Abstract
Background
The prorenin receptor (PRR) plays a critical role in ureteric bud (UB) branching morphogenesis. DOT1 Like (DOT1L), a histone methyltransferase specific for Histone 3 lysine 79 (H3K79), is important for differentiation of the UB-derived renal collecting duct cells. In this study, we tested whether DOT1L/H3 dimethyl K79 (H3m2K79) are regulated by PRR deletion in the UB and UB-derived collecting ducts in the embryonic mouse kidneys.
Methods
Mutant Hoxb7Cre+/PRRflox/flox (PRRUB-/-) and control PRRUB+/+, mice were studied on embryonic (E) day E17.5. DOT1L mRNA and protein expression in the kidney was examined by real-time qRT-PCR and immunohistochemistry, respectively. H3m2K79 protein expression was determined by immunohistochemistry and Western blot analysis.
Results
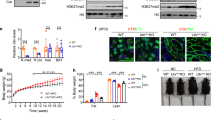
DOT1L mRNA levels were decreased in mutant compared to control mice (0.68 ± 0.06 vs. 1.0 ± 0.01, p < 0.01). DOT1L and H3m2K79 immunostaining was reduced in the mutant vs. control kidneys (Dot1: 0.62 ± 0.03 vs. 1.0 ± 0.01, p < 0.05; H3m2K79: 0.64 ± 0.04 vs.1.1 ± 0.01. p < 0.05.). Western blot analysis revealed decreased H3m2K79 protein levels in mutant compared to control kidneys (1.0 ± 0.06 vs. 1.5 ± 0.02, p < 0.05).
Conclusion
Targeted deletion of the PRR in the UB and UB-derived collecting ducts results in reduced DOT1L gene/protein and H3m2K79 protein expression in the embryonic mouse metanephroi in vivo.
Impact
-
The role of histone methylation in mediating the effect of the prorenin receptor on the ureteric bud branching (UB) morphogenesis and urine acidification during kidney development is unknown.
-
We demonstrate that histone H3 lysine (K) 79 dimethylation by methyltransferase Dot1 is reduced in the embryonic kidney of mice that lack the prorenin receptor in the UB lineage.
Similar content being viewed by others
Introduction
Branching morphogenesis of the ureteric bud (UB) is a key developmental process that directs organogenesis of the kidney.1,2 Disruption of UB branching results in a variety of congenital anomalies of the kidney and urinary tract (CAKUT), including kidney agenesis (absence of the kidney), kidney hypoplasia (small kidney with reduced nephron number), hypodysplasia (small kidney with disorganized tissue histology), and ureter defects that can cause reflux of the urine or obstruction of the urine outflow.2 Collectively, CAKUT account for ~40% of end-stage kidney disease in children.3
We previously demonstrated that conditional ablation of the prorenin receptor (PRR) in the developing UB in mice (PRRUB−/−) using HOXB7Cre driver reduces UB branching resulting in renal hypodysplasia, decreased nephron number at birth, polyuria and reduced ability to acidify the urine on postnatal (P) day P30.4 The PRR is the cell-surface receptor for renin and prorenin, and an accessory subunit of the vacuolar proton pump V-ATPase encoded for by ATP6AP2 gene.5 In the adult rat collecting duct, PRR is most abundant at the apical surface of acid-secreting type α intercalated cells (α-ICs) where it colocalizes with the V-ATPase and may be activated in a paracrine fashion by prorenin or renin released by adjacent principal cells.6
Disruptor of Telomere Silencing (DOT1L) stimulates methylation of the histone mark H3K79 and generally indicates an active gene transcriptional state.7 DOT1L is expressed in the UB and collecting ducts in mice.8 Loss of DOT1L/H3K79me in principal cells of the UB-derived collecting ducts in mice results in increased number of acid-secreting intercalated cells and upregulates V-ATPase B1 subunit expression in these cells.9 In addition, DOT1L regulates ATP6V1B1 gene transcription rate in kidney inner medullary collecting duct (IMCD3) cells in vitro.9 In this work, we tested the hypothesis that DOT1L/H3 dimethyl K79 (H3m2K79) are regulated by PRR deletion in the UB and UB-derived collecting ducts of the embryonic mouse kidneys.
Methods
Animals
All experiments involving mice were approved by Tulane Institutional Animal Care and Use Committee. To delete PRR conditionally in the UB, we used the HOXB7Cre,GFP transgene, which drives Cre expression in the Wolffian duct and UB epithelium from E9.5 onward.10 The resulting HOXB7Cre+,GFP+/PRRflox/flox mice represent UB-specific PRR-knockout mice (PRRUB-/-).4 Control mice consisted of HOXB7Cre+,GFP+/PRR+/+ (PRRUB+/+) littermates. Embryo sex was not determined.
Isolation of embryonic kidneys
Embryonic kidneys were dissected from PRRUB-/- and control mice on embryonic (E) day E17.5. Images were acquired using an Olympus IX70 inverted phase-contrast microscope.
Quantitative reverse-transcription polymerase chain reaction (qRT-PCR)
qRT-PCR was performed in the Mx3000P equipment (Stratagene, La Jolla, CA) using MxPro QPCR software (Stratagene) as previously described.4 mRNA was extracted from snap-frozen E17.5 PRRUB-/- and control kidneys [n = 3 mice (6 kidneys) per group]. Total mRNA was extracted and purified using QIAwave RNA Mini Kit (50),Cat. No. / ID: 74534(QIAGEN) according to the manufacture’s instruction. Reverse Transcription was performed using LunaScript RT SuperMix Kit (NEB #E3010)(New England Bio Labs) according to the manufacture’s protocol. The Taqman probes (Dot1 Mm01173481_m1 and GAPDH Mm99999915_g1) were ordered from Thermal Scientific for qRT-PCR assay. The quantity of DOT1 mRNA was normalized by that of GAPDH mRNA expression. RNA samples were analyzed in triplicates in each run. PCR reaction was performed twice.
Immunohistochemistry
Kidneys from E17.5 PRRUB-/- and control [n = 3 mice (6 kidneys) per group] were cut in the longitudinal midplane, processed through the paraffin, and embedded on the cut surface. Kidneys were sectioned at 4-µm and two consecutive sections adjacent to the longitudinal midplane were processed for immunostaining with each antibody (n = 12 sections per group). Immunostaining was performed by the immunofluorescent standard technique with Vectastain Elite kit (Vector Laboratories, Burlingame, CA). Primary antibodies included rabbit anti-DOT1L (Ab64077, Abcam, 1:1000), anti-H3 di-methyl K79 (Rabbit polyclonal, ab3594, Abcam), and anti-Aqp2 (goat anti-Aqp2; sc-9882, Santa Cruz, 1:100). Secondary antibodies were detected with Alexa Fluor dyes (Invitrogen). Specificity of immunostaining was documented by the omission of the primary antibody. The intensity of respective antibody immunofluorescence in kidney sections (n = 3 mice per group) was examined using Slide book 4.0 software (Intelligent Imaging Innovations, Denver, CO). All counts were performed in a blinded fashion.
Western blot analysis
Kidneys from E17.5 PRRUB–/– and control [n = 3 mice (6 kidneys) per group] were pooled and homogenized in cold lysis buffer containing a cocktail of enzyme inhibitors using RIPA Lysis and Extraction Buffer Catalog number: 89901 and Halt Protease Inhibitor Cocktail Part No. 78430 from Thermo Scientific™. The samples were centrifuged, and the supernatants containing proteins (30 μg/lane) were resolved on 4–12% gradient SDS-polyacrylamide gels and transferred to nitrocellulose membranes. After blocking nonspecific binding, the membranes were incubated with anti-H3 di-methyl K79 (Rabbit polyclonal, ab3594, Abcam) or anti-total histone H3 (1/1000, Upstate) antibodies at room temperature for 1 h. After washes in phosphate-buffered saline/Tween, the nitrocellulose membrane was exposed for 1 h at room temperature to the secondary antibody. Immunoreactive bands were visualized using the enhanced chemiluminescence detection system (ECL, Amersham, NJ).
Statistical analysis
Statistical analyses were carried out upon all biologic replicates with Student’s t test or a one-way ANOVA. Data are presented as Mean ± SEM. A p value of <0.05 was considered statistically significant.
Results
Targeted deletion of the PRR in the UB lineage in PRRUB-/- mice reduces DOT1L mRNA and protein expression in the embryonic kidney
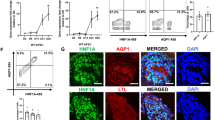
To determine whether lack of the PRR in the UB and UB-derived collecting ducts reduces expression of the histone H3K79 methyltransferase DOT1L, we examined DOT1L mRNA and protein expression in the kidney at E17.5. Kidney section surface area was smaller in the mutant compared to control mice (220600 ± 20120 vs. 533800 ± 72170 pixels, p < 0.05), consistent with renal hypoplasia observed in mutant mice in our previous study.4 (Fig. 1). In the embryonic kidney, besides abundant expression in the UB lineage, DOT1L is expressed in the cap mesenchyme, nephrons and at lower levels in cortical stromal cells.8 To determine the effect of PRR deficiency on DOT1L expression more specifically in the UB lineage, we quantitated DOT1L immunofluorescence intensity in the medulla and papilla areas where DOT1L staining should mainly represent expression in the UB/collecting ducts. DOT1L immunostaining, factored per kidney section surface area, was also reduced in the mutant vs. control kidneys (280 ± 35 vs. 400 ± 45 pixels, p < 0.01) (Fig. 1). DOT1L mRNA levels, factored per expression of the housekeeping gene GAPDH, were decreased in mutant compared to control mice (0.68 ± 0.06 vs. 1.0 ± 0.01, p < 0.01) (Fig. 1).
a a,b- DOT1L immunofluorescence is reduced in the papilla/medulla of the PRRUB−/− compared to control mice; c,d- Double immunofluorescence staining shows Aqp2-positive principal cells of the collecting duct (green) and reduction in DOT1L immunofluorescence in the intercalated cells of the collecting duct (red) in mutant kidney. b Data point graph shows reduced DOT1L fluorescent intensity in mutant compared to control kidneys. c Bar graph shows reduced DOT1L/GAPDH mRNA ratios in mutant compared to control kidneys.
Targeted deletion of the PRR in the UB lineage in PRRUB–/– mice reduces H3m2K79 protein expression in the embryonic kidney
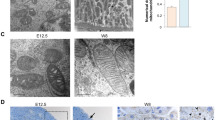
To determine whether reduced expression of DOT1L in PRRUB-/- kidneys is associated with decreased expression of its target H3K79m2, we measured the levels of H3K79m2 protein in PRRUB-/- and control kidneys at E17.5 by immunofluorescence and Western blot analysis. H3K79m2 immunostaining, factored per kidney section surface area, was reduced in the medulla and papilla areas of the mutant vs. control kidneys (215 ± 46 vs. 550 ± 42 pixels, p < 0.01) (Fig. 2). Western blot analysis revealed decreased H3K79m2 protein levels in mutant compared to control kidneys (1.0 ± 0.06 vs. 1.5 ± 0.02 densitometric units, p < 0.05) (Fig. 3).
a a,b- H3m2K79 immunofluorescence is reduced in the papilla/medulla of the PRRUB−/− compared to control mice; c,d- Double immunofluorescence staining shows Aqp2-positive principal cells of the collecting duct (green) and reduction in H3K79m2 immunofluorescence in the intercalated cells of the collecting duct (red) in mutant kidney. b Data point graph shows reduced H3K79m2 fluorescent intensity in mutant compared to control kidneys.
Discussion
The present study demonstrates that targeted deletion of the PRR in the UB lineage in PRRUB-/- mice reduces DOT1L mRNA and protein expression and decreases expression of its target H3K79m2 protein levels in the embryonic kidney.
Conditional ablation of the PRR in the developing UB in PRRUB−/− mice inhibits UB branching, resulting in renal hypodysplasia and reduced ability to acidify the urine.4 Histone H3K79 methyltransferase DOT1L regulates differentiation of the UB-derived collecting duct cells during development in mice.11 In this study, we examined whether reduced UB branching and reduced urine acidification capacity in PRRUB−/− mice is associated with a decrease in DOT1L/H3K79 methylation in the embryonic kidney.
Regulation of acid-base balance by the kidney is essential for health. In the collecting duct, α-intercalated cells (α-ICs) secrete protons into the tubular lumen through apical membrane V-ATPase.12 The PRR, encoded by ATP6AP2, is an accessory subunit of the V-ATPase.5 Differentiation of ICs from precursor UB cells requires the winged helix transcription factor FOXI1 as FOXI1UB−/− mice develop distal renal tubular acidosis (dRTA) due to absence of ICs.13 PRR deletion in the collecting duct in PRRUB−/− mice causes decreased expression of FOXI1, Cl-/HCO3- exchanger (AE1) and α-IC-specific V-ATPase subunit α4.4 Thus, PRR is a direct or indirect regulator of gene expression in α-ICs during terminal differentiation of collecting duct cells. As mutations in genes encoding V-ATPase α4 subunit or AE1 result in genetic forms of dRTA in humans,14 the role of PRR in acid-base homeostasis may be clinically relevant.
Histone methylation regulates gene expression by controlling access to DNA-binding transcription factors.15 Disruptor of telomeric silencing (DOT1L) is methyltransferase specific for histone H3K79.16 DOT1L regulates gene transcription via histone methylation and global knockout of DOT1L results in embryonic lethality in mice.17 Targeted deletion of DOT1L in principal cells of the collecting duct in mice results in increased number of acid-secreting intercalated cells and upregulates V-ATPase B1 subunit expression in these cells most likely by increasing ATP6V1V1 gene transcription rate.9
Our present findings of decreased DOT1L mRNA and protein expression in the embryonic PRRUB-/- compared to control kidney, coupled with diminished expression of its target H3K79m2 protein levels, suggest that reduced H3K79m2 methylation may alter the expression of H3K79m2-target genes important for UB branching or differentiation of acid-secreting cells in the collecting duct. One possible mechanism how lack of UB PRR may decrease ability of the kidney to acidify the urine is by reduced methylation of FOXI1 gene and its downstream targets, V-ATPase α4 and AE1 (Fig. 4). Here, DOT1L-mediated H3K79m2 may serve as a signal for repression of IC lineage during terminal differentiation of collecting duct cells.
Deletion of PRR in the UB in PRRUB-/- mice results in reduced DOT1L/H3K79me2 levels in the embryonic kidney. Decrease in DOT1L-mediated H3K79m2 may repress expression of genes promoting UB branching or genes promoting differentiation of UB cells into intercalated cell (IC) lineage (Foxi1). Decreased IC number may contribute, in part, to defects in urinary acidification capacity in PRRUB-/- mice.
In conclusion, targeted deletion of the PRR in the UB and UB-derived collecting ducts results in reduced DOT1L gene/protein and H3m2K79 protein expression in the embryonic mouse metanephroi in vivo. DOT1L mutations have not been described in human CAKUT. However, expression of many CAKUT-associated genes is altered in mice with targeted deletion of DOT1L in nephron progenitor lineage.8 Given that only 20–30% of human CAKUT cases are caused by known monogenic mutations, majority of CAKUT are likely due to undiscovered gene mutations or epigenetic modifications of gene expression. Future studies will determine whether H3K79 methylation downstream of the UB PRR plays a role in epigenetic regulation of collecting duct development and function.
Data availability
All data generated or analysed during this study are included in this published article.
References
Costantini, F. & Kopan, R. Patterning a complex organ: branching morphogenesis and nephron segmentation in kidney development. Dev. Cell 18, 698–712 (2010).
Song, R. & Yosypiv, I. V. Genetics of congenital anomalies of the kidney and urinary tract. Pediatr. Nephrol. 26, 353–364 (2011).
Ardissino, G. et al. Epidemiology of chronic renal failure in children: Data From the ItalKid project. Pediatrics 111, e382–e387 (2003).
Song, R., Preston, G., Ichihara, A. & Yosypiv, I. V. Deletion of the prorenin receptor from the ureteric bud causes renal hypodysplasia. PLoS ONE 8, e63835 (2013).
Nguyen, G. et al. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J. Clin. Invest 109, 1417–1427 (2002).
Advani, A. et al. The (Pro)renin receptor site-specific and functional linkage to the vacuolar H+-ATPase in the kidney. Hypertension 54, 261–269 (2009).
Wong, M., Polly, P. & Liu, T. The histone methyltransferase DOT1L: regulatory functions and a cancer therapy target. Am. J. Cancer Res. 5, 2823–2837 (2015).
Wang, F. et al. Targeted disruption of the histone lysine 79 methyltransferase Dot1L in nephron progenitors causes congenital renal dysplasia. Epigenetics 16, 1235–1250 (2021).
Xiao, Z., Chen, L., Zhou, Q. & Zhang, W. Dot1l deficiency leads to increased intercalated cells and upregulation of V-ATPase B1 in mice. Exp. Cell Res 344, 167–175 (2016).
Zhao, H. et al. Role of fibroblast growth factor receptors 1 and 2 in the ureteric bud. Dev. Biol. 276, 403–415 (2004).
Wu, H. et al. Aqp2-expressing cells give rise to renal intercalated cells. J. Am. Soc. Nephrol. 24, 243–252 (2004). (2013).
Wagner, C. A. et al. Renal vacuolar H+-ATPase. Physiol. Rev. 84, 1263–1314 (2004).
Blomqvist, S. R. et al. Distal renal tubular acidosis in mice that lack the forkhead transcription factor Foxi1. J. Clin. Invest 113, 1560–1570 (2004).
Karet, F. E. Inherited distal renal tubular acidosis. J. Am. Soc. Nephrol. 13, 2178–2184 (2002).
Harikumar, A. & Meshorer, E. Chromatin remodeling and bivalent histone modifications in embryonic stem cells. EMBO Rep. 16, 1609–1619 (2015).
Zhang, W., Hayashizaki, Y. & Kone, B. C. Structure and regulation of the mDot1 gene, a mouse histone H3 methyltransferase. Biochem J. 377, 641–651 (2004).
Jones, B. et al. The histone H3K79 methyltransferase Dot1L is essential for mammalian development and heterochromatin structure. PLoS Genet 4, 1–11 (2008).
Funding
This work was supported by NIH Grant DK-071699 to Ihor Yosypiv.
Author information
Authors and Affiliations
Contributions
I.V.Y. conceived and designed research, interpreted results of experiments, edited and revised manuscript. R.S. performed experiments, analyzed data and drafted manuscript. I.V.Y. and R.S. approved final version of manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
All experiments involving mice were approved by Tulane Institutional Animal Care and Use Committee.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Song, R., Yosypiv, I.V. Deletion of the prorenin receptor in the ureteric bud in mice inhibits Dot1/H3K79 pathway. Pediatr Res (2024). https://doi.org/10.1038/s41390-024-03026-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41390-024-03026-5