2004).
Klotz, R. et al. Circulating tumor cells exhibit metastatic tropism and reveal brain metastasis drivers. Cancer Discov. 10, 86–103 (2020).
Krebs, M. G. et al. Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. J. Clin. Oncol. 29, 1556–1563 (2011).
Mohler, J. L. et al. Prostate cancer, Version 2.2019, NCCN clinical practice guidelines in oncology. J. Natl Compr. Canc Netw. 17, 479–505 (2019).
Grillet, F. et al. Circulating tumour cells from patients with colorectal cancer have cancer stem cell hallmarks in ex vivo culture. Gut 66, 1802–1810 (2017).
Troncarelli Flores, B. C. et al. Molecular and kinetic analyses of circulating tumor cells as predictive markers of treatment response in locally advanced rectal cancer patients. Cells. 8 (2019).
American Society of Clinical Oncology 2007 Update of Recommendations for the Use of Tumor Markers in Breast Cancer. J Oncol Pract. 3, 336–339 (2007).
Oakes, S. A. Endoplasmic reticulum proteostasis: a key checkpoint in cancer. Am. J. Physiol. Cell Physiol. 312, C93–C102 (2017).
Paterlini-Brechot, P. & Benali, N. L. Circulating tumor cells (CTC) detection: clinical impact and future directions. Cancer Lett. 253, 180–204 (2007).
Polyak, K. & Weinberg, R. A. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat. Rev. Cancer 9, 265–273 (2009).
IJ, F. The pathogenesis of cancer metastasis the ‘seed and soil’ hypothesis revisited. Nat. Rev. Cancer 3, 453–458 (2003).
Szczerba, B. M. et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature 566, 553–557 (2019).
Labernadie, A. et al. A mechanically active heterotypic E-cadherin/N-cadherin adhesion enables fibroblasts to drive cancer cell invasion. Nat. Cell Biol. 19, 224–237 (2017).
Labelle, M. & Hynes, R. O. The initial hours of metastasis: the importance of cooperative host-tumor cell interactions during hematogenous dissemination. Cancer Discov. 2, 1091–1099 (2012).
Aceto, N. et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 158, 1110–1122 (2014).
Carter, L. et al. Molecular analysis of circulating tumor cells identifies distinct copy-number profiles in patients with chemosensitive and chemorefractory small-cell lung cancer. Nat. Med. 23, 114–119 (2017).
Lucci, A. et al. Circulating tumor cells and early relapse in node-positive melanoma. Clin. Cancer Res. 26, 1886–1895 (2020).
Riethdorf, S. et al. Detection and HER2 expression of circulating tumor cells: prospective monitoring in breast cancer patients treated in the neoadjuvant GeparQuattro trial. Clin. Cancer Res. 16, 2634–2645 (2010).
Mazel, M. et al. Frequent expression of PD-L1 on circulating breast cancer cells. Mol. Oncol. 9, 1773–1782 (2015).
Bates, M. et al. Circulating tumour cells: The Good, the Bad and the Ugly. Biochim. Biophys. Acta Rev. Cancer 1878, 188863 (2023).
Kanwar, N. et al. Heterogeneity of circulating tumor cell-associated genomic gains in breast cancer and its association with the host immune response. Cancer Res. 81, 6196–6206 (2021).
Chambers, A. F., Groom, A. C. & MacDonald, I. C. Dissemination and growth of cancer cells in metastatic sites. Nat. Rev. Cancer 2, 563–572 (2002).
Nikanjam, M., Kato, S. & Kurzrock, R. Liquid biopsy: current technology and clinical applications. J. Hematol. Oncol. 15, 131 (2022).
Peinado, H., Lavotshkin, S. & Lyden, D. The secreted factors responsible for pre-metastatic niche formation: old sayings and new thoughts. Semin Cancer Biol. 21, 139–146 (2011).
Psaila, B. & Lyden, D. The metastatic niche: adapting the foreign soil. Nat. Rev. Cancer 9, 285–293 (2009).
Liu, Y. & Cao, X. Characteristics and significance of the pre-metastatic niche. Cancer Cell. 30, 668–681 (2016).
Huang, Z. et al. Epithelial-mesenchymal transition: the history, regulatory mechanism, and cancer therapeutic opportunities. MedComm 3, e144 (2022).
Zheng, X. et al. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature 527, 523–530 (2015).
Bakir, B., Chiarella, A. M., Pitarresi, J. R. & Rustgi, A. K. EMT, MET, plasticity, and tumor metastasis. Trends Cell Biol. 30, 764–776 (2020).
Xiong, J. et al. Epidermal growth factor promotes transforming growth factor-beta1-induced epithelial-mesenchymal transition in HK-2 cells through a synergistic effect on Snail. Mol. Biol. Rep. 41, 241–250 (2014).
Mani, S. A. et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 133, 704–715 (2008).
Orrapin, S. et al. Clinical implication of circulating tumor cells expressing epithelial mesenchymal transition (EMT) and cancer stem cell (CSC) markers and their perspective in HCC: a systematic review. Cancers (Basel) 14 (2022).
Mitra, A., Mishra, L. & Li, S. EMT, CTCs and CSCs in tumor relapse and drug-resistance. Oncotarget 6, 10697–10711 (2015).
Agnoletto, C. et al. Heterogeneity in circulating tumor cells: the relevance of the stem-cell subset. Cancers (Basel) 11 (2019).
Aktas, B. et al. Stem cell and epithelial-mesenchymal transition markers are frequently overexpressed in circulating tumor cells of metastatic breast cancer patients. Breast Cancer Res. 11, R46 (2009).
Theodoropoulos, P. A. et al. Circulating tumor cells with a putative stem cell phenotype in peripheral blood of patients with breast cancer. Cancer Lett. 288, 99–106 (2010).
Gradilone, A. et al. Circulating tumor cells (CTCs) in metastatic breast cancer (MBC): prognosis, drug resistance and phenotypic characterization. Ann. Oncol. 22, 86–92 (2011).
Weber, G. F. Metabolism in cancer metastasis. Int J. Cancer 138, 2061–2066 (2016).
Chen, J. et al. Metabolic reprogramming-based characterization of circulating tumor cells in prostate cancer. J. Exp. Clin. Cancer Res. 37, 127 (2018).
Lu, M. et al. Elevated G6PD expression contributes to migration and invasion of hepatocellular carcinoma cells by inducing epithelial-mesenchymal transition. Acta Biochim. Biophys. Sin. (Shanghai). 50, 370–380 (2018).
Knott, S. R. V. et al. Asparagine bioavailability governs metastasis in a model of breast cancer. Nature 554, 378–381 (2018).
Diamantopoulou, Z. et al. The metastatic spread of breast cancer accelerates during sleep. Nature 607, 156–162 (2022).
Piskounova, E. et al. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature 527, 186–191 (2015).
Amintas, S. et al. Circulating tumor cell clusters: united we stand divided we fall. Int. J. Mol. Sci. 21 (2020).
Peeters, D. J. et al. Circulating tumour cells and lung microvascular tumour cell retention in patients with metastatic breast and cervical cancer. Cancer Lett. 356, 872–879 (2015).
Denes, V. et al. Metastasis blood test by flow cytometry: in vivo cancer spheroids and the role of hypoxia. Int. J. Cancer 136, 1528–1536 (2015).
Gkountela, S. et al. Circulating tumor cell clustering shapes DNA methylation to enable metastasis seeding. Cell 176, 98–112e114 (2019).
Labelle, M., Begum, S. & Hynes, R. O. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 20, 576–590 (2011).
Liu, X. et al. Immune checkpoint HLA-E:CD94-NKG2A mediates evasion of circulating tumor cells from NK cell surveillance. Cancer Cell. 41, 272–287e279 (2023).
Fu, A. et al. Tumor-resident intracellular microbiota promotes metastatic colonization in breast cancer. Cell 185, 1356–1372e1326 (2022).
Ubellacker, J. M. et al. Lymph protects metastasizing melanoma cells from ferroptosis. Nature 585, 113–118 (2020).
Aguirre-Ghiso, J. A. Models, mechanisms and clinical evidence for cancer dormancy. Nat. Rev. Cancer 7, 834–846 (2007).
Pantel, K. & Brakenhoff, R. H. Dissecting the metastatic cascade. Nat. Rev. Cancer 4, 448–456 (2004).
Kauffman, E. C., Robinson, V. L., Stadler, W. M., Sokoloff, M. H. & Rinker-Schaeffer, C. W. Metastasis suppression: the evolving role of metastasis suppressor genes for regulating cancer cell growth at the secondary site. J. Urol. 169, 1122–1133 (2003).
Aguirre Ghiso, J. A., Kovalski, K. & Ossowski, L. Tumor dormancy induced by downregulation of urokinase receptor in human carcinoma involves integrin and MAPK signaling. J. Cell Biol. 147, 89–104 (1999).
Liu, D., Ghiso, J. A., Estrada, Y. & Ossowski, L. EGFR is a transducer of the urokinase receptor initiated signal that is required for in vivo growth of a human carcinoma. Cancer Cell. 1, 445–457 (2002).
Aguirre-Ghiso, J. A. Inhibition of FAK signaling activated by urokinase receptor induces dormancy in human carcinoma cells in vivo. Oncogene 21, 2513–2524 (2002).
Aguirre-Ghiso, J. A., Estrada, Y., Liu, D. & Ossowski, L. ERK(MAPK) activity as a determinant of tumor growth and dormancy; regulation by p38(SAPK). Cancer Res. 63, 1684–1695 (2003).
Aguirre-Ghiso, J. A., Ossowski, L. & Rosenbaum, S. K. Green fluorescent protein tagging of extracellular signal-regulated kinase and p38 pathways reveals novel dynamics of pathway activation during primary and metastatic growth. Cancer Res. 64, 7336–7345 (2004).
Aguirre-Ghiso, J. A., Liu, D., Mignatti, A., Kovalski, K. & Ossowski, L. Urokinase receptor and fibronectin regulate the ERK(MAPK) to p38(MAPK) activity ratios that determine carcinoma cell proliferation or dormancy in vivo. Mol. Biol. Cell. 12, 863–879 (2001).
Wang, H. et al. The osteogenic niche is a calcium reservoir of bone micrometastases and confers unexpected therapeutic vulnerability. Cancer Cell. 34, 823–839 (2018).
Correia, A. L. et al. Hepatic stellate cells suppress NK cell-sustained breast cancer dormancy. Nature 594, 566–571 (2021).
Dai, J. et al. Astrocytic laminin-211 drives disseminated breast tumor cell dormancy in brain. Nat. Cancer 3, 25–42 (2022).
Shen, S., Vagner, S. & Robert, C. Persistent cancer cells: the deadly survivors. Cell 183, 860–874 (2020).
Gerstberger, S., Jiang, Q. & Ganesh, K. Metastasis. Cell 186, 1564–1579 (2023).
Deneve, E. et al. Capture of viable circulating tumor cells in the liver of colorectal cancer patients. Clin. Chem. 59, 1384–1392 (2013).
Matouk, I. J. et al. The role of the oncofetal H19 lncRNA in tumor metastasis orchestrating the EMT-MET decision. Oncotarget 7, 3748–3765 (2016).
Bergers, G. & Benjamin, L. E. Tumorigenesis and the angiogenic switch. Nat. Rev. Cancer 3, 401–410 (2003).
Rabinovsky, R., Uhr, J. W., Vitetta, E. S. & Yefenof, E. Cancer dormancy: lessons from a B cell lymphoma and adenocarcinoma of the prostate. Adv. Cancer Res. 97, 189–202 (2007).
Farrar, J. D. et al. Cancer dormancy. VII. A regulatory role for CD8+ T cells and IFN-gamma in establishing and maintaining the tumor-dormant state. J. Immunol. 162, 2842–2849 (1999).
Zhou, W. et al. The lncRNA H19 mediates breast cancer cell plasticity during EMT and MET plasticity by differentially sponging miR-200bc and let-7b. Sci. Signal. 10, eaak9557 (2017).
Jin, X. et al. A metastasis map of human cancer cell lines. Nature 588, 331–336 (2020).
Echeverria, G. V. et al. High-resolution clonal mapping of multi-organ metastasis in triple negative breast cancer. Nat. Commun. 9, 5079 (2018).
Basnet, H. et al. Flura-seq identifies organ-specific metabolic adaptations during early metastatic colonization. Elife. 8 (2019).
Lawson, D. A. et al. Single-cell analysis reveals a stem-cell program in human metastatic breast cancer cells. Nature 526, 131–135 (2015).
Speak, A. O. et al. A high-throughput in vivo screening method in the mouse for identifying regulators of metastatic colonization. Nat. Protoc. 12, 2465–2477 (2017).
Zhang, X. Interactions between cancer cells and bone microenvironment promote bone metastasis in prostate cancer. Cancer Commun. (Lond.). 39, 76 (2019).
Lin, H. et al. GATA2-mediated transcriptional activation of Notch3 promotes pancreatic cancer liver metastasis. Mol. Cells 45, 329–342 (2022).
Kastelan, S. et al. Liver metastasis in uveal melanoma—treatment options and clinical outcome. Front. Biosci. (Landmark Ed.). 27, 72 (2022).
Chen, W., Hoffmann, A. D., Liu, H. & Liu, X. Organotropism: new insights into molecular mechanisms of breast cancer metastasis. NPJ Precis. Oncol. 2, 4 (2018).
Cosphiadi, I. et al. Bone metastasis in advanced breast cancer: analysis of gene expression microarray. Clin. Breast Cancer 18, e1117–e1122 (2018).
Valastyan, S. & Weinberg, R. A. Tumor metastasis: molecular insights and evolving paradigms. Cell 147, 275–292 (2011).
Azubuike, U. F. & Tanner, K. Biophysical determinants of cancer organotropism. Trends Cancer 9, 188–197 (2023).
Schouten, L. J., Rutten, J., Huveneers, H. A. & Twijnstra, A. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer 94, 2698–2705 (2002).
Boire, A., Brastianos, P. K., Garzia, L. & Valiente, M. Brain metastasis. Nat. Rev. Cancer 20, 4–11 (2020).
Mirza, S., Jain, N. & Rawal, R. Evidence for circulating cancer stem-like cells and epithelial-mesenchymal transition phenotype in the pleurospheres derived from lung adenocarcinoma using liquid biopsy. Tumour Biol. 39, 1010428317695915 (2017).
Sihto H, L. J. et al. Breast cancer biological subtypes and protein expression predict for the preferential distant metastasis sites: a nationwide cohort study. Breast Cancer Res. 13, R87 (2011).
Witzel, I. et al. Breast cancer brain metastases: biology and new clinical perspectives. Breast Cancer Res. 18, 8 (2016).
Wang, Y. et al. Longitudinal detection of subcategorized CD44v6(+) CTCs and circulating tumor endothelial cells (CTECs) enables novel clinical stratification and improves prognostic prediction of small cell lung cancer: a prospective, multi-center study. Cancer Lett. 571, 216337 (2023).
Zhang, L. et al. The identification and characterization of breast cancer CTCs competent for brain metastasis. Sci. Transl. Med. 5, 180ra148 (2013).
Parri, M. & Chiarugi, P. Rac and Rho GTPases in cancer cell motility control. Cell Commun. Signal. 8, 23 (2010).
Chen, M. et al. Identification of RAC1 in promoting brain metastasis of lung adenocarcinoma using single-cell transcriptome sequencing. Cell Death Dis. 14, 330 (2023).
Chen, E. I. et al. Adaptation of energy metabolism in breast cancer brain metastases. Cancer Res. 67, 1472–1486 (2007).
Garcia-Espinosa, M. A. et al. Cerebral glucose metabolism and the glutamine cycle as detected by in vivo and in vitro 13C NMR spectroscopy. Neurochem Int. 45, 297–303 (2004).
Rao, J., Oz, G. & Seaquist, E. R. Regulation of cerebral glucose metabolism. Minerva Endocrinol. 31, 149–158 (2006).
Neumann, D., Schlattner, U. & Wallimann, T. A molecular approach to the concerted action of kinases involved in energy homoeostasis. Biochem Soc. Trans. 31, 169–174 (2003).
Aljohani, H. M. et al. Genetic mutations associated with lung cancer metastasis to the brain. Mutagenesis 33, 137–145 (2018).
Homma, S. et al. Nrf2 enhances cell proliferation and resistance to anticancer drugs in human lung cancer. Clin. Cancer Res. 15, 3423–3432 (2009).
Bowley, T. Y. et al. The RPL/RPS gene signature of melanoma CTCs associates with brain metastasis. Cancer Res. Commun. 2, 1436–1448 (2022).
Guimaraes, J. C. & Zavolan, M. Patterns of ribosomal protein expression specify normal and malignant human cells. Genome Biol. 17, 236 (2016).
Bos, P. D. et al. Genes that mediate breast cancer metastasis to the brain. Nature 459, 1005–1009 (2009).
Yuzhalin, A. E. & Yu, D. Brain metastasis organotropism. Cold Spring Harb. Perspect. Med. 10 (2020).
Zhou, W. et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell. 25, 501–515 (2014).
Harati, R. et al. Combinatorial targeting of microRNA-26b and microRNA-101 exerts a synergistic inhibition on cyclooxygenase-2 in brain metastatic triple-negative breast cancer cells. Breast Cancer Res. Treat. 187, 695–713 (2021).
Okajima, T. et al. Molecular cloning of brain-specific GD1alpha synthase (ST6GalNAc V) containing CAG/Glutamine repeats. J. Biol. Chem. 274, 30557–30562 (1999).
Basile, J. R., Barac, A., Zhu, T., Guan, K. L. & Gutkind, J. S. Class IV semaphorins promote angiogenesis by stimulating Rho-initiated pathways through plexin-B. Cancer Res. 64, 5212–5224 (2004).
Valiente, M. et al. Serpins promote cancer cell survival and vascular co-option in brain metastasis. Cell 156, 1002–1016 (2014).
Massague, J. & Obenauf, A. C. Metastatic colonization by circulating tumour cells. Nature 529, 298–306 (2016).
Nadal, R. et al. Combined analysis of copy number alterations by single-nucleotide polymorphism array and MYC status in non-metastatic breast cancer patients: comparison according to the circulating tumor cell status. Tumour Biol. 36, 711–718 (2015).
Schwartz, H. et al. Incipient melanoma brain metastases instigate astrogliosis and neuroinflammation. Cancer Res. 76, 4359–4371 (2016).
Chen, Q. et al. Carcinoma-astrocyte gap junctions promote brain metastasis by cGAMP transfer. Nature 533, 493–498 (2016).
Zhang, L. et al. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature 527, 100–104 (2015).
Peinado, H. et al. Pre-metastatic niches: organ-specific homes for metastases. Nat. Rev. Cancer 17, 302–317 (2017).
Altorki, N. K. et al. The lung microenvironment: an important regulator of tumour growth and metastasis. Nat. Rev. Cancer 19, 9–31 (2019).
Kaplan, R. N. et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438, 820–827 (2005).
Said, N., Sanchez-Carbayo, M., Smith, S. C. & Theodorescu, D. RhoGDI2 suppresses lung metastasis in mice by reducing tumor versican expression and macrophage infiltration. J. Clin. Invest. 122, 1503–1518 (2012).
Hiratsuka, S. et al. The S100A8-serum amyloid A3-TLR4 paracrine cascade establishes a pre-metastatic phase. Nat. Cell Biol. 10, 1349–1355 (2008).
Erler, J. T. et al. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 15, 35–44 (2009).
El Rayes, T. et al. Lung inflammation promotes metastasis through neutrophil protease-mediated degradation of Tsp-1. Proc. Natl Acad. Sci. USA 112, 16000–16005 (2015).
De Cock, J. M. et al. Inflammation triggers Zeb1-dependent escape from tumor latency. Cancer Res. 76, 6778–6784 (2016).
Ghajar, C. M. et al. The perivascular niche regulates breast tumour dormancy. Nat. Cell Biol. 15, 807–817 (2013).
Kim, T. H. et al. Integrin (alpha6beta4) signals through Src to increase expression of S100A4, a metastasis-promoting factor: implications for cancer cell invasion. Mol. Cancer Res. 7, 1605–1612 (2009).
Hoshino, A. et al. Tumour exosome integrins determine organotropic metastasis. Nature 527, 329–335 (2015).
Fong, M. Y. et al. Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat. Cell Biol. 17, 183–194 (2015).
Fina, E. et al. Gene signatures of circulating breast cancer cell models are a source of novel molecular determinants of metastasis and improve circulating tumor cell detection in patients. J. Exp. Clin. Cancer Res. 41, 78 (2022).
Wculek, S. K. & Malanchi, I. Neutrophils support lung colonization of metastasis-initiating breast cancer cells. Nature 528, 413–417 (2015).
Taftaf, R. et al. ICAM1 initiates CTC cluster formation and trans-endothelial migration in lung metastasis of breast cancer. Nat. Commun. 12, 4867 (2021).
Chen, Q., Zhang, X. H. & Massague, J. Macrophage binding to receptor VCAM-1 transmits survival signals in breast cancer cells that invade the lungs. Cancer Cell. 20, 538–549 (2011).
Oskarsson, T. et al. Breast cancer cells produce tenascin C as a metastatic niche component to colonize the lungs. Nat. Med. 17, 867–874 (2011).
Malanchi, I. et al. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature 481, 85–89 (2011).
Lalor, P. F., Lai, W. K., Curbishley, S. M., Shetty, S. & Adams, D. H. Human hepatic sinusoidal endothelial cells can be distinguished by expression of phenotypic markers related to their specialised functions in vivo. World J. Gastroenterol. 12, 5429–5439 (2006).
Paku S, D. B., Tóth, R. & Timár, J. Organ-specificity of the extravasation process: an ultrastructural study. Clin. Exp. Metastasis. 18, 481–492 (2000).
Xie, Z. et al. Exosome-delivered CD44v6/C1QBP complex drives pancreatic cancer liver metastasis by promoting fibrotic liver microenvironment. Gut 71, 568–579 (2022).
Li, S. et al. MAPK4 silencing in gastric cancer drives liver metastasis by positive feedback between cancer cells and macrophages. Exp. Mol. Med. 55, 457–469 (2023).
Mann, J., Reeves, H. L. & Feldstein, A. E. Liquid biopsy for liver diseases. Gut 67, 2204–2212 (2018).
Qi, L. N. et al. Circulating tumor cells undergoing EMT provide a metric for diagnosis and prognosis of patients with hepatocellular carcinoma. Cancer Res. 78, 4731–4744 (2018).
Zhang, H. et al. CD133 positive cells isolated from A549 cell line exhibited high liver metastatic potential. Neoplasma 61, 153–160 (2014).
Wu, Z. et al. TPO-induced metabolic reprogramming drives liver metastasis of colorectal cancer CD110+ tumor-initiating cells. Cell. Stem Cell. 17, 47–59 (2015).
Li, M. et al. Metabolomic analysis of circulating tumor cells derived liver metastasis of colorectal cancer. Heliyon. 9 (2023).
Ju, H. Q. et al. Modulation of redox homeostasis by inhibition of MTHFD2 in colorectal cancer: mechanisms and therapeutic implications. J. Natl Cancer Inst. 111, 584–596 (2019).
Maddocks, O. D. et al. Serine starvation induces stress and p53-dependent metabolic remodelling in cancer cells. Nature 493, 542–546 (2013).
Pollari, S. et al. Enhanced serine production by bone metastatic breast cancer cells stimulates osteoclastogenesis. Breast Cancer Res. Treat. 125, 421–430 (2011).
Li, S. et al. Tumour-derived exosomes in liver metastasis: a Pandora’s box. Cell Prolif, e13452 (2023).
Costa-Silva, B. et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol. 17, 816–826 (2015).
Lukanidin, E. & Sleeman, J. P. Building the niche: the role of the S100 proteins in metastatic growth. Semin. Cancer Biol. 22, 216–225 (2012).
Sun, H. et al. Hypoxia-inducible exosomes facilitate liver-tropic premetastatic niche in colorectal cancer. Hepatology 74, 2633–2651 (2021).
McDonald, B. et al. Systemic inflammation increases cancer cell adhesion to hepatic sinusoids by neutrophil mediated mechanisms. Int J. Cancer 125, 1298–1305 (2009).
Shasha, T., Gruijs, M. & van Egmond, M. Mechanisms of colorectal liver metastasis development. Cell Mol. Life Sci. 79, 607 (2022).
Li, C. et al. hnRNPA2B1-mediated extracellular vesicles sorting of miR-122-5p potentially promotes lung cancer progression. Int. J. Mol. Sci. 22 (2021).
Zeng, Z. et al. Cancer-derived exosomal miR-25-3p promotes pre-metastatic niche formation by inducing vascular permeability and angiogenesis. Nat. Commun. 9, 5395 (2018).
Yokota, Y. et al. Serum exosomal miR-638 is a prognostic marker of HCC via downregulation of VE-cadherin and ZO-1 of endothelial cells. Cancer Sci. 112, 1275–1288 (2021).
Fang, J. H. et al. Hepatoma cell-secreted exosomal microRNA-103 increases vascular permeability and promotes metastasis by targeting junction proteins. Hepatology 68, 1459–1475 (2018).
Takano Y, M. T. et al. Circulating exosomal microRNA-203 is associated with metastasis possibly via inducing tumor-associated macrophages in colorectal cancer. Oncotarget 8, 78598–78613 (2017).
Giannou, A. D. et al. Tissue resident iNKT17 cells facilitate cancer cell extravasation in liver metastasis via interleukin-22. Immunity 56, 125–142e112 (2023).
Mundy, G. R. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat. Rev. Cancer 2, 584–593 (2002).
Suva, L. J., Washam, C., Nicholas, R. W. & Griffin, R. J. Bone metastasis: mechanisms and therapeutic opportunities. Nat. Rev. Endocrinol. 7, 208–218 (2011).
Coleman, R. E. Skeletal complications of malignancy. Cancer 80, 1588–1594 (1997).
Chai, S. et al. Identification of epithelial and mesenchymal circulating tumor cells in clonal lineage of an aggressive prostate cancer case. NPJ Precis Oncol. 6, 41 (2022).
Awolaran, O., Brooks, S. A. & Lavender, V. Breast cancer osteomimicry and its role in bone specific metastasis; an integrative, systematic review of preclinical evidence. Breast 30, 156–171 (2016).
Anborgh, P. H., Mutrie, J. C., Tuck, A. B. & Chambers, A. F. Role of the metastasis-promoting protein osteopontin in the tumour microenvironment. J. Cell Mol. Med. 14, 2037–2044 (2010).
Kelly, T. et al. Expression of heparanase by primary breast tumors promotes bone resorption in the absence of detectable bone metastases. Cancer Res. 65, 5778–5784 (2005).
Sanders, J. L. et al. Extracellular calcium-sensing receptor expression and its potential role in regulating parathyroid hormone-related peptide secretion in human breast cancer cell lines. Endocrinology 141, 4357–4364 (2000).
Waning, D. L. & Guise, T. A. Molecular mechanisms of bone metastasis and associated muscle weakness. Clin. Cancer Res. 20, 3071–3077 (2014).
Yue, Z. et al. RSPO2 and RANKL signal through LGR4 to regulate osteoclastic premetastatic niche formation and bone metastasis. J. Clin. Invest. 132 (2022).
Theil, G., Lindner, C., Bialek, J. & Fornara, P. Association of circulating tumor cells with inflammatory and biomarkers in the blood of patients with metastatic castration-resistant prostate cancer. Life (Basel). 11 (2021).
Zhang, X. H. et al. Latent bone metastasis in breast cancer tied to Src-dependent survival signals. Cancer Cell. 16, 67–78 (2009).
Yoneda, T., Williams, P. J., Hiraga, T., Niewolna, M. & Nishimura, R. A bone-seeking clone exhibits different biological properties from the MDA-MB-231 parental human breast cancer cells and a brain-seeking clone in vivo and in vitro. J. Bone Min. Res. 16, 1486–1495 (2001).
Gay, L. J. & Felding-Habermann, B. Contribution of platelets to tumour metastasis. Nat. Rev. Cancer 11, 123–134 (2011).
Gersuk, G. M. et al. Inhibition of human natural killer cell activity by platelet-derived growth factor (PDGF). III. Membrane binding studies and differential biological effect of recombinant PDGF isoforms. Scand. J. Immunol. 33, 521–532 (1991).
Carvalho, I. et al. Overexpression of platelet-derived growth factor receptor alpha in breast cancer is associated with tumour progression. Breast Cancer Res. 7, R788–R795 (2005).
Leblanc, R. et al. Interaction of platelet-derived autotaxin with tumor integrin alphaVbeta3 controls metastasis of breast cancer cells to bone. Blood 124, 3141–3150 (2014).
Jackson, W. 3rd et al. Role of megakaryocytes in breast cancer metastasis to bone. Cancer Res. 77, 1942–1954 (2017).
Nguyen, D. X., Bos, P. D. & Massague, J. Metastasis: from dissemination to organ-specific colonization. Nat. Rev. Cancer 9, 274–284 (2009).
Sun, Y. X. et al. Skeletal localization and neutralization of the SDF-1(CXCL12)/CXCR4 axis blocks prostate cancer metastasis and growth in osseous sites in vivo. J. Bone Min. Res. 20, 318–329 (2005).
Tang, Y. et al. Pre-metastatic niche triggers SDF-1/CXCR4 axis and promotes organ colonisation by hepatocellular circulating tumour cells via downregulation of Prrx1. J. Exp. Clin. Cancer Res. 38, 473 (2019).
Wu, Q. et al. SCUBE2 mediates bone metastasis of luminal breast cancer by modulating immune-suppressive osteoblastic niches. Cell Res. 33, 464–478 (2023).
Smid, M. et al. Genes associated with breast cancer metastatic to bone. J. Clin. Oncol. 24, 2261–2267 (2006).
Sleeman, J. P. The lymph node pre-metastatic niche. J. Mol. Med. (Berl.). 93, 1173–1184 (2015).
Kurokawa, Y. Experiments on lymph node metastasis by intralymphatic inoculation of rat ascites tumor cells, with special reference to lodgement, passage, and growth of tumor cells in lymph nodes. Gan 61, 461–471 (1970).
Preynat-Seauve, O. et al. Extralymphatic tumors prepare draining lymph nodes to invasion via a T-cell cross-tolerance process. Cancer Res. 67, 5009–5016 (2007).
Nevala, W. K. et al. Evidence of systemic Th2-driven chronic inflammation in patients with metastatic melanoma. Clin. Cancer Res. 15, 1931–1939 (2009).
Lund, A. W. et al. VEGF-C promotes immune tolerance in B16 melanomas and cross-presentation of tumor antigen by lymph node lymphatics. Cell Rep. 1, 191–199 (2012).
Scarlett, U. K. et al. Ovarian cancer progression is controlled by phenotypic changes in dendritic cells. J. Exp. Med. 209, 495–506 (2012).
Nunez, N. G. et al. Tumor invasion in draining lymph nodes is associated with Treg accumulation in breast cancer patients. Nat. Commun. 11, 3272 (2020).
Shabaneh, T. B. et al. Oncogenic BRAF(V600E) Governs Regulatory T-cell Recruitment during Melanoma Tumorigenesis. Cancer Res. 78, 5038–5049 (2018).
du Bois, H., Heim, T. A. & Lund, A. W. Tumor-draining lymph nodes: At the crossroads of metastasis and immunity. Sci. Immunol. 6, eabg3551 (2021).
Skobe, M. et al. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat. Med. 7, 192–198 (2001).
Hirakawa, S. et al. VEGF-C-induced lymphangiogenesis in sentinel lymph nodes promotes tumor metastasis to distant sites. Blood 109, 1010–1017 (2007).
Hirakawa, S. et al. VEGF-A induces tumor and sentinel lymph node lymphangiogenesis and promotes lymphatic metastasis. J. Exp. Med. 201, 1089–1099 (2005).
Fleury, M. E., Boardman, K. C. & Swartz, M. A. Autologous morphogen gradients by subtle interstitial flow and matrix interactions. Biophys. J. 91, 113–121 (2006).
Zhang, Q. et al. ETV4 mediated tumor-associated neutrophil infiltration facilitates lymphangiogenesis and lymphatic metastasis of bladder cancer. Adv. Sci. (Weinh.). 10, e2205613 (2023).
Pan, L. et al. Distribution of circulating tumor cell phenotype in early cervical cancer. Cancer Manag. Res. 11, 5531–5536 (2019).
Markiewicz, A. et al. Mesenchymal phenotype of CTC-enriched blood fraction and lymph node metastasis formation potential. PLoS ONE 9, e93901 (2014).
Yi-Wen, W. et al. Stem cell-like circulating tumor cells indicate poor prognosis in gastric cancer. Arch. Med. Sci. 18, 1297–1307 (2022).
Pang, S. et al. Molecular profiles of single circulating tumor cells from early breast cancer patients with different lymph node statuses. Thorac. Cancer 14, 156–167 (2023).
Zhou M, X. P., Chen, L., Zhang, P. & Xu, F. Correlation between the expression of CD24 on circulating tumor cells and prognosis in breast cancer. Am. J. Transl. Res. 15, 1941–1952 (2023).
Li, Z. et al. Predictive value of folate receptor-positive circulating tumor cells for the preoperative diagnosis of lymph node metastasis in patients with lung adenocarcinoma. Small Methods 5, e2100152 (2021).
Jiang, S. et al. Identify the clinicopathological characteristics of lung carcinoma patients being false negative in folate receptor based circulating tumor cell detection. Small Methods 7, e2300055 (2023).
Saloustros, E. et al. Cytokeratin-19 mRNA-positive circulating tumor cells during follow-up of patients with operable breast cancer: prognostic relevance for late relapse. Breast Cancer Res. 13, R60 (2011).
Wang, X. M. et al. KRT19 and CEACAM5 mRNA-marked circulated tumor cells indicate unfavorable prognosis of breast cancer patients. Breast Cancer Res. Treat. 174, 375–385 (2019).
Chen, C. C. et al. Simultaneous detection of multiple mRNA markers CK19, CEA, c-Met, Her2/neu and hMAM with membrane array, an innovative technique with a great potential for breast cancer diagnosis. Cancer Lett. 240, 279–288 (2006).
Yie, S. M. et al. Detection of Survivin-expressing circulating cancer cells in the peripheral blood of breast cancer patients by a RT-PCR ELISA. Clin. Exp. Metastasis. 23, 279–289 (2006).
Ke, F. et al. Application of suspension array assay to detect marker genes expression of circulating tumor cells for early prediction of breast cancer metastasis. Zhonghua Yi Xue Za Zhi. 87, 2257–2261 (2007).
Shen, C., Hu, L., Xia, L. & Li, Y. The detection of circulating tumor cells of breast cancer patients by using multimarker (Survivin, hTERT and hMAM) quantitative real-time PCR. Clin. Biochem. 42, 194–200 (2009).
Espana, L. et al. Overexpression of Bcl-xL in human breast cancer cells enhances organ-selective lymph node metastasis. Breast Cancer Res. Treat. 87, 33–44 (2004).
Ignatiadis, M., Sledge, G. W. & Jeffrey, S. S. Liquid biopsy enters the clinic—implementation issues and future challenges. Nat. Rev. Clin. Oncol. 18, 297–312 (2021).
Vasseur, A. et al. Clinical utility of circulating tumor cells: an update. Mol. Oncol. 15, 1647–1666 (2020).
De Rubis, G., Rajeev Krishnan, S. & Bebawy, M. Liquid biopsies in cancer diagnosis, monitoring, and prognosis. Trends Pharmacol. Sci. 40, 172–186 (2019).
Ren, L. et al. Clinical significance of a circulating tumor cell-based classifier in stage IB lung adenocarcinoma. Ann. Surg. 277, e439–e448 (2023).
Zhang, Z. et al. Relationship between circulating tumour cell count and prognosis following chemotherapy in patients with advanced non-small-cell lung cancer. Respirology 21, 519–525 (2016).
Ye, Z. et al. Detecting and phenotyping of aneuploid circulating tumor cells in patients with various malignancies. Cancer Biol. Ther. 20, 546–551 (2019).
Gogoi, P. et al. Development of an automated and sensitive microfluidic device for capturing and characterizing circulating tumor cells (CTCs) from Clinical Blood Samples. PLoS ONE 11, e0147400 (2016).
Chen, B. et al. Targeting negative surface charges of cancer cells by multifunctional nanoprobes. Theranostics 6, 1887–1898 (2016).
Lankiewicz, S., Rivero, B. G. & Bocher, O. Quantitative real-time RT-PCR of disseminated tumor cells in combination with immunomagnetic cell enrichment. Mol. Biotechnol. 34, 15–27 (2006).
Werner, S. L. et al. Analytical validation and capabilities of the epic CTC platform: enrichment-free circulating tumour cell detection and characterization. J. Circ. Biomark. 4, 3 (2015).
Campton, D. E. et al. High-recovery visual identification and single-cell retrieval of circulating tumor cells for genomic analysis using a dual-technology platform integrated with automated immunofluorescence staining. BMC Cancer 15, 360 (2015).
Nagrath, S. et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature 450, 1235–1239 (2007).
Huang, L. R., Cox, E. C., Austin, R. H. & Sturm, J. C. Continuous particle separation through deterministic lateral displacement. Science 304, 987–990 (2004).
Carlo, D. D., Irimia, D., Tompkins, R. G. & Toner, M. Continuous inertial focusing, ordering, and separation of particles in microchannels. Proc. Natl Acad. Sci. USA 104, 18892–18897 (2007).
Karabacak, N. M. et al. Microfluidic, marker-free isolation of circulating tumor cells from blood samples. Nat. Protoc. 9, 694–710 (2014).
Parkinson, D. R. et al. Considerations in the development of circulating tumor cell technology for clinical use. J. Transl. Med. 10, 138 (2012).
Chen, Z. et al. Surgical stress and cancer progression: the twisted tango. Mol. Cancer 18, 132 (2019).
Gall, T. M. et al. Reduced dissemination of circulating tumor cells with no-touch isolation surgical technique in patients with pancreatic cancer. JAMA Surg. 149, 482–485 (2014).
Kejik, Z. et al. Circulating tumour cells (CTCs) in NSCLC: from prognosis to therapy design. Pharmaceutics 13 (2021).
Scully, O. J., Bay, B.-H., Yip, G. & Yu, Y. Breast cancer metastasis. Cancer Genomics Proteom. 9, 311–320 (2012).
Espejo-Cruz, M. L. et al. Circulating tumor cells in hepatocellular carcinoma: a comprehensive review and critical appraisal. Int. J. Mol. Sci. 22 (2021).
Nieto, M. A. Epithelial plasticity: a common theme in embryonic and cancer cells. Science 342, 1234850 (2013).
Santamaria, P. G., Moreno-Bueno, G., Portillo, F. & Cano, A. EMT: Present and future in clinical oncology. Mol. Oncol. 11, 718–738 (2017).
Nieto, M. A., Huang, R. Y., Jackson, R. A. & Thiery, J. P. Emt: 2016. Cell 166, 21–45 (2016).
Smith, B. N. & Bhowmick, N. A. Role of EMT in metastasis and therapy resistance. J. Clin. Med. 5 (2016).
Yu, M. et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science 339, 580–584 (2013).
Saleem, R. A. et al. Essential structural and functional determinants within the forkhead domain of FOXC1. Nucleic Acids Res. 32, 4182–4193 (2004).
Berry, F. B., Saleem, R. A. & Walter, M. A. FOXC1 transcriptional regulation is mediated by N- and C-terminal activation domains and contains a phosphorylated transcriptional inhibitory domain. J. Biol. Chem. 277, 10292–10297 (2002).
Ray, P. S. et al. FOXC1 is a potential prognostic biomarker with functional significance in basal-like breast cancer. Cancer Res. 70, 3870–3876 (2010).
Xu, Z. Y. et al. FOXC1 contributes to microvascular invasion in primary hepatocellular carcinoma via regulating epithelial-mesenchymal transition. Int. J. Biol. Sci. 8, 1130–1141 (2012).
Powell, A. A. et al. Single cell profiling of circulating tumor cells: transcriptional heterogeneity and diversity from breast cancer cell lines. PLoS ONE 7, e33788 (2012).
Wei, C. et al. Crosstalk between cancer cells and tumor associated macrophages is required for mesenchymal circulating tumor cell-mediated colorectal cancer metastasis. Mol. Cancer 18, 64 (2019).
Chen, Z. et al. Branched-chain aminotransferase 1 promotes Schwann cell migration and proliferation to accelerate facial nerve regeneration through the Twist/FoxC1 and Sox2 pathways. Int J. Biol. Macromol. 242, 124870 (2023).
Driemel, C. et al. Context-dependent adaption of EpCAM expression in early systemic esophageal cancer. Oncogene 33, 4904–4915 (2014).
Pang, M. F. et al. TGF-beta1-induced EMT promotes targeted migration of breast cancer cells through the lymphatic system by the activation of CCR7/CCL21-mediated chemotaxis. Oncogene 35, 748–760 (2016).
Wang, D. et al. C/EBPdelta-Slug-Lox1 axis promotes metastasis of lung adenocarcinoma via oxLDL uptake. Oncogene 39, 833–848 (2020).
Yang, L. et al. The effect of aspirin on circulating tumor cells in metastatic colorectal and breast cancer patients: a phase II trial study. Clin. Transl. Oncol. 20, 912–921 (2018).
Li, X. et al. Anticancer effects of curcumin on nude mice bearing lung cancer A549 cell subsets SP and NSP cells. Oncol. Lett. 16, 6756–6762 (2018).
Liang, Z. et al. Curcumin reverses tobacco smoke‑induced epithelial‑mesenchymal transition by suppressing the MAPK pathway in the lungs of mice. Mol. Med Rep. 17, 2019–2025 (2018).
Huang, G. et al. TGF-beta signal rewiring sustains epithelial-mesenchymal transition of circulating tumor cells in prostate cancer xenograft hosts. Oncotarget 7, 77124–77137 (2016).
Ito, M. et al. Impact of circulating tumour cells on survival of eribulin-treated patients with metastatic breast cancer. Med Oncol. 36, 89 (2019).
Shiao, S. L., Chu, G. C. & Chung, L. W. Regulation of prostate cancer progression by the tumor microenvironment. Cancer Lett. 380, 340–348 (2016).
Ao, Z. et al. Identification of cancer-associated fibroblasts in circulating blood from patients with metastatic breast cancer. Cancer Res. 75, 4681–4687 (2015).
Liu, M., Yang, J., Xu, B. & Zhang, X. Tumor metastasis: Mechanistic insights and therapeutic interventions. MedComm 2, 587–617 (2021).
Hosaka, K. et al. Pericyte-fibroblast transition promotes tumor growth and metastasis. Proc. Natl Acad. Sci. USA 113, E5618–E5627 (2016).
Hosaka, K. et al. Tumour PDGF-BB expression levels determine dual effects of anti-PDGF drugs on vascular remodelling and metastasis. Nat. Commun. 4, 2129 (2013).
Kreutz, D. et al. Curcumin exerts its antitumor effects in a context dependent fashion. J. Proteom. 182, 65–72 (2018).
Fang, T. et al. Tumor-derived exosomal miR-1247-3p induces cancer-associated fibroblast activation to foster lung metastasis of liver cancer. Nat. Commun. 9, 191 (2018).
Osmulski, P. A. et al. Contacts with macrophages promote an aggressive nanomechanical phenotype of circulating tumor cells in prostate cancer. Cancer Res. 81, 4110–4123 (2021).
Yang, Y. et al. The PDGF-BB-SOX7 axis-modulated IL-33 in pericytes and stromal cells promotes metastasis through tumour-associated macrophages. Nat. Commun. 7, 11385 (2016).
Tseng, J. Y. et al. Interleukin-17A modulates circulating tumor cells in tumor draining vein of colorectal cancers and affects metastases. Clin. Cancer Res. 20, 2885–2897 (2014).
Xia, J. et al. Versatile ginsenoside Rg3 liposomes inhibit tumor metastasis by capturing circulating tumor cells and destroying metastatic niches. Sci. Adv. 8, eabj1262 (2022).
Pavco, P. A. et al. Antitumor and antimetastatic activity of ribozymes targeting the messenger RNA of vascular endothelial growth factor receptors. Clinical cancer research: an official journal of the American Association for Cancer Research. Clin. Cancer Res. 6, 2094–2103 (2000).
Wu, Y. et al. Anti-vascular endothelial growth factor receptor-1 antagonist antibody as a therapeutic agent for cancer. Clin. Cancer Res. 12, 6573–6584 (2006).
Bae, D. G., Kim, T. D., Li, G., Yoon, W. H. & Chae, C. B. Anti-flt1 peptide, a vascular endothelial growth factor receptor 1-specific hexapeptide, inhibits tumor growth and metastasis. Clin. Cancer Res. 11, 2651–2661 (2005).
Shen, L. et al. Antiangiogenic and antitumoral effects mediated by a vascular endothelial growth factor receptor 1 (VEGFR-1)-targeted DNAzyme. Mol. Med. 19, 377–386 (2013).
Liu, Z. et al. Protein tyrosine phosphatase receptor type O expression in the tumor niche correlates with reduced tumor growth, angiogenesis, circulating tumor cells and metastasis of breast cancer. Oncol. Rep. 33, 1908–1914 (2015).
Dong, H. et al. Tumor-derived exosomal protein tyrosine phosphatase receptor type O polarizes macrophage to suppress breast tumor cell invasion and migration. Front Cell Dev. Biol. 9, 703537 (2021).
Li, D. et al. Cancer-specific calcium nanoregulator suppressing the generation and circulation of circulating tumor cell clusters for enhanced anti-metastasis combinational chemotherapy. Acta Pharm. Sin. B. 11, 3262–3271 (2021).
Ortiz-Otero, N. et al. TRAIL-coated leukocytes to kill circulating tumor cells in the flowing blood from prostate cancer patients. BMC Cancer 21, 898 (2021).
Liang, Z. Q. et al. Exploring the anti-metastatic effects of Astragalus mongholicus Bunge-Curcuma aromatica Salisb. on colorectal cancer: A network-based metabolomics and pharmacology approach. Phytomedicine 114, 154772 (2023).
Fu, S. et al. Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity. Nature 473, 528–531 (2011).
Altamirano, J. et al. The inotropic effect of cardioactive glycosides in ventricular myocytes requires Na+-Ca2+ exchanger function. J. Physiol. 575, 845–854 (2006).
Cavey, M. & Lecuit, T. Molecular bases of cell-cell junctions stability and dynamics. Cold Spring Harb. Perspect. Biol. 1, a002998 (2009).
Kim, S. A. et al. Calcium-dependent dynamics of cadherin interactions at cell-cell junctions. Proc. Natl Acad. Sci. USA 108, 9857–9862 (2011).
Salado, C. et al. Resveratrol prevents inflammation-dependent hepatic melanoma metastasis by inhibiting the secretion and effects of interleukin-18. J. Transl. Med. 9, 59 (2011).
Restivo, A. et al. Aspirin as a neoadjuvant agent during preoperative chemoradiation for rectal cancer. Br. J. Cancer 113, 1133–1139 (2015).
Schwarz, S. et al. Glycosaminoglycans as tools to decipher the platelet tumor cell interaction: a focus on P-selectin. Molecules 25 (2020).
Labuschagne, C. F. et al. Cell clustering promotes a metabolic switch that supports metastatic colonization. Cell Metab. 30, 720–734e725 (2019).
Liu, T. et al. Circulating glioma cells exhibit stem cell-like properties. Cancer Res. 78, 6632–6642 (2018).
Kakar, S. S. et al. Withaferin a alone and in combination with cisplatin suppresses growth and metastasis of ovarian cancer by targeting putative cancer stem cells. PLoS ONE 9, e107596 (2014).
Andrade, F. et al. Polymeric micelles targeted against CD44v6 receptor increase niclosamide efficacy against colorectal cancer stem cells and reduce circulating tumor cells in vivo. J. Control Release 331, 198–212 (2021).
Li, Y. et al. Nanoparticle-delivered miriplatin ultrasmall dots suppress triple negative breast cancer lung metastasis by targeting circulating tumor cells. J. Control Release 329, 833–846 (2021).
Liu, S. et al. Expression of intercellular adhesion molecule 1 by hepatocellular carcinoma stem cells and circulating tumor cells. Gastroenterology 144, 1031–1041e1010 (2013).
Li, Y. et al. USP1 maintains the survival of liver circulating tumor cells by deubiquitinating and stabilizing TBLR1. Front. Oncol. 10, 554809 (2020).
Isakoff, S. J. Triple-negative breast cancer: role of specific chemotherapy agents. Cancer J. 16, 53–61 (2010).
Yuan, Z. et al. Ginsenoside Rg3 promotes cytotoxicity of Paclitaxel through inhibiting NF-kappaB signaling and regulating Bax/Bcl-2 expression on triple-negative breast cancer. Biomed. Pharmacother. 89, 227–232 (2017).
Hu, M. et al. Circulating tumor cells in colorectal cancer in the era of precision medicine. J. Mol. Med. (Berl.). 100, 197–213 (2022).
Zavridou, M. et al. Prognostic significance of gene expression and DNA methylation markers in circulating tumor cells and paired plasma derived exosomes in metastatic castration resistant prostate cancer. Cancers (Basel) 13 (2021).
Markou, A., Tzanikou, E. & Lianidou, E. The potential of liquid biopsy in the management of cancer patients. Semin. Cancer Biol. 84, 69–79 (2022).
Ahn, J. C. et al. Detection of circulating tumor cells and their implications as a biomarker for diagnosis, prognostication, and therapeutic monitoring in hepatocellular carcinoma. Hepatology 73, 422–436 (2021).
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (82330065, 30900650, 81372501, 81572260, 81172232, and 31430030) and the Guangzhou or Guangdong Science and Technology Planning Program (2023B1111020005, 2023B03J0106, 2021B1212040017, 20170402094, 2018A050506036 and 2020B1515120032).
Author information
Authors and Affiliations
Contributions
Z.K., W.H., and Y.Z. proposed the study conception. Q.Z., B.L., X.S., Y.L., Y.W., Z.X., and L.R. searched the references. Q.Z., B.L., and X.S. drafted the manuscript. Q.Z., Y.L., and T.F. drawn the figures and tables. Each author made substantial contributions to the manuscript in writing and editing. All authors have read and approved the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhan, Q., Liu, B., Situ, X. et al. New insights into the correlations between circulating tumor cells and target organ metastasis. Sig Transduct Target Ther 8, 465 (2023). https://doi.org/10.1038/s41392-023-01725-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41392-023-01725-9
This article is cited by
-
A Senescence-Associated Secretory Phenotype of Bone Marrow Mesenchymal Stem Cells Inhibits the Viability of Breast Cancer Cells
Stem Cell Reviews and Reports (2024)