Abstract
Background
Active surveillance (AS) is generally recognized as the preferred option for men with low-risk prostate cancer. Current guidelines use prostate-specific antigen (PSA) of 10–20 ng/mL or low-volume biopsy Gleason grade group (GG) 2 as features that, in part, define the favorable intermediate-risk disease and suggest that AS may be considered for some men in this risk category.
Methods
We identified 26,548 men initially managed with AS aged <80 years, with clinically localized prostate cancer (cT1-2cN0M0), PSA ≤ 20 ng/mL, biopsy GG ≤ 2 with percent positive cores ≤33% and who converted to treatment with radical prostatectomy from the surveillance, epidemiology, and end results prostate with the watchful waiting database. Multivariable logistic regression was performed to determine predictors of adverse pathology at RP according to PSA level (<10 vs 10–20 ng/mL) and GG (1 vs 2).
Results
Of 1731 men with GG 1 disease and PSA 10–20 ng/mL, 382 (22.1%) harbored adverse pathology compared to 2340 (28%) of 8,367 men with GG 2 and a PSA < 10 ng/mL who had adverse pathology at RP. On multivariable analysis, the odds of harboring adverse pathology with a PSA 10–20 ng/mL (odds ratio [OR] 1.87, 95% confidence interval [CI] 1.71–2.05, p < 0.001) was less than that of GG 2 (OR 2.56, 95%CI 2.40–2.73, p < 0.001) after adjustment.
Conclusions
Our results support extending AS criteria more permissively to carefully selected men with PSA 10–20 ng/mL and GG 1 disease.
Similar content being viewed by others
Introduction
The current treatment paradigm for men with localized prostate cancer involves risk stratification to distinguish men whose disease can be safely managed with active surveillance (AS) from those more likely to benefit from immediate definitive treatment [1,2,3]. The use of prostate-specific antigen (PSA) as a threshold to define eligibility for AS varies widely, with PSA > 10 ng/mL used to exclude men in certain large AS series [4]. The absolute value of PSA can be a driver of both patient and physician anxiety to offer AS [5]. Contemporary risk stratification tools demonstrate that a higher PSA incurs an increased risk of high-grade cancer; however, it is unclear how applicable a threshold PSA of 10 ng/mL is in an AS population where the volume of biopsy-detected disease is by definition very low.
The National Comprehensive Cancer Network (NCCN) prostate cancer guidelines use Gleason grade group (GG) 1 or 2, <50% biopsy cores positive and PSA 10–20 ng/mL as features that define “favorable intermediate-risk” disease and suggest that AS may be an option for some men within this risk category [6], prompting a pressing need to define AS eligibility more precisely within this group.
Prior analyses using surveillance, epidemiology, and end results (SEER) registry data or similar US datasets to evaluate prostate cancer management and outcomes in AS, have lacked a validated indicator of AS use, and analyses have defined “conservative management” based on the absence of identifiable active treatment, or similar proxies which are not generally adequate. To address this, we utilized the newly released SEER prostate with watchful waiting (WW) database which includes an explicit indicator for AS [7].
In this study, we aimed to determine the risk of pathological upgrading or upstaging according to PSA level (<10 vs 10–20 ng/mL) and grade group (GG) (1 vs 2) in men with intermediate-risk localized prostate cancer who underwent radical prostatectomy (RP) using the SEER-WW database.
Subjects and methods
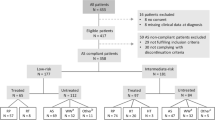
Men were identified from the SEER-WW database which includes a dichotomous variable for AS/WW (yes or no/unknown) for men diagnosed with prostate cancer from 18 SEER registries between January 2010 and December 2015 [8]. Over this period, SEER covered approximately 30% of the US population. SEER-WW includes data on the documented initial management intent of the treating physician recorded in the medical record and whether the patient was converted to definitive treatment within one year of diagnosis, which is unique and the primary focus of this dataset compared to prior SEER datasets. The cohort was then restricted to men aged <80 years with clinically localized prostate cancer (cT1-2cN0M0), PSA ≤ 20 ng/mL, biopsy GG ≤ 2 with percent positive cores (PPC) ≤ 33% who underwent RP (n = 29,120). Patients with no surgical pathology information were excluded (n = 2572), yielding a final cohort of 26,548 for the analyses. The primary outcome was adverse pathology, defined as pathological upgrading (GG ≥ 3) or any upstaging to non-localized disease (≥pT3a).
Statistical analysis
An inherent limitation of the SEER-WW database is the extent of missing data with only 46% of cases with complete data on basic clinical characteristics for risk stratification [7]. Before granting access to the database, the SEER Program requires investigators to acknowledge that the amount of missing data is considerable. To address this limitation, we performed multiple imputations using the Amelia II, R package (version 1.7.5), which uses an expectation-maximization with bootstrapping algorithm. We have described this in detail in prior publications using the SEER-WW dataset [7, 9]. Briefly, imputed variables were clinical T stage, biopsy GG, PSA, number of positive cores, and PPC. Year of diagnosis, race/ethnicity, age, insurance, marital status, and initial treatment were used as additional covariates for the multiple imputation model. We handled the SEER registry as a cross-sectional variable and year of diagnosis as a time-series variable. PSA was log-transformed. Race/ethnicity, initial treatment, health insurance, and marital status were inputted as nominal variables, and clinical T stage, biopsy GG, and a number of positive cores were inputted as ordered variables in the model. We generated five imputations under 1000 maximum resampling. Owing to a lack of granularity in staging information of T1NOS and T2NOS codes, the data were separated into two datasets (T1 and T2) for the multiple imputation models. These two datasets were then recombined to make the final multiple imputation datasets.
Descriptive statistics were generated to report the demographic, clinical, and pathologic characteristics of the study cohort. Means and standard deviations (SD) were reported for continuous variables. Multivariable logistic regression was performed to determine predictors of adverse pathology at RP. Odds ratios (OR) and 95% confidence intervals (CI) were reported for the regression models. Statistical analyses were performed using R version 3.6 and all p values were 2-sided and p < 0.05 was considered statistically significant. This study was granted an exemption and consent was waived by the University of California, San Francisco Institutional Review Board, given the use of publicly available data. This study followed the strengthening the reporting of observational studies in epidemiology (STROBE) reporting guidelines for cohort studies [10].
Results
In total 26,548 men were included in the final cohort (<80 years, with clinically localized prostate cancer (cT1-2cN0M0), PSA ≤ 20 ng/mL, biopsy GG ≤ 2 with percent positive cores (PPC) ≤ 33% who underwent RP and had surgical pathology available).
Table 1 summarizes baseline characteristics and pathological outcomes of the study cohort according to biopsy GG and PSA level. Of the men with GG 1 disease, 15,301 had a PSA < 10 ng/mL, and 1731 had a PSA 10–20 ng/mL. In men with GG 2 disease, 8367 had a PSA < 10 ng/mL, and 1149 had a PSA 10–20 ng/mL. In the 1731 men who had GG 1 disease and a PSA 10–20 ng/mL, mean (±SD) PSA was 13.1 ng/mL ±2.7, 259 (15.0%) were Black, 1298 (75.0%) had clinical T1 disease and mean was PPC 16.6 ± 8.1. In men diagnosed with GG 2 disease, 8,367 had a PSA < 10 ng/mL, and 1149 had a PSA 10–20 ng/mL. Of the 8367 men with GG 2 disease and a PSA < 10 ng/mL, the mean PSA was 5.4 ng/mL ±1.9, 1038 (12.4%) were Black, 5872 (70.2%) had clinical T1 disease and mean PPC 19.2 ± 7.7.
Compared to men with PSA < 10 ng/mL and GG 2 disease, a significantly smaller proportion of men with PSA 10–20 ng/mL and GG 1 disease experienced pathological upgrading (16.3% vs 11.6%), pathological upstaging (16.7% vs 14.6%) and adverse pathology (28.0% vs 22.1%, all p < 0.001). On multivariable analysis, PSA 10–20 ng/mL (OR 1.87, 95% CI 1.71–2.05, p < 0.001) had lower odds of adverse pathology at RP than those with GG 2 (OR 2.56, 95% CI 2.40–2.73, p < 0.001) after adjustment for age, year of diagnosis, race, clinical stage and PPC (Table 2). As a sensitivity analysis, the interaction between PSA 10–20 ng/mL and GG 2 on the primary endpoint of adverse pathology at RP was found to be not significant (OR 0.90, 95% CI 0.75–1.07, p = 0.23) (Supplementary Table 1).
Discussion
In this study, we used data from the SEER-WW database, a US population-level database with an explicit indicator of AS/WW, and found that men with GG 1 disease and a PSA of 10–20 ng/mL on AS have a lower risk of adverse pathology at RP compared to men with GG 2 and a PSA < 10 ng/mL. Despite concerns about extending AS criteria to intermediate-risk patients [11], the evidence to support the use of PSA as a threshold to identify appropriate patients and exclude those at higher risk for progression remains unclear. Nonetheless, we found that a larger proportion of men with GG 1 and PSA 10–20 ng/mL received AS than men with GG 2 and PSA < 10 ng/mL in the SEER-WW database.
A cross-sectional study in the National Prostate Cancer Register of Sweden found the use of AS increased from 31% in 2009 to 53% in 2014 in men with GG 1 prostate cancer and PSA 10–20 ng/mL [12]. A further study using this nationwide, population-based cohort compared 5087 men with GG 1 disease and a PSA < 10 ng/mL with men diagnosed with GG 2 disease or PSA 10–20 ng/mL and who underwent RP within 6 months of diagnosis. The outcomes were upgrading from GG 3 to 5 and a compositive outcome of adverse pathology (defined as GG 3–5, extracapsular extension, seminal vesical invasion, or positive lymph nodes). Overall, men with GG 1 disease and a PSA 10–15 ng/mL and PSA density <0.15 ng/mL/cm3 did not significantly differ in upgrading or adverse pathology findings compared to men with NCCN low-risk prostate cancer [13]. However, the sample size was small in that analysis compared to our study sample.
Results from 698 patients in Sunnybrook AS cohort 82 patients had a baseline PSA > 10 ng/mL and 157 with a PSA rise to >10 ng/mL during surveillance found that PSA on multivariate analysis was not clearly predictive of future adverse histology at RP [14]. There was also a trend for patients in this cohort with a PSA rising over 10 ng/mL on AS to have a higher Gleason score on follow-up biopsy. Furthermore, a higher incidence of high-grade disease and positive margins at RP among those whose PSA rose over 10 ng/mL on surveillance confirms that PSA monitoring can still yield important information in identifying significant cancers. However, the authors found that no patients who started AS with a PSA over 10 ng/mL had high-grade (Gleason ≥8) disease [14]. This is consistent with prior reports [15, 16] and suggests that for low-risk disease baseline PSA may carry limited prognostic value, but is insufficient to discriminate whether surveillance is a safe strategy.
In addition to upgrading and adverse pathology at RP, longer-term outcomes for men on AS are important, particularly if extending criteria to intermediate-risk patients. Data from the Sunnybrook AS cohort found that 15-year metastasis-free survival was 94% in men with GG 1 regardless of whether PSA was <10 or 10–20 ng/ml vs 84% 15-year metastasis-free survival in those with GG 2 and PSA < 20 ng/ml [17]. These findings also lend further support to the concept of risk stratification in AS based on multivariable instruments rather than binary cut points.
Several limitations are inherent to our analysis, which need to be acknowledged. The SEER-WW dataset does not contain data on PSA density which may reduce the bias of PSA values induced by prostate volume. Recent data suggests that men on AS with GG 2 disease and higher PSA density at baseline have a shorter time to definitive treatment compared to men with GG 1 disease and lower PSA density [18]. AS should be informed by incorporating clinical parameters such as PSA density, PSA kinetics, MRI, and genomic biomarkers into patient-centered decision-making [19]. While the SEER-WW dataset is population-based within SEER registry regions, it does not represent the entire United States and does not include the newly added Massachusetts or Wisconsin SEER registry regions. SEER-WW includes the initial management intent and whether the patient was converted to definitive treatment within 1 year of diagnosis, therefore our results may not be generalizable to a broader population of men on AS where decisions on follow up or treatment should be dynamically informed by the unique longitudinal trajectory of each patient with PSA density, PSA kinetics, MRI and biopsy [18, 19]. Although SEER-WW specifies the initial documented management intent of AS/WW by the treating physician, it does indicate the rationale for subsequent definitive treatment. Our primary outcome was adverse pathology at surgery and is therefore only an intermediate endpoint. The long-term oncologic outcomes of these men are currently unknown. Furthermore, due to imperfect data collection/reporting of pN + in SEER-WW, we were unable to assess pathologic nodal disease at RP as an outcome. Despite this, our study has numerous strengths including a large, racially diverse cohort derived from a dataset with an explicit variable for AS/WW and represents comprehensive nationwide data on current practice patterns on AS.
Conclusions
In conclusion, our results support extending the criteria for AS to carefully selected men with PSA 10–20 ng/mL for GG 1 prostate cancer and should be accompanied by informed decision-making.
Data availability
Data used in this study are publicly available.
References
Chen RC, Rumble RB, Loblaw DA, Finelli A, Ehdaie B, Cooperberg MR, et al. Active surveillance for the management of localized prostate cancer (cancer care Ontario guideline): American society of clinical oncology clinical practice guideline endorsement. J Clin Oncol. 2016;34:2182–90.
Sanda MG, Cadeddu JA, Kirkby E, Chen RC, Crispino T, Fontanarosa J, et al. Clinically localized prostate cancer: AUA/ASTRO/SUO guideline. Part I: risk stratification, shared decision making, and care options. J Urol. 2018;199:683–90.
Mottet N, Bellmunt J, Bolla M, Briers E, Cumberbatch MG, De Santis M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2017;71:618–29.
Tosoian JJ, Carter HB, Lepor A, Loeb S. Active surveillance for prostate cancer: current evidence and contemporary state of practice. Nat Rev Urol. 2016;13:205–15.
Kinsella N, Stattin P, Cahill D, Brown C, Bill-Axelson A, Bratt O, et al. Factors influencing men’s choice of and adherence to active surveillance for low-risk prostate cancer: a mixed-method systematic review. Eur Urol. 2018;74:261–80.
Schwarz DS, Blower MD. The endoplasmic reticulum: structure, function and response to cellular signaling. Cell Mol Life Sci. 2016;73:79–94.
Jeong CW, Washington SL 3rd, Herlemann A, Gomez SL, Carroll PR, Cooperberg MR. The new surveillance, epidemiology, and end results prostate with watchful waiting database: opportunities and limitations. Eur Urol. 2020;78:335–44.
Surveillance, Epidemiology, and End Results (SEER) Program: Prostate with Watchful Waiting Database (2010–2016). https://seer.cancer.gov/seerstat/databases/prostate-ww/index.html Accessed 26 Dec 2020.
Washington SL, Jeong CW, Lonergan PE, Herlemann A, Gomez SL, Carroll PR, et al. Regional variation in active surveillance for low-risk prostate cancer in the US. JAMA Netw Open. 2020;3:e2031349.
von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147:573–7.
Patel HD, Tosoian JJ, Carter HB, Epstein JI. Adverse pathologic findings for men electing immediate radical prostatectomy: defining a favorable intermediate-risk group. JAMA Oncol. 2018;4:89–92.
Loeb S, Folkvaljon Y, Curnyn C, Robinson D, Bratt O, Stattin P. Uptake of active surveillance for very-low-risk prostate cancer in Sweden. JAMA Oncol. 2017;3:1393–8.
Loeb S, Folkvaljon Y, Bratt O, Robinson D, Stattin P. Defining intermediate risk prostate cancer suitable for active surveillance. J Urol. 2019;201:292–9.
Toren P, Wong LM, Timilshina N, Alibhai S, Trachtenberg J, Fleshner N, et al. Active surveillance in patients with a PSA >10 ng/mL. Can Urol Assoc J. 2014;8:E702–707.
Fall K, Garmo H, Andren O, Bill-Axelson A, Adolfsson J, Adami HO, et al. Prostate-specific antigen levels as a predictor of lethal prostate cancer. J Natl Cancer Inst. 2007;99:526–32.
Ng MK, Van AsN, Thomas K, Woode-Amissah R, Horwich A, Huddart R, et al. Prostate-specific antigen (PSA) kinetics in untreated, localized prostate cancer: PSA velocity vs PSA doubling time. BJU Int. 2009;103:872–6.
Musunuru HB, Yamamoto T, Klotz L, Ghanem G, Mamedov A, Sethukavalan P, et al. Active surveillance for intermediate risk prostate cancer: survival outcomes in the sunnybrook experience. J Urol. 2016;196:1651–8.
Stavrinides V, Papageorgiou G, Danks D, Giganti F, Pashayan N, Trock B, et al. Mapping PSA density to outcome of MRI-based active surveillance for prostate cancer through joint longitudinal-survival models. Prostate Cancer Prostatic Dis. 2021.
Lonergan PE, Washington SL 3rd, Cowan JE, Zhao S, Nguyen HG, Shinohara K, et al. Risk factors for biopsy reclassification over time in men on active surveillance for early stage prostate cancer. J Urol. 2020;204:1216–21.
Funding
CWJ was supported by a grant from the National R&D Program for Cancer Control, Ministry of Health and Welfare, Republic of Korea (HA17C0039).
Author information
Authors and Affiliations
Contributions
Concept: CWJ, SLW, MRC; data acquisition: CWJ, SLW; statistical analysis: CWJ; manuscript drafting: PEL, CWJ; obtaining funding: CWJ. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The study was performed in accordance with the Declaration of Helsinki. Ethical committee approval was waived as this is publicly available data.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lonergan, P.E., Jeong, C.W., Washington, S.L. et al. Active surveillance in intermediate-risk prostate cancer with PSA 10–20 ng/mL: pathological outcome analysis of a population-level database. Prostate Cancer Prostatic Dis 25, 690–693 (2022). https://doi.org/10.1038/s41391-021-00448-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-021-00448-8
This article is cited by
-
Active surveillance should not be routinely considered in ISUP grade group 2 prostate cancer
BMC Urology (2023)
-
Prediction of clinically significant prostate cancer through urine metabolomic signatures: A large-scale validated study
Journal of Translational Medicine (2023)
-
Diagnostic significance of reassessment of prostate biopsy specimens by experienced urological pathologists at a high-volume institution
Virchows Archiv (2022)