Abstract
Background
The aim of this systematic review and meta-analysis was to analyse the efficacy of azithromycin in acute bronchiolitis and wheezing.
Methods
PubMed, Scopus, and Web of Science databases were searched for randomized controlled trials comparing azithromycin to placebo in children <2 years of age. Main outcomes were progress of acute wheezing episode and recurrence of wheezing. We used random-effects model to calculate mean difference (MD) with 95% confidence interval (CI) or risk ratios (RR) with CI.
Results
We screened 1604 abstracts and included 7 studies. Risk of bias was low in three and had some concerns in four studies. Need for intensive care unit treatment was assessed in four studies (446 children) and the risk difference was 0.0% (CI –2.0 to 2.0; low quality evidence). Hospitalization duration was –0.27 days shorter in the azithromycin group (MD-0.27, CI –0.47 to –0.07; three studies; moderate quality evidence). Azithromycin did not prevent recurrence of wheezing (RR 0.84, CI 0.45–1.56; three studies), hospital readmissions (RR 1.14, CI 0.82–1.60; four studies).
Conclusions
We found moderate quality evidence that azithromycin may reduce hospitalization duration. Low certainty evidence suggests that azithromycin does not reduce the need for intensive care unit treatment. Furthermore, azithromycin did not prevent wheezing recurrence.
Impact
-
Azithromycin may reduce hospitalization time in acute bronchiolitis and wheezing episodes among children aged less than two.
-
Azithromycin administrated during the acute wheezing period, does not have preventive effect on wheezing recurrence.
-
Azithromycin seemed to have similar adverse event profile than placebo.
-
Future studies with clinically relevant outcomes, and sufficient sample sizes are needed, before implementing azithromycin into clinical use.
Similar content being viewed by others
Introduction
One-third of children suffer from wheezing during the first three years of life.1 Up to 26% of children present with recurrent wheeze (≥ 3 episodes) by age 6.2 Acute bronchiolitis causes the majority of hospital admissions in infants under 12 months in the United States and over three million hospital admissions annually worldwide, and predisposes to subsequent recurrent wheezing and asthma development.3,4,5 Bronchiolitis in infants under 12 months is mainly caused by respiratory syncytial virus (RSV), whereas rhinovirus (RV) prevails in older children.6,7
Because of the strong association to viral respiratory infections, guidelines do not suggest antibiotics for wheezing, yet they are widely used.8 Only supportive treatment of breathing and oxygenation, fluid replacement and alleviating symptoms, usually with inhaled short acting beta-agonists for children older than 12 months are widely accepted. Previously explored pharmacological prevention of post-RSV wheezing include montelukast and corticosteroids both inhaled and systemic. The results have remained mostly negative, possibly owing to the fact that the inflammation pattern in bronchiolitis and wheezy bronchitis is predominantly non-eosinophilic. Inhaled beta-agonists do not reduce hospital admissions or length-of-stay.9,10 Novel treatment strategies are needed to attenuate and prevent the symptoms of wheezing.
The exact shares of viral and bacterial aetiologies of wheezing remain obscure, as viral isolation is costly and not part of the routine examination.11 In the COPSAC2000 birth cohort the prevalence of co-infection of virus and bacteria was 55% in acute wheezing episodes among young children (85% either Haemophilus influenzae, Streptococcus pneumoniae or Moraxella catarrhalis), suggesting that antibiotics might have a role in treating such episodes to some extent.12 In adults macrolides have been successfully used in the treatment of chronic pulmonary diseases, such as diffuse panbronchiolitis, asthma, bronchiectasis, cystic fibrosis, and acute exacerbations of chronic obstructive pulmonary disease.13 A recent study respectively found that azithromycin treatment in children with poorly controlled asthma resulted in reduced asthma symptoms and exacerbations.14 Pulmonary diseases with neutrophil dominance are the most responsive to macrolide treatment. Macrolides have been long used for their antibacterial activity that extends over Mycoplasma pneumoniae and chlamydia pneumoniae, and their additional antiviral and anti-inflammatory effects have been studied during the past two decades.15,16,17,18,19 The anti-inflammatory properties are linked to the modulation of interleukin and TNF-α release and thus to the inhibition of the chemotaxis, oxidative burst and endothelial adhesion of neutrophiles. Immunomodulatory effects on several other cells, including fibroblasts, epithelial and endothelial cells, macrophages, and dendritic cells, have also been reported. Azithromycin has the highest tissue penetration of macrolides, accumulating particularly in phagocytes, which carry azithromycin to the inflammation site.19 Azithromycin also increases epithelial integrity.20
In addition to these well-targeted effects on the previously mentioned adult inflammatory respiratory diseases, azithromycin is a well-tolerated and safe drug, and therefore has attracted interest in its suitability for treating wheezing in children. In the past two decades a handful of studies have investigated this issue, but the results remain controversial. A couple of studies have demonstrated promising protection against subsequent wheezing episodes in infants treated with azithromycin during the initial episode.9,21 Stokholm et al. found out that azithromycin caused an impressive shortening of 63% in the asthma-like symptom episode.22 Bacharier et al. reported that the risk of progression to severe LRTI was 36% lower among azithromycin group compared to placebo.18
In this meta-analysis we assess the clinical effect of azithromycin in treating acute viral bronchiolitis or other wheezing episode (all later referred as wheeze or wheezing) and preventing recurrent episodes in children ages less than 24 months.10,23,24
Methods
Search strategy
A comprehensive search was conducted in December 2022 to following databases: PubMed (MEDLINE), Scopus, and Web of Science. The complete search strategy is presented in the supplementary file 1. We did not use any filters in the search in the PubMed and Web of Science databases. In Scopus we used language filter and filtered only to articles. There were no limitations regarding the time of publication.
Inclusion and exclusion criteria
We included randomized controlled trials (RCTs) comparing azithromycin treatment regardless of dose, route of administration and course duration to placebo or no intervention added to standard care in children aged less than 24 months suffering from acute wheezing. Acute wheezing was defined as clinically or parentally diagnosed obstructive expiratory respiratory disorder and it included diagnoses of bronchiolitis, and recurrent wheezing. We included studies with children less than two years of age, as the prevalence and incidence of wheezing is highest in this age-group. Study outcomes had to be clinical as studies only focused on laboratory parameters were excluded. Furthermore, all studies that did not report original data or were observational were excluded. Non-English reports were also excluded.
Review process
Covidence software was used in the screening and extracting process. Every abstract and full text was screened by two individual authors (RMU and IK) Disagreement between the two screening authors was resolved by a third author opinion or mutual consensus. Two authors (RMU and IK) performed data extraction independently. Following information was extracted: authors, year of publication, country where the study was conducted, study period, study design, original inclusion criteria, the definition of intervention group and control group, total number of patients included in the study, number of patients in the intervention and control groups, and outcome measures.
Main outcome
Our main outcomes were: (1) how azithromycin impacted the progress of acute wheezing episode, meaning need for hospitalization, need for pediatric intensive care unit treatment and length of hospital stay. (2) Did azithromycin have an impact on the recurrence of wheezing, including novel wheezing episodes, need for readmission to hospital and asthma diagnoses during the follow-up. Our secondary outcome was the treatment related adverse events. The main effect measures were risk ratios (RR) with 95% confidence interval (CI) for dichotomous outcomes and mean difference (MD) with CI for continuous outcomes. Risk difference (RD) with CI was used for outcomes with extremely low event rates.
Risk of bias
Cochrane risk of bias tool 2.0 was used to evaluate the quality of included studies, and risk of bias figures were reported accordingly. Risk of bias was performed by one author (IK). Robvis package in R version 4.2.2 was used to produce the figures.
Statistical analysis
The RevMan version 5.4.1 were used for the meta-analysis. Data analysis was performed according to Cochrane Handbook of Systematic Reviews Guidelines. Forest plots were presented for all pooled outcomes. We chose a random-effects model for main analyses due to assumed high heterogeneity based on the inclusion criteria and interventions (azithromycin dose, route of administration, course length and study setting). Statistical heterogeneity was tested and I2 results are reported in the forest plots. The model decision between fixed-effects and random-effects was however unrelated to statistical heterogeneity (I2 value). We used Mantel-Haenszel method in analyses. Furthermore, in one study we pooled high dose and standard dose azithromycin groups together for analysis in terms of reported mean and SD and Cochrane formula was used for this calculation.
We have reported our findings according to Preferred Reporting Items in Systematic Reviews and Meta-Analysis (PRISMA). PRISMA checklist is found in the supplementary materials. The body of evidence for each of the main outcomes were assessed according to GRADE (Grading of Recommendations, Assessment, Development and Evaluations) framework.
Protocol registration
We registered our protocol in Prospero (registration no. CRD42023392184).
Results
Search results
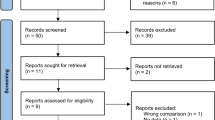
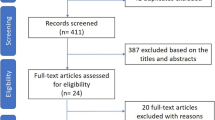
After screening 1604 abstracts and further assessment of 30 full reports, we included 7 studies for systematic review and meta-analysis (Fig. 1).
Characteristics of the included studies and patients
All included studies were double blinded RCTs and they were performed in the USA, Brazil, Australia, and Netherlands. (Table 1) The azithromycin dosing was mostly 10 mg/kg daily, and the duration varied from 3 days to 14 days. Two studies used a strategy with single or weekly repeated higher doses (30 mg/kg). (Table 1) Most of the patients were otherwise healthy children diagnosed with an acute bronchiolitis, mild or severe. (Table 1) Three studies focused on RSV cases and four included all viral aetiologies (Supplementary Table 1 and Table 1). The inclusion and exclusion criteria in the included studies were rather similar (Supplementary Table 1).
Risk of bias
Risk of bias was assessed to be low in three studies and had some concerns in four studies (Fig. 2) Most of the bias was due to selection of reported results. One study had some concerns with missing outcome data. However, none of these were judged to create such issues to validity that the study would have assessed to be in high risk of bias.
Clinical course of acute wheezing episodes
Four studies (446 children) analyzed the need for PICU admission and one child in the azithromycin group (0.5%) and four children in the control group (1.8%) were admitted to PICU (pooled RD 0.0%, CI –2.0% to 2.0%; Fig. 3). Evidence quality for PICU admissions was ranked as low. Three studies with 325 children analyzed the overall length of hospitalization period, and the mean difference was -0.27 days (CI –0.47 to –0.07 days; Fig. 4) favoring azithromycin group. Evidence quality for hospitalization duration was ranked as moderate (Table 2).
Prevention of recurrent wheezing
Two studies analyzed the readmission rate at three months follow-up and 13.8% (12/87) were readmitted in the azithromycin group and 13.8% in the control group (11/80), RR 1.01 (CI 0.48–2.15; Supplementary Fig. 1). Three studies assessed the need for readmission at six months and rates were 23.2% in the azithromycin group and 20.6% in the control group, RR 1.16 (CI 0.79–1.69; Supplementary Fig. 1). One study further assessed the readmission during one-year follow-up and the corresponding rates were 10.5% and 5.0% (RR 2.11, CI 0.21–21.36; Supplementary Fig. 1). When all of the previous timepoints were pooled together the risk ratio for readmission to hospital was 1.14 (CI 0.82–1.60; Supplementary Fig. 1). Evidence quality was ranked as low (Table 2).
Wheezing recurrence without the need for inpatient admission was analyzed in three studies and in three different time points. One study assessed recurrence at three months and the RR for wheezing was 0.48 (CI 0.22–1.06); Supplementary Fig. 2. One study assessed the recurrence during one year follow-up (RR 0.74, CI 0.35–1.54; Supplementary Fig. 2). One study analyzed recurrence up to 4 years of follow-up and the RR was 1.31 (CI 0.92-1.85). After pooling of these studies, the risk ratio for at least one recurrence of wheezing episode was 0.84 (CI 0.45–1.56; Supplementary Fig. 2). Evidence quality was ranked as very low (Table 2).
Two studies assessed the clinically diagnosed asthma. At one year, the RR was 0.42 (CI 0.09–1.92, one study) and at up to four years 1.49 (CI 0.68–3.28, one study). Pooled estimate for asthma did not show any difference between the groups (0.94, CI 0.29–3.10; Supplementary Fig. 3). Evidence quality was ranked as low.
Adverse events
Three studies with 515 children analyzed treatment related serious adverse events and reported 16 (6.2%) events among the 257 children in the azithromycin group and 17 (6.6%) events among the 258 children in the control group (RD 0.0%, CI –2.0% to 2.0%; Supplementary Fig. 4). Two studies (268 children) focused on gastrointestinal adverse events and did not find significant difference between the groups (RD 2.0%, CI –1.0% to 5.0%; Supplementary Fig. 4). Evidence quality regarding the adverse events was ranked as low (Table 2).
Discussion
In this systematic review and meta-analysis, we found low quality evidence that azithromycin does not reduce intensive care unit admissions. According to moderate quality evidence azithromycin reduces hospitalization time by –0.27 days. We also found that azithromycin does not prevent recurrent episodes of wheezing. Adverse events were similar between azithromycin and placebo.
Based on four studies azithromycin shortened the length of stay with –0.27 days—a difference that does not significantly benefit in the clinical practice. However, this was the only outcome in the review, where azithromycin showed efficacy and the evidence certainty was ranked as moderate. As the treatment durations in clinical practice have high variation, a mean reduction of six hours would not make an important impact, that would justify the addition of antibiotic to treatment of acute bronchiolitis. Need for intensive care unit admissions did not show evidence of a difference. However, the administration of azithromycin was started in hospital, and thus it is rather late. For the greatest theoretical anti-inflammatory and anti-viral benefit, azithromycin should be administered early at the onset of symptoms before the virus reaches its replication peak. Bacharier et al. included older children in their trial, where azithromycin was initiated immediately after symptom onset, it prevented the escalation of symptoms and reduced the need for hospitalization or corticosteroid treatment by 36%.18
Severe bronchiolitis episodes are associated with subsequent recurrent wheezing and asthma and passive immunization with recombinant anti-RSV antibody reduces this risk in late preterm infants.25,26 Azithromycin has been suggested to have the mechanistic rationale to benefit in the prevention of post-RSV recurrent wheezing too, because it inhibits neutrophilic airway inflammation, the dominant pattern seen in viral bronchiolitis.16 Azithromycin could also have an effect to aiway microbiota, and for example, prevent the harmful bacteria (such as Moraxella) associated and speculated to play an role in asthma development.27,28,29 On the other hand the use of antibiotics in early childhood, including during wheezing, is a known risk factor for asthma development.30 In this meta-analysis, four studies reported the recurrence of wheezing during their follow-ups from 3 months to 4 years, with no signal of a long-term protection with azithromycin.9,18,21,30 Although azithromycin has a long half-life because of its intracellular accumulation, maintaining measurable quantities in the airway macrophages for three weeks after the last dose of an 8-day course, recolonization of the airways seems inevitable after the treatment.31 Thus it seems unreasonable to expect any long-term protection against subsequent wheezing. This is in accordance with our finding that azithromycin could potentially decrease the recurrence of wheezing a few months onwards, but not up to 6 months or longer. A possible explanation for this could be that the short duration of given azithromycin treatment in the acute wheezing episode temporarily supresses local inflammation, but the effect vanishes. However, a repeated dosing or longer prophylactic courses would cause higher burden of antimicrobial resistance and influence both the microbiota in airways and gut also negatively.
Adverse events, which was the secondary outcome of this meta-analysis, were reported in 3 studies and they did not differ between the arms. Treatment related adverse event rates were approximately 6% in both the azithromycin and placebo groups. The detected gastrointestinal symptoms were mild diarrhea and vomiting, but the patients recovered and were able to continue the trial.
The prevalence of asthma increases with age. The immunology of asthma is heterogeneous; and the type of airway inflammation may be eosinophilic or neutrophilic.32 A recent Indian study found that elementary school aged children with poorly treated asthma might benefit from azithromycin along with standard treatment. They compared azithromycin (10 mg/kg) three times weekly for 3 months with standard treatment to standard treatment alone.14 Thus it could be possible that azithromycin would be beneficial in older children than included in this current meta-analysis.
The main weakness of this meta-analysis was the heterogeneity of the patients, ranging from mild wheezing to severe bronchiolitis requiring mechanical ventilation. Furthermore, due to heterogenous reporting, we were unable to estimate the impact of azithromycin based on the aetiology, and the lack of bacterial samples also is a limitation. RSV and non-RSV cases of wheezing are known to respond for example differently to oral corticosteroids. Further limitation is the low evidence quality as it varied between moderate and very low.
Conclusion
Our meta-analysis, including seven double blinded RCTs, showed that azithromycin therapy on wheezing did not reduce subsequent wheezing episodes. The hospital length of stay was shorter compared to placebo. Adverse event rates were similar between the arms. Further studies with azithromycin administered already at onset of the respiratory infection symptoms are required to properly assess the antiviral and anti-inflammatory potential of makrolides with different dosings and viral etiology reporting is also warranted.
Data availability
All data generated during the review process are available upon request from the corresponding author.
References
Mallol, J. et al. International prevalence of recurrent wheezing during the first year of life: Variability, treatment patterns and use of health resources. Thorax 65, 1004–1009, https://doi.org/10.1136/thx.2009.115188 (2010).
Ly, N. P., Gold, D. R., Weiss, S. T. & Celedón, J. C. Recurrent wheeze in early childhood and asthma among children at risk for atopy. Pediatrics 117, e1132–e1138, https://doi.org/10.1542/peds.2005-2271 (2006).
Leader, S. & Kohlhase, K. Recent trends in severe respiratory syncytial virus (RSV) among US infants, 1997 to 2000. J. Pediatr. 143, 127–132, https://doi.org/10.1067/S0022-3476(03)00510-9 (2003).
Chang, A. B., Chang, C. C., O’Grady, K. & Torzillo, P. J. Lower respiratory tract infections. Pediatr. Clin. North Am. 56, 1303–1321, https://doi.org/10.1016/j.pcl.2009.09.003 (2009).
Zorc, J. J. & Hall, C. B. Bronchiolitis: Recent evidence on diagnosis and management. Pediatrics 125, 342–349, https://doi.org/10.1542/peds.2009-2092 (2010).
Jartti, T. & Korppi, M. Rhinovirus-induced bronchiolitis and asthma development. Pediatr. Allergy Immunol. 22, 350–355, https://doi.org/10.1111/j.1399-3038.2011.01170.x (2011).
Beigelman, A. & Bacharier, L. B. The role of early life viral bronchiolitis in the inception of asthma. Curr. Opin. Allergy Clin. Immunol. 13, 211–216, https://doi.org/10.1097/ACI.0b013e32835eb6ef (2013).
Bisgaard, H. & Szefler, S. Prevalence of asthma-like symptoms in young children. Pediatr. Pulmonol. 42, 723–728, https://doi.org/10.1002/ppul.20644 (2007).
Beigelman, A. et al. Randomized trial to evaluate azithromycin’s effects on serum and upper airway IL-8 levels and recurrent wheezing in infants with respiratory syncytial virus bronchiolitis. J. Allergy Clin. Immunol. 135, 1171–1178.e1, https://doi.org/10.1016/j.jaci.2014.10.001 (2015).
McCallum G. B. et al. Three-weekly doses of azithromycin for indigenous infants hospitalized with bronchiolitis: A multicentre, randomized, placebo-controlled trial. Front Pediatr. 3. https://doi.org/10.3389/fped.2015.00032 (2015).
Mummidi, P. S., Tripathy, R., Dwibedi, B., Mahapatra, A. & Baraha, S. Viral aetiology of wheezing in children under five. Indian J. Med. Res. 145, 189–193, https://doi.org/10.4103/ijmr.IJMR_840_15 (2017).
Bisgaard, H. et al. Association of bacteria and viruses with wheezy episodes in young children: Prospective birth cohort study. BMJ (Online) 341, 770, https://doi.org/10.1136/bmj.c4978 (2010).
Martinez, F. Role of macrolide therapy in chronic obstructive pulmonary disease. Int J. Chron. Obstruct Pulmon Dis. ume 3, 331–350, https://doi.org/10.2147/COPD.S681 (2008).
Ghimire, J. J. et al. Azithromycin for poorly controlled asthma in children. Chest 161, 1456–1464, https://doi.org/10.1016/j.chest.2022.02.025 (2022).
Pinto, L. A. et al. Effect of clarithromycin on the cell profile of bronchoalveolar lavage fluid in mice with neutrophil-predominant lung disease. Rev. Hosp. Clin. Fac. Med Sao Paulo 59, 99–103, https://doi.org/10.1590/S0041-87812004000300002 (2004).
Friedlander, A. L. & Albert, R. K. Chronic Macrolide Therapy in Inflammatory Airways Diseases. Chest 138, 1202–1212, https://doi.org/10.1378/chest.10-0196 (2010).
Kanoh, S. & Rubin, B. K. Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clin. Microbiol Rev. 23, 590–615, https://doi.org/10.1128/CMR.00078-09 (2010).
Bacharier, L. B. et al. Early administration of azithromycin and prevention of severe lower respiratory tract illnesses in preschool children with a history of such illnesses. JAMA 314, 2034, https://doi.org/10.1001/jama.2015.13896 (2015).
Kricker, J. A. et al. NoNantimicrobial Actions Of Macrolides: Overview and perspectives for future development. Pharm. Rev. 73, 1404–1433, https://doi.org/10.1124/pharmrev.121.000300 (2021).
Halldorsson, S. et al. Azithromycin maintains airway epithelial integrity during Pseudomonas aeruginosa infection. Am. J. Respir. Cell Mol. Biol. 42, 62–68, https://doi.org/10.1165/rcmb.2008-0357OC (2010).
Luisi, F. L. et al. Azithromycin administered for acute bronchiolitis may have a protective effect on subsequent wheezing. J. Brasileiro de. Pneumologia 46, e20180376–e20180376, https://doi.org/10.36416/1806-3756/e20180376 (2020).
Stokholm, J. et al. Azithromycin for episodes with asthma-like symptoms in young children aged 1–3 years: a randomised, double-blind, placebo-controlled trial. Lancet Respir. Med. 4, 19–26, https://doi.org/10.1016/S2213-2600(15)00500-7 (2016).
Kneyber, M. C. J., van Woensel, J. B. M., Uijtendaal, E., Uiterwaal, C. S. P. M. & Kimpen, J. L. L. Azithromycin does not improve disease course in hospitalized infants with respiratory syncytial virus (RSV) lower respiratory tract disease: A randomized equivalence trial. Pediatr. Pulmonol. 43, 142–149, https://doi.org/10.1002/ppul.20748 (2008).
Kong, M. et al. Azithromycin treatment vs placebo in children with respiratory syncytial virus–induced respiratory failure. JAMA Netw. Open 3, e203482, https://doi.org/10.1001/jamanetworkopen.2020.3482 (2020).
Simoes, E. A. F. et al. Palivizumab prophylaxis, respiratory syncytial virus, and subsequent recurrent wheezing. J. Pediatr. 151, 34–42.e1, https://doi.org/10.1016/j.jpeds.2007.02.032 (2007).
Yoshihara, S. et al. Effect of palivizumab prophylaxis on subsequent recurrent wheezing in preterm infants. Pediatrics 132, 811–818, https://doi.org/10.1542/peds.2013-0982 (2013).
Zhou, Y. et al. Azithromycin therapy during respiratory syncytial virus bronchiolitis: Upper airway microbiome alterations and subsequent recurrent wheeze. J. Allergy Clin. Immunol. 138, 1215–1219.e5, https://doi.org/10.1016/j.jaci.2016.03.054 (2016).
Teo, S. M. et al. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe 17, 704–715, https://doi.org/10.1016/j.chom.2015.03.008 (2015).
Teo, S. M. et al. Airway microbiota dynamics uncover a critical window for interplay of pathogenic bacteria and allergy in childhood respiratory disease. Cell Host Microbe 24, 341–352.e5, https://doi.org/10.1016/j.chom.2018.08.005 (2018).
Beigelman A. et al. Azithromycin to prevent recurrent wheeze following severe respiratory syncytial virus bronchiolitis. NEJM Evid. 1. https://doi.org/10.1056/EVIDoa2100069 (2022).
Matzneller, P. et al. Blood, tissue, and intracellular concentrations of azithromycin during and after end of therapy. Antimicrob. Agents Chemother. 57, 1736–1742, https://doi.org/10.1128/AAC.02011-12 (2013).
Porsbjerg, C., Melén, E., Lehtimäki, L. & Shaw, D. Asthma. Lancet 401, 858–873, https://doi.org/10.1016/S0140-6736(22)02125-0 (2023).
McCallum, G. B. et al. A single dose of azithromycin does not improve clinical outcomes of children hospitalised with bronchiolitis: A randomised, placebo-controlled trial. PLoS One 8, e74316, https://doi.org/10.1371/journal.pone.0074316 (2013).
Funding
Open access funding provided by University of Eastern Finland (including Kuopio University Hospital).
Author information
Authors and Affiliations
Contributions
I.K. and M.R. conceptualized the study. R.-M.U. and I.K. conducted the search and screening process. I.K. performed statistical analyses and visualization. R.-M.U. wrote the first draft. M.R. and I.K. critically evaluated the revised the manuscript. M.R. provided resources. All authors have approved the final work to be submitted for publication and take full responsibility on the results.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ukkonen, RM., Renko, M. & Kuitunen, I. Azithromycin for acute bronchiolitis and wheezing episodes in children – a systematic review with meta-analysis. Pediatr Res (2023). https://doi.org/10.1038/s41390-023-02953-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41390-023-02953-z