Abstract
Background
Androgen deprivation therapy (ADT) has adverse effects on body composition, including muscle wasting and body fat accumulation, which may be attenuated by nutrition therapy. This systematic review summarises available evidence on the effects of dietary interventions on lean mass, fat mass and body mass index (BMI) in men treated with ADT for prostate cancer.
Methods
MEDLINE, Embase, Web of Science and ClinicalTrials.org were searched from inception through December 2020. We included all controlled trials evaluating effects of supplementation or dietary interventions on body composition in men with prostate cancer receiving continuous ADT. Methodological quality of the studies was assessed using the Cochrane Collaboration’s risk of bias tool. Meta-analysis was performed using a random effects model to calculate standardised mean differences between intervention and comparator groups. (PROSPERO; CRD42020185777).
Results
Eleven studies (n = 536 participants) were included. Seven studies investigated the effects of dietary advice interventions, e.g. individual or group counselling, and four studies included a nutritional supplement. Eight studies combined the dietary intervention with exercise. Nine studies reported sufficient data for inclusion in the meta-analysis. Dietary advice and supplementation interventions combined were not associated with significant changes in lean mass (0.05 kg; 95% CI: −0.17, 0.26; p = 0.674; n = 355), fat mass (−0.22 kg; 95% CI: −0.45, 0.01; p = 0.064; n = 336) or BMI (−0.16 kg*m−2; 95% CI: −0.37, 0.04; p = 0.121; n = 399). Dietary advice interventions alone were associated with a significant fat mass reduction (−0.29 kg; 95% CI: −0.54, −0.03; p = 0.028; n = 266).
Conclusions
Most studies were dietary advice interventions targeting caloric restriction, which showed the potential to reduce fat mass but did not increase lean mass in men treated with ADT. Future interventions should investigate whether a combination of dietary advice and protein supplementation with concomitant resistance exercise could counteract ADT-induced muscle wasting.
Similar content being viewed by others
Introduction
Prostate cancer is the second most commonly diagnosed cancer in men globally and its incidence is projected to rise as the world population ages [1, 2]. Androgen deprivation therapy (ADT) reduces prostate cancer growth and disease-specific mortality, and thus is considered the standard treatment for advanced prostate cancer [3]. However, the reduction of testosterone to castrate levels causes severe adverse effects, such as sexual dysfunction and fatigue, and is further associated with adverse changes in body composition, including reduced bone mineral density, increased fat mass and loss of skeletal muscle mass [4, 5]. In turn, long-term treatment with ADT is associated with a higher risk for developing metabolic syndrome, osteoporosis and cardiovascular disease [6,7,8]. Moreover, these body composition changes are often accompanied by a reduction in muscle strength, physical function and quality of life [9, 10].
Age-related loss of skeletal muscle mass and strength is associated with an increased risk for morbidity and mortality in the general population [11, 12]. In men treated with ADT, this ageing process is exacerbated due to suppression of testosterone, an anabolic steroid that promotes muscle growth [13] but is also involved in prostate cancer pathogenesis [14]. The accelerated deterioration of body composition is supported by studies showing a 2–4% decrease of lean mass and a concomitant 14% increase in fat mass in men with prostate cancer after 36 weeks on ADT, often resulting in sarcopenic obesity [5, 15]. Furthermore, ADT-induced lean mass changes appear to affect the limbs more than the trunk [5]. Exacerbated muscle wasting of the limbs may augment the decline in physical function and contribute to the loss of autonomy in men with advanced disease. Indeed, long-term ADT is associated with decreased biomechanical function of the lower-limb muscles during walking [16]. In addition, the risk of prostate cancer recurrence after primary treatment reportedly increases by 21% per 5 kg*m−2 growth in BMI, suggesting a higher risk of disease-specific mortality for patients with obesity [17]. In fact, higher values of both skeletal muscle mass and muscle density have been associated with a reduced mortality risk for men with advanced disease [18, 19]. As treatment with ADT might continue for several years, there is a growing need for interventions that alleviate the disease burden caused by prostate cancer and its treatment.
To treat catabolic alterations in patients with cancer, a combination of nutrition therapy and physical exercise is recommended [20]. While resistance exercise is well established as an effective strategy to promote muscle growth in healthy ageing adults [21], a previous meta-analysis found that in men treated with ADT supervised exercise interventions failed to induce changes in lean mass [22]. However, the authors purposefully excluded interventions with a diet component and argued that concomitant protein supplementation may increase lean mass in this population. Indeed, it has been demonstrated that protein intake can promote skeletal muscle growth and enhance the effects of resistance exercise in healthy individuals [23, 24] and patients with cancer [25]. Physiologically, the stimulation of muscle protein synthesis by dietary protein is driven by the increased availability of amino acids [24]. Increasing the dietary protein intake has shown to be an effective and inexpensive strategy to counteract age-related loss of skeletal muscle mass [24], and therefore may benefit men treated with ADT. Furthermore, dietary interventions aiming to decrease fat intake or restrict calories have been associated with body mass reductions in men with prostate cancer [26]. However, these results are not specific to men treated with ADT and caution is warranted as the interpretation of body mass alone, without accounting for body composition, may be misleading due to the complex metabolic state induced by ADT. Whether dietary interventions can attenuate or even reverse the adverse effects of ADT on body composition, specifically lean mass, remains therefore unclear.
The objective of the present systematic review and meta-analysis was to summarise the available evidence on the potential benefits of nutritional supplementation or dietary advice interventions for men treated with ADT for prostate cancer to counteract treatment-related changes in body composition, specifically muscle wasting and increased body fat.
Methods
Literature search
A systematic search of peer-reviewed literature was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [27]. This project was registered in the international prospective register of systematic reviews (PROSPERO; CRD42020185777). The search included the databases MEDLINE, Embase and Web of Science, as well as the clinical trial register ClinicalTrials.gov. Databases were searched from their inception until December 21st, 2020. The search string included terms related to prostate cancer (e.g. prostatic tumour) and nutrition (e.g. diet, supplementation). The individual search strings for each database are presented in Table 1. No language restrictions were applied. In addition, reference lists of included studies and reviews were searched by hand for relevant studies.
Study selection
Duplicate articles were removed using EndNote (version X9, Clarivate, Philadelphia, PA, USA). Two authors (LU, MW) independently screened the titles and abstracts of identified studies to assess eligibility. The full texts of relevant articles were retrieved and assessed for eligibility. Disagreements were resolved through consultation with a third author (NF).
Eligibility criteria
The inclusion criteria followed the PICOS (participants, interventions, comparisons, outcomes, study design) model [28]. Studies were eligible if they included: (P) men with clinically diagnosed prostate cancer who received any form of ADT (i.e. hormone therapy or bilateral orchiectomy) and continued the treatment for the duration of the study; (I) a dietary intervention component aimed to improve body composition outcomes, either a direct (e.g. nutritional supplementation) or indirect (e.g. nutrition advice) manipulation of dietary intake, regardless of a physical activity component; (C) a comparator group with either a placebo or no dietary intervention, regardless of a physical activity component; (O) an objective measure of body composition via direct (e.g. dual-energy X-ray absorptiometry (DXA)) or indirect (e.g. bioelectrical impedance analysis, anthropometry) methods, including at least one of the following outcomes: lean mass, fat mass or body mass index (BMI); (S) a controlled study design, either a randomised controlled trial (RCT) or controlled trial, with outcomes assessed pre and post intervention, i.e. no cross-sectional studies. Dietary interventions solely aimed at inhibiting cancer progress were excluded. Eligibility was irrespective of participants’ age, disease stage or concomitant treatments. Abstracts were eligible if sufficient information about the intervention was available from study protocols and the authors provided additional data.
Data collection
Data from all included studies were extracted into a purposefully developed spreadsheet by two authors (LU, MW). Extracted data included: (1) general information (authors, year of publication, aim, study design); (2) participant information (sample size, age, type of ADT, time on ADT); (3) intervention details (intervention protocol, setting, duration); (4) findings (body composition outcomes, compliance, adverse events). Authors were contacted for further information or to clarify study procedures, if needed. If additional data was provided, it was included in the analysis [29,30,31].
Risk of bias assessment
The Cochrane Collaboration’s tool for assessing risk of bias [32] was used to assess the methodological quality of the included studies. Two authors (LU, MW) independently examined the studies for the following potential sources of bias: selection (random sequence generation; allocation concealment), performance (blinding of participants and personnel), detection (blinding of outcome assessment), attrition (incomplete outcome data), reporting (selective reporting) and other biases (e.g. reporting of intervention design and adherence). Risk of bias was only assessed for full-text publications.
Data analysis
The analyses were performed using R version 4.0.3 [33], RStudio [34] and the metafor package version 2.4.0 [35]. Post-intervention values of lean mass, fat mass and BMI of the included studies were pooled using a random effects model. If studies reported both absolute and relative body composition measures, only absolute measures were included in the analysis. Statistical heterogeneity (τ2) was assessed using the restricted maximum-likelihood estimator and, additionally, Cochran’s Q and I2 were reported. Large heterogeneity was determined as I2 > 50% [36]. Cook’s distances were used to identify influential outliers with values greater than the median plus six times the interquartile range of the Cook’s distances considered influential. Publication bias was assessed using funnel plots. Rank correlation and regression tests using the standard error of the observed outcomes as predictor were used to check for funnel plot asymmetry [37, 38]. Results are presented with 95% confidence intervals (CI). Findings from studies without adequate data for inclusion in the meta-analysis were reported narratively.
Results
Overview
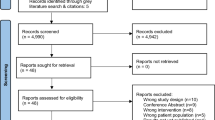
The results of the literature search are summarised in Fig. 1. The search identified 32,382 articles, of which 13,875 were duplicates and 18,442 were removed after screening of titles and abstracts. Full texts were retrieved and assessed for 68 articles, of which ten were deemed eligible. One additional article was identified through hand searching of reference lists, resulting in a total of eleven articles with data from 536 participants included in this review.
Study characteristics
The characteristics of the eleven included studies are presented in Supplementary Table 1. All studies were RCTs published from 2009 onwards. Duration of the interventions ranged from 3 to 12 months. Seven studies investigated the effects of a dietary advice intervention [29, 30, 39,40,41,42,43] and four studies included nutritional supplementation [31, 44,45,46]. Dietary advice consisted of general healthy-eating guidelines in five studies [29, 39, 40, 42, 43], and a low-carbohydrate [30] or low-glycaemic index diet [41] in one study each. Nutritional supplements contained protein powder, whey in two studies [31, 44] and soy in one study [45], and vitamin D in one study [46]. Dietary advice was delivered using various formats, including written general nutrition recommendations or specific instructions, individual or group counselling or a combination of these. Eight studies combined the dietary intervention with a physical activity component, which included supervised exercise in five studies [29, 31, 39, 40, 44] and exercise recommendations in three studies [30, 41, 42]. Supervised exercise protocols included resistance exercise, either alone or combined with aerobic exercise, in all five studies, while studies providing exercise recommendations to participants only included aerobic exercise. The comparator consisted of a usual care group in all studies but two supplementation studies [45, 46], where participants in the comparator group received a placebo. The detailed intervention protocols are outlined in Table 2. Body composition was assessed using DXA in five studies [29,30,31, 43, 44], bioelectrical impedance analysis in three studies [40, 41, 46] and skinfold thickness measurements in one study [42]. Two studies [39, 45] reported BMI as the only body composition outcome.
Risk of bias in studies
A summary of the risk of bias in the included studies is shown in Fig. 2. Most studies reported appropriate allocation concealment and blinding of study personnel conducting outcome assessments. The most common sources of methodological bias were lack of blinding of participants and study personnel during the intervention [29, 30, 39,40,41,42,43,44], selective reporting [44,45,46] and incomplete reporting of outcome data [44, 45]. The risk of bias of one study [31] published only as an abstract could not be determined.
Changes in lean mass
Seven studies [29,30,31, 40, 42, 43, 46] including 355 participants reported lean mass pre and post intervention. The results from the pooled analysis showed that dietary interventions did not significantly increase lean mass. The pooled mean difference in total lean mass was 0.05 kg (95% CI: −0.16, 0.25; p = 0.674) with low heterogeneity (I2 = 0%) (Fig. 3A). The exclusion of two supplementation interventions [31, 46] from the pooled analysis did not change the significance of the results.
A Lean mass, B fat mass, and C body mass index (BMI) in men treated with ADT for prostate cancer. Forest plots showing the results of meta-analyses of post intervention values for each outcome using a random effects model. Because changes in BMI cannot clearly be defined as favourable for either group, we refrained from using the term ‘favours’. Effects situated left of the middle line present a higher BMI in the comparator group, whereas effects situated right of the middle line present a higher BMI in the intervention group. CI confidence interval, ES effect size, RE random effects.
Changes in fat mass
Seven studies [29,30,31, 40,41,42,43] including 336 participants reported fat mass pre and post intervention. The results from the pooled analysis showed that dietary interventions did not significantly decrease fat mass. The pooled mean difference in total fat mass was −0.22 kg (95% CI: −0.45, 0.01; p = .064) with low heterogeneity (I2 = 11%) (Fig. 3B). The exclusion of the only supplementation intervention [31] from the pooled analysis resulted in a significant pooled mean difference of −0.29 kg (95% CI: −0.54, −0.03; p = .028; n = 266) with low heterogeneity (I2 = 7%) for dietary advice interventions.
Changes in BMI
Eight studies [29,30,31, 39,40,41,42,43] including 399 participants reported BMI pre and post intervention. The results from the pooled analysis showed no significant effect of dietary interventions on BMI. The pooled mean difference in BMI was −0.16 kg*m−2 (95% CI: −0.37, 0.04; p = 0.121) with low heterogeneity (I2 = 6%) (Fig. 3C). The exclusion of the only supplementation intervention [31] from the pooled analysis did not change the significance of the results.
Narrative reporting of study results
Two studies [44, 45] on the effects of nutritional supplementation on body composition outcomes did not report post-intervention measures. Sharma et al. [45] examined the effect of daily intake of 20 g soy protein containing 160 mg isoflavones compared to 20 g whole milk powder over 12 weeks. They assessed body composition using BMI, which they report did not change significantly in either group. In addition, a four-armed RCT that investigated the effect of resistance training combined with 50 g whey protein isolate daily over 12 weeks, compared to either of those interventions alone and a comparator group, found that protein supplementation did not influence body composition outcomes [44]. In this study, body composition was assessed using DXA, but post-intervention measures were only reported as pooled data for training and non-training groups.
Discussion
ADT elicits adverse effects on body composition that include increased body fat accumulation, a concomitant BMI rise and accelerated muscle wasting [47]. Because ADT causes such drastic metabolic alterations, dietary interventions have been proposed as a way to mitigate treatment-related side effects. The evidence on the potential benefits of such interventions is limited though. We present the first meta-analysis of prospectively collected data on the effects of dietary interventions on body composition outcomes in men treated with ADT for prostate cancer. Eleven RCTs that reported the effects of dietary advice or nutritional supplementation on lean mass, fat mass and BMI were identified. The results from our meta-analysis show that interventions using dietary advice have the potential to reduce fat mass in men treated with ADT, whereas lean mass and BMI remain mostly unaltered irrespective of the intervention type. However, the effect on fat mass did not persist when supplementation studies were added to the analysis, which may be due to the considerable heterogeneity of intervention designs and aims.
Loss of lean mass is a metabolic alteration commonly observed in patients with cancer and can be caused by the tumour, treatments, modified diet or physical inactivity [20, 48]. There is consistent evidence that low muscle mass is associated with declines in physical function and quality of life, increased frailty and a higher mortality risk [20, 49]. Treatment with ADT augments these catabolic processes due to the inhibition of androgen signalling, which plays a critical role in the regulation of muscle protein synthesis, and thereby exacerbates ageing-related muscle wasting [13]. Our results indicate that none of the dietary interventions reversed this process by increasing lean mass. Considering the adverse metabolic state induced by ADT and the loss of lean mass reported by Galvão et al. [5], simply preserving pre-intervention values of lean mass may already be a success. Preservation of lean mass while achieving fat mass loss was only reported by one intervention, which combined dietary and walking advice [42], but this finding may have been influenced by the outcome measure as they reported relative lean mass in contrast to absolute lean mass in the other included studies. Low protein intake, which is frequently found among patients with cancer, can further contribute to the loss of skeletal muscle induced by ADT. Current guidelines on nutrition for patients with cancer recommend a daily protein intake of 1–1.5 g/kg bodyweight to preserve lean mass [20]. We examined the changes in dietary intake in all six studies that reported diet assessment outcomes (see Table 2) and found that only two interventions, a low-carbohydrate diet [30] and protein supplementation [44], were associated with increased protein intake, while all interventions reported reductions in total energy intake except for the aforementioned protein supplementation [44]. A substantial reduction in total energy intake while failing to increase dietary protein intake may promote loss of lean mass. Such a trend was prevalent in one study [43], which reported a 2.5% reduction in lean mass after 12 weeks of a Mediterranean-style diet aimed at decreasing dietary fat intake and increasing intake of fruits, vegetables and fibre. The authors argue that the dietary protein intake may have been insufficient to preserve lean mass during intentional weight loss.
Nutrient deficiencies of patients with cancer may be caused by high energy demands of the tumour or impeded gastrointestinal uptake, and supplementation of specific nutrients has been suggested as a potential treatment [20, 24]. Despite our comprehensive search, we identified only two studies [31, 44] that supplemented dietary protein and provided lean mass measures. Dawson et al. [44] did not observe an effect of daily supplementation of 50 g whey protein isolate, divided into two doses, on lean mass over 12 weeks with or without supervised resistance exercise, however, post-intervention measures for each group were not provided. While the average daily protein intake during the intervention was significantly higher in the supplementation groups, the supplementation only group nevertheless had the lowest dietary protein to bodyweight ratio of all groups. It is unclear whether the data were skewed by one participant in this group, who reportedly refused to take the supplement after 2 weeks but was still included in the analysis. The net protein increase in the intervention groups was less than half of the supplemented 50 g per day, highlighting the need for frequent diet assessments to avoid unintentional changes in the habitual diet of the participants. Interestingly, the combined supplementation and exercise group, which showed the highest dietary protein intake with 1.4 g/kg bodyweight, consumed one of the two daily doses immediately after the supervised exercise sessions, presumably increasing compliance with the intervention. It is worth noting that, despite evidence that protein supplementation after resistance exercise evokes an acute muscle protein synthesis response in men on ADT [50], the only study with such a design that could be included in the meta-analysis was by Dalla Via et al. [31]. They observed no significant changes in lean mass in participants of a 12-month intervention that combined daily supplementation of whey protein, calcium carbonate and vitamin D with supervised resistance exercise. Participants were also instructed to consume the supplement within 2 h post-exercise on training days to increase compliance, but whether the daily dose of 25 g protein was sufficient to reach the recommended intake level remains unclear because diet assessment were not reported. Despite the lack of significant effects, the protocols used by both studies present promising approaches as concomitant resistance exercise has been shown to enhance the stimulatory effect of amino acid intake on muscle protein synthesis [24], a physiological mechanism not aided by any of the other interventions. In addition, Inglis et al. [46] reported a significant lean mass increase following 12 weeks of high-dose vitamin D supplementation, but the effect did not persist after 24 weeks. Evidence from patients with advanced cancer of various types shows that supplementation of the amino acid-related nutrients beta-hydroxy-beta-methylbutyrate, arginine and glutamine was associated with a significant lean mass increase after only 4 weeks compared to patients who received a supplement containing non-essential amino acids [51]. Altogether, protein supplementation could help to balance deficits, but timing, quantity and composition of the supplement may be crucial and future studies should investigate whether this approach would counteract the chronic effects of ADT on muscle physiology.
Regarding fat mass, our findings show a beneficial effect of the dietary interventions with significant reductions reported in four studies [29, 30, 41, 42], while three studies observed no differences between intervention and comparator groups [31, 40, 43]. None of the studies reported fat mass gains in the intervention groups but in several comparator groups. The pooled analysis showed that dietary advice interventions were associated with a significant fat mass reduction but the effect was no longer present when the results from Dalla Via et al. [31], the only protein supplementation intervention that measured fat mass, were included. This may be explained by considerable heterogeneity among the intervention designs. Because weight gain is a common side effect of ADT [47], most dietary advice interventions focused on calorie restriction to achieve a negative energy balance, whereas none of the supplementation interventions aimed to change total energy intake. In fact, all studies reported a baseline BMI above the 25 kg*m−2 cut-off for overweight [52]. This puts men treated with ADT at an increased risk for obesity, metabolic syndrome, cardiovascular disease and frailty [53]. Chaplow et al. [29] reported that fat mass reduction was associated with improved mobility performance following a combined diet and exercise intervention. Overall, these findings highlight the promising potential of interventions that promote changes in dietary behaviour such as reducing calories to mitigate ADT-related side effects, irrespective of their effect on lean mass.
Despite changes in fat mass in some studies, we observed no differences in BMI between groups irrespective of the intervention type. Among the studies not included in our meta-analysis, Sharma et al. [45] administered a soy protein supplement containing isoflavones, which are known for their phytoestrogenic effects, but did not affect BMI. Because the comparator group received milk protein, the lack of a between-group effect may be explained by a similar nutrient content of both supplements. Also, BMI as an outcome measure is inadequate to capture potential physiological changes that may have been induced by the supplements, because it neglects the distribution of tissues, such as muscle and fat mass, that differ in their relationship to cancer prognosis [49]. The typical changes in body composition associated with ADT, as well as those intended by diet and exercise interventions, may in fact result in a constant BMI despite substantial changes of total lean and fat mass. Therefore, researchers should use measures that quantify tissue distribution such as DXA, which is considered the ideal method for patients with cancer [54].
Knowledge of ADT-related side effects among men with prostate cancer is lacking, with a study revealing that 65% of men who recently started ADT were unaware that muscle wasting may occur [55]. This lack of information prevents men from engaging in beneficial behaviours, such as regular exercise or a healthy diet, and puts interventions that address these issues into focus. It is well established that by modifying both energy intake and expenditure, interventions that combine exercise and diet generally achieve better weight management results than either of those alone [56]. Exercise protocols of included studies ranged from supervised exercise, alone or combined with aerobic exercise, to walking recommendations, and three studies included no exercise. Resistance exercise in particular has been shown to positively affect body composition and muscle strength in men treated with ADT [53], while considered safe and feasible even for patients with bone metastases [57], which supports the argument to include exercise protocols in future trials. We are aware of a number of ongoing trials, including combined dietary and exercise advice interventions [58, 59], creatine supplementation with resistance exercise [60], and beta-hydroxy-methylbutyrate supplementation (NCT01607879). Future studies should explore options for individualised diet interventions that match the dietary advice or supplement to the nutritional requirements and deficits of the patient, and monitor dietary intake frequently to allow for adjustments if needed.
The strengths of this review include the comprehensive search, which was performed using broad search terms that would encompass all potentially relevant articles. Only prospective, controlled trials with measurements both at baseline and post-intervention were included. The results of this review are limited by the heterogeneity of dietary intervention designs and methods used for body composition assessment. Treatment duration with ADT has been shown to influence both the rate and the total loss of lean mass [15, 47], yet time on ADT at enrolment was not reported for all studies. Most studies included men with a minimum of 3 months on ADT except for one [41], which included hormone-naïve men due to receive ADT and administered the treatment as part of the study. This study, however, did not report lean mass, and changes in fat mass and BMI in the comparator group were similar to other studies, therefore we argue that treatment duration did likely not affect the results. Also, the small number of eligible studies did not allow for subgroup analyses, though neither the rank correlation nor the regression test indicated any funnel plot asymmetry. In addition, not all studies monitored dietary intake or compliance with the diet intervention, limiting the conclusions to be drawn as it remains unclear to which extent diet was modified. Furthermore, the results from two out of three studies that investigated protein supplementation, which is of particular interest for patients with potential nutrient deficiencies, were not included in the meta-analyses due to insufficient reporting of outcome data [44, 45].
Conclusions
Dietary interventions have the potential to mitigate the adverse changes in lean and fat mass experienced by men treated with ADT. This systematic review and meta-analysis summarises the current body of evidence on the effect of dietary interventions on body composition outcomes. While our results show that dietary advice interventions successfully reduced body fat, the benefits for lean mass were less pronounced. Additional protein supplementation may be required to preserve lean mass during intentional body fat reduction. Considering the benefits of increased muscle mass for morbidity and mortality, future studies should investigate the effects of interventions that combine healthy-eating advice with protein supplementation to achieve energy reduction while balancing nutritional deficits to stimulate muscle protein synthesis. Further research should also examine whether additional resistance exercise enhances the effects of dietary interventions.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clinic. 2018;68:394–424.
Rawla P. Epidemiology of prostate cancer. World J Oncol. 2019;10:63–89.
Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, et al. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2014;65:467–79.
Downing A, Wright P, Hounsome L, Selby P, Wilding S, Watson E, et al. Quality of life in men living with advanced and localised prostate cancer in the UK: a population-based study. Lancet Oncol. 2019;20:436–47.
Galvao DA, Spry NA, Taaffe DR, Newton RU, Stanley J, Shannon T, et al. Changes in muscle, fat and bone mass after 36 weeks of maximal androgen blockade for prostate cancer. BJU Int. 2008;102:44–7.
Braga-Basaria M, Dobs AS, Muller DC, Carducci MA, John M, Egan J, et al. Metabolic syndrome in men with prostate cancer undergoing long-term androgen-deprivation therapy. J Clin Oncol: Off J Am Soc Clin Oncol. 2006;24:3979–83.
Muniyan S, Xi L, Datta K, Das A, Teply BA, Batra SK, et al. Cardiovascular risks and toxicity—the Achilles heel of androgen deprivation therapy in prostate cancer patients. Biochim Biophys Acta Rev Cancer. 2020;1874:188383.
Poulsen MH, Frost M, Abrahamsen B, Gerke O, Walter S, Lund L. Osteoporosis and prostate cancer; a 24-month prospective observational study during androgen deprivation therapy. Scand J Urol. 2019;53:34–9.
Gonzalez BD, Jim HSL, Small BJ, Sutton SK, Fishman MN, Zachariah B, et al. Changes in physical functioning and muscle strength in men receiving androgen deprivation therapy for prostate cancer: a controlled comparison. Support Care Cancer: Off J Multinatl Assoc Support Care Cancer. 2016;24:2201–7.
Huang YT, Li CC, Chou YH, Ke HL, Chen CY. Health-related quality of life of exposed versus non-exposed androgen deprivation therapy patients with prostate cancer: a cross-sectional study. Int J Clin Pharm. 2019;41:993–1003.
Li R, Xia J, Zhang XI, Gathirua-Mwangi WG, Guo J, Li Y, et al. Associations of muscle mass and strength with all-cause mortality among US older adults. Med Sci Sports Exerc. 2018;50:458–67.
Landi F, Liperoti R, Fusco D, Mastropaolo S, Quattrociocchi D, Proia A, et al. Sarcopenia and mortality among older nursing home residents. J Am Med Dir Assoc. 2012;13:121–6.
Vingren J, Kraemer W, Ratamess N, Anderson J, Volek J, Maresh C. Testosterone physiology in resistance exercise and training: the up-stream regulatory elements. Sports Med. 2010;40:1037–53.
Huggins C, Stevens R, Hodges CV. Studies on prostatic cancer: II. The effects of castration on advanced carcinoma of the prostate gland. Arch Surg. 1941;43:209–23.
Smith MR, Saad F, Egerdie B, Sieber PR, Tammela TL, Ke C, et al. Sarcopenia during androgen-deprivation therapy for prostate cancer. J Clin Oncol: Off J Am Soc Clin Oncol. 2012;30:3271–6.
Cheung AS, Gray H, Schache AG, Hoermann R, Lim Joon D, Zajac JD, et al. Androgen deprivation causes selective deficits in the biomechanical leg muscle function of men during walking: a prospective case–control study. J Cachexia Sarcopenia Muscle. 2017;8:102–12.
Cao Y, Ma J. Body mass index, prostate cancer-specific mortality, and biochemical recurrence: a systematic review and meta-analysis. Cancer Prev Res. 2011;4:486–501.
Muñoz-Rodríguez J, Domínguez A, Rosado MA, Centeno C, Parejo V, Costa-Trachsel I, et al. Effect of muscle density in patients with metastatic prostate cancer administered androgen deprivation therapy. Endocrinol Diabetes Nutr. 2020;68:92–8.
Pak S, Kim MS, Park EY, Kim SH, Lee KH, Joung JY. Association of body composition with survival and treatment efficacy in castration-resistant prostate cancer. Front Oncol. 2020;10:558.
Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, Bozzetti F, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr. 2017;36:11–48.
Peterson MD, Sen A, Gordon PM. Influence of resistance exercise on lean body mass in aging adults: a meta-analysis. Med Sci Sports Exerc. 2011;43:249–58.
Chen Z, Zhang Y, Lu C, Zeng H, Schumann M, Cheng S. Supervised physical training enhances muscle strength but not muscle mass in prostate cancer patients undergoing androgen deprivation therapy: a systematic review and meta-analysis. Front Physiol. 2019;10:843.
Cermak NM, Res PT, de Groot LC, Saris WH, van Loon LJ. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: a meta-analysis. Am J Clin Nutr. 2012;96:1454–64.
Wolfe RR. The role of dietary protein in optimizing muscle mass, function and health outcomes in older individuals. Br J Nutr. 2012;108 Suppl 2:S88–93.
Cereda E, Turri A, Klersy C, Cappello S, Ferrari A, Filippi AR, et al. Whey protein isolate supplementation improves body composition, muscle strength, and treatment tolerance in malnourished advanced cancer patients undergoing chemotherapy. Cancer Med. 2019;8:6923–32.
Mohamad H, McNeill G, Haseen F, N’Dow J, Craig LC, Heys SD. The effect of dietary and exercise interventions on body weight in prostate cancer patients: a systematic review. Nutr Cancer. 2015;67:43–60.
Page M, McKenzie J, Bossuyt P, Boutron I, Hoffmann T, Mulrow C, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. MetaArXiv. 2020. Available from: osf.io/preprints/metaarxiv/v7gm2.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–34.
Chaplow ZL, Focht BC, Lucas AR, Grainger E, Simpson C, Buell J, et al. Effects of a lifestyle intervention on body composition in prostate cancer patients on androgen deprivation therapy. JCSM Clin Rep. 2020;5:52–60.
Freedland SJ, Howard L, Allen J, Smith J, Stout J, Aronson W, et al. A lifestyle intervention of weight loss via a low-carbohydrate diet plus walking to reduce metabolic disturbances caused by androgen deprivation therapy among prostate cancer patients: carbohydrate and prostate study 1 (CAPS1) randomized controlled trial. Prostate Cancer Prostatic Dis. 2019;22:428–37.
Dalla Via J, Owen PJ, Daly RM, Mundell NL, Livingston PL, Rantalainen T, et al. Effects of a multicomponent exercise program combined with a multi-nutrient supplement on musculoskeletal health in men with prostate cancer receiving androgen deprivation therapy: a 12-month randomised controlled trial. Australas J Ageing. 2020;39:19–21.
Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ (Clin Res Ed). 2011;343:d5928.
R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2013.
RStudio Team. RStudio: integrated development for R. Boston, MA: RStudio, Inc.; 2015.
Viechtbauer W. Conducting meta-analyses in R with the metafor Package. J Stat Software. 2010;1:1–48.
Deeks J, Higgins J, Altman D. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al., editors. Cochrane handbook for systematic reviews of interventions. 2nd ed. Chichester (UK): John Wiley & Sons; 2019. p. 241–84.
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101.
Sterne JA, Egger M. Regression methods to detect publication and other bias in meta-analysis. In: Rothstein HR, Sutton AJ, Borenstein M, editors. Publication bias in meta-analysis: prevention, assessment and adjustment. Chichester (UK): Wiley; 2005. p. 99–110.
Bourke L, Doll H, Crank H, Daley A, Rosario D, Saxton JM. Lifestyle intervention in men with advanced prostate cancer receiving androgen suppression therapy: a feasibility study. Cancer Epidemiol Biomark Prev. 2011;20:647–57.
Gilbert SE, Tew GA, Fairhurst C, Bourke L, Saxton JM, Winter EM, et al. Effects of a lifestyle intervention on endothelial function in men on long-term androgen deprivation therapy for prostate cancer. Br J Cancer. 2016;114:401–8.
Nobes JP, Langley SE, Klopper T, Russell-Jones D, Laing RW. A prospective, randomized pilot study evaluating the effects of metformin and lifestyle intervention on patients with prostate cancer receiving androgen deprivation therapy. BJU Int. 2011;109:1495–502.
O’Neill RF, Haseen F, Murray LJ, O’Sullivan JM, Cantwell MM. A randomised controlled trial to evaluate the efficacy of a 6-month dietary and physical activity intervention for patients receiving androgen deprivation therapy for prostate cancer. J Cancer Survivorship: Res Pract. 2015;9:431–40.
Baguley BJ, Skinner TL, Jenkins DG, Wright ORL. Mediterranean-style dietary pattern improves cancer-related fatigue and quality of life in men with prostate cancer treated with androgen deprivation therapy: a pilot randomised control trial. Clin Nutr. 2021;40:245–54.
Dawson JK, Dorff TB, Todd Schroeder E, Lane CJ, Gross ME, Dieli-Conwright CM. Impact of resistance training on body composition and metabolic syndrome variables during androgen deprivation therapy for prostate cancer: a pilot randomized controlled trial. BMC Cancer. 2018;18.
Sharma P, Wisniewski A, Braga-Basaria M, Xu X, Yep M, Denmeade S, et al. Lack of an effect of high dose isoflavones in men with prostate cancer undergoing androgen deprivation therapy. J Urol. 2009;182:2265–73.
Inglis JE, Fernandez ID, van Wijngaarden E, Culakova E, Reschke JE, Kleckner AS, et al. Effects of high-dose vitamin D supplementation on phase angle and physical function in patients with prostate cancer on ADT. Nutr Cancer. 2020:1–8. https://doi.org/10.1080/01635581.2020.1819348. Online ahead of print.
Haseen F, Murray LJ, Cardwell CR, O’Sullivan JM, Cantwell MM. The effect of androgen deprivation therapy on body composition in men with prostate cancer: systematic review and meta-analysis. J Cancer Surviv: Res Pract. 2010;4:128–39.
Bloch W. Tumour muscle crosstalk more as regulation of muscle wasting—role of exercise. Acta Physiol. 2017;219:704–5.
Cespedes Feliciano EM, Kroenke CH, Caan BJ. The obesity paradox in cancer: how important is muscle? Annu Rev Nutr. 2018;38:357–79.
Hanson ED, Nelson AR, West DWD, Violet JA, O’Keefe L, Phillips SM, et al. Attenuation of resting but not load-mediated protein synthesis in prostate cancer patients on androgen deprivation. J Clin Endocrinol Metab. 2017;102:1076–83.
May PE, Barber A, D’Olimpio JT, Hourihane A, Abumrad NN. Reversal of cancer-related wasting using oral supplementation with a combination of beta-hydroxy-beta-methylbutyrate, arginine, and glutamine. Am J Surg Pathol. 2002;183:471–9.
World Health Organization. Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. 1995.
Lam T, Birzniece V, McLean M, Gurney H, Hayden A, Cheema BS. The adverse effects of androgen deprivation therapy in prostate cancer and the benefits and potential anti-oncogenic mechanisms of progressive resistance training. Sports Med Open. 2020;6:13.
Di Sebastiano KM, Mourtzakis M. A critical evaluation of body composition modalities used to assess adipose and skeletal muscle tissue in cancer. Appl Physiol Nutr Metab. 2012;37:811–21.
Walker LM, Tran S, Wassersug RJ, Thomas B, Robinson JW. Patients and partners lack knowledge of androgen deprivation therapy side effects. Urol Oncol. 2013;31:1098–105.
Jakicic JM, Clark K, Coleman E, Donnelly JE, Foreyt J, Melanson E, et al. Appropriate intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2001;33:2145–56.
Cormie P, Newton RU, Spry N, Joseph D, Taaffe DR, Galvao DA. Safety and efficacy of resistance exercise in prostate cancer patients with bone metastases. Prostate Cancer Prostatic Dis. 2013;16:328–35.
Algotar A, Hsu CH, Chow S, Dougherty S, Babiker HM, Marrero D, et al. Comprehensive Lifestyle Improvement Program for Prostate Cancer (CLIPP): protocol for a feasibility and exploratory efficacy study in men on androgen deprivation. JMIR Res Protoc. 2019;8:116–23.
Manson A, Myers J, Billinger S, Ward J, Parker W, Hamilton-Reeves J, et al. Feasibility of an intervention for men on androgen deprivation therapy: a research protocol. Res Nurs Health. 2019;42:324–33.
Fairman CM, Kendall KL, Newton RU, Hart NH, Taaffe DR, Chee R, et al. Examining the effects of creatine supplementation in augmenting adaptations to resistance training in patients with prostate cancer undergoing androgen deprivation therapy: a randomised, double-blind, placebo-controlled trial. BMJ Open. 2019;9:e030080.
Funding
Open Access funding enabled and organised by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
MS, CMF, LU, NF: study conception and design. LU, MW, NF and MS: search, screening, data extraction and analysis. CMF, AH and WB: substantial input regarding the study design and the interpretation of the data. LU and MS drafted and finalised the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Umlauff, L., Weber, M., Freitag, N. et al. Dietary interventions to improve body composition in men treated with androgen deprivation therapy for prostate cancer: a solution for the growing problem?. Prostate Cancer Prostatic Dis 25, 149–158 (2022). https://doi.org/10.1038/s41391-021-00411-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-021-00411-7