Abstract
Background
The typical characteristic of pediatric obstructive sleep apnea syndrome (OSAS) is systemic inflammation and adenotonsillar hypertrophy (ATH), but the inflammatory markers and mechanism of adenotonsillar proliferation are unclear.
Methods
IHC, qPCR, and western blotting were used to identify the expression of CHI3L1 in the tonsils of children with OSAS. The primary tonsil lymphocytes (PTLCs) from children with OSAS were cultured and recombinant human CHI3L1 protein was added to culture media. After the stimulation with CHI3L1 protein of different concentrations and time points, lymphocyte proliferation was assessed by CCK-8 kits and flow cytometry. The activation of ERK1/2 and the effects on the proliferation of PTLCs were observed by western blotting.
Results
The expression of CHI3L1 was higher in the OSAS group than in the PS group. CHI3L1 (100 ng/mmol for 24 h) resulted in a significant increase in the proliferation rate. The ERK1/2 activator (PMA) promoted the proliferation of PTLCs and inhibitor AG126 significantly inhibited proliferation.
Conclusions
CHI3L1 can promote the proliferation of tonsil lymphocytes via ERK1/2 pathways. This result indicates that CHI3L1 may play an important role in the pathogenesis of OSAS in children. Inhibition of CHI3L1 or ERK1/2 may be potential therapeutic targets for CHI3L1-induced proliferation in childhood OSAS.
Impact
-
CHI3L1 may be an inflammatory marker in childhood OSAS.
-
CHI3L1 can promote the proliferation of PTLCs in a concentration and time-dependent condition.
-
CHI3L1 can promote the proliferation of tonsil lymphocytes via ERK1/2 pathways.
Similar content being viewed by others
Introduction
Obstructive sleep apnea syndrome (OSAS) in children is a disorder of breathing during sleep characterized by prolonged partial upper airway obstruction and/or intermittent complete obstruction (obstructive apnea) that disrupts normal ventilation during sleep and normal sleep patterns, accompanied by symptoms or signs, such as habitual snoring, disturbed sleep, and daytime neurobehavioral problems and other symptoms. The prevalence of OSAS in children ranges from 1.2 to 5.7% and the peak ages are between 2 and 8 years. The most important influencing factor for pediatric OSAS is considered to be adenotonsillar hypertrophy (ATH). Adenotonsillectomy (AT) is the first line of treatment at present.1 There is no effective medication for pediatric OSAS so far.
CHI3L1 is a chitinase-like glycoprotein consisting of 383 amino acids with a molecular weight of 40 kDa, so it is also called YKL40. CHI3L1 is involved in promoting cell proliferation, regulating migration, and invasion.2,3 Serum chitinase-like protein YKL40 is linked to some inflammatory diseases, such as asthma, allergic rhinitis, and chronic rhinosinusitis (CRS) with nasal polyps.4,5,6 A study reported that serum YKL40 level was elevated in adults with OSAS and correlated with the severity of the disease.7 Serum YKL40 level was correlated with apnea hypopnea index (AHI) in adults with OSAS and serum YKL40 level was a potential biomarker for OSAS.7,8 We have found that there was also a higher CHI3L1/YKL40 level in the blood serum of children with OSAS. The study has not been reported whether CHI3L1 can promote inflammatory hyperplasia in lymphoid tissue. The proliferation of lymphoid tissue, which is the typical characteristic in children with OSAS, has not been linked with CHI3L1. This study intends to explore the possible mechanism of CHI3L1 in children with OSAS and search for a specific medication for pediatric OSAS.
Methods
Sixty-four children aged 2–14 years with clinical symptoms related to sleep-disordered breathing were recruited from January 2019 to December 2020. All the pediatric patients were confirmed as OSAS or primary snoring (PS) and AT was performed because of ATH.
The diagnosis of OSAS was according to Chinese guidelines for the diagnosis and treatment of childhood obstructive sleep apnea and obstructive apnea hypopnea index (OAHI) >1 was diagnosed as OSAS.9 The research is in compliance with ethical standards and meets the requirements of the Ethics Committee (IEC-FOM-013-2.0). Informed consent was obtained from the participants whose residual tonsil tissue was collected.
Reagents
Recombinant human CHI3L1 protein/YKL40 was purchased from R&D systems (2599-CH-050). Antibody (Ab) of CHI3L1 was purchased from Abcam. Abs of proliferating cell nuclear antigen (PCNA), phospho-extracellular-regulated kinase (ERK), ERK, and β-actin were purchased from ABclonal (Wuhan). ERK activator (12-O-tetradecanoyl phorbol-13-acetate (PMA)) and ERK1 (p44) and ERK2 (p42) inhibitor (AG126) were purchased from APExBIO (Houston). Fetal bovine serum (FBS) and Roswell Park Memorial Institute (RPMI) 1640 medium were purchased from Gibco (Invitrogen).
Immunohistochemistry (IHC)
The sections of tonsil tissues were pretreated using heat-mediated antigen retrieval with sodium citrate buffer (pH = 6) for 20 min. Then the sections were incubated in 0.3% hydrogen peroxide for 10 min to block endogenous peroxidase and then incubated with PCNA (ABclonal, 1:3000) and CHI3L1 (ab77528, 1:1000) for 120 min at room temperature and detected using a horseradish peroxidase (HRP)-conjugated compact polymer system. 3,3′-Diaminobenzidine was used as the chromogen. The sections were then counterstained with hematoxylin. The sections were observed and photos were taken under a confocal laser scanning microscope.
Real-time PCR of tonsil tissues
Tonsil tissues were collected after surgical removal and immediately placed in liquid nitrogen. Total RNA was extracted from the tissue of tonsils using the RNA simple Total RNA Kit (DP419, TIANGEN, Beijing). In all, 1 μg of total RNA was used for synthesizing the cDNA using the AMV First Strand cDNA Synthesis Kit; B532445, Sangon Biotech, Shanghai). The reaction was carried out at 42 °C 45 min, 85 °C 5 min, 4 °C. One microliter of cDNA was used for the real-time PCR reaction using 2xSG Fast qPCR Master Mix (Low Rox, SYBR; B639272, Sangon Biotech, Shanghai). All primers were synthesized by Sangon (Shanghai) and the primers were as follows: CHI3L1 (forward) 5′-CTTTCCTGGTCGTCGTATCCTA-3′, (reverse) 5′-CACAGTCCATAGAATCCTCGG-3′ and ACTB; (forward) 5′-GGCCAACCGCGAGAAGATGAC-3’, (reverse) 5′-GGATAGCACAGCCTGGATAGCAAC-3′. The PCR reaction parameters were 95 °C 3 min, followed by 40 cycles at 95 °C 3 s, 60 °C 30 s, then 95 °C 15 s, 60 °C 30 s, 95 °C 1 s. ACTB was used as an internal control. The mRNA expression of CHI3L1 was normalized to ACTB and expressed as relative fold of change using the formula of 2−ΔΔCt.
Western blotting of tonsil tissues
Tonsil tissues were collected after surgical removal and immediately placed in liquid nitrogen. The protein of tonsil tissues was extracted in RIPA lysis buffer with phenylmethanesulfonylfluoride (PMSF). Protein concentration was determined using a bicinchoninic acid protein assay (Biosharp, Guangzhou). 5× sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis sample loading buffer was added to the protein samples and stored at −20 °C. In all, 10 μl protein samples were equally loaded on 10% SDS-polyacrylamide gel and transferred to 0.22 μm polyvinylidene fluoride membrane (Millipore). After blocking with 5% nonfat milk in TBST, the membranes were incubated with primary Ab against CHI3L1 (1:1000) and β-actin (1:2000 dilution) overnight at 4 °C followed by HRP-conjugated secondary Ab (1:5000 dilution, ABclonal). Detection was visualized using an ECL assay kit (Biosharp Life Sciences). The protein expression levels of CHI3L1 were analyzed with the NIH ImageJ program and were expressed as fold changes compared to the levels of β-actin.
Primary tonsil lymphocyte (PTLC) culture
Tonsils of OSAS patients were removed by otorhinolaryngologic surgeons according to the standard operation. The capsule of tonsils was separated and tissues were cut into small pieces and washed with phosphate-buffered saline (PBS) containing antibiotics and immediately delivered in icy PBS plus antibiotics to the laboratory within 10 min. The dissected tissue pieces were transferred into a 70 μm cell strainer in a 6 mm Petri dish with sterile, icy PBS plus antibiotics and then dissociated by grinding the tissue through the sieve with a syringe plunge. The suspension was pelleted (1000 rpm for 5 min) and then the supernatant was abandoned. Tonsil lymphocytes were isolated from the mixed cell suspension by density gradient centrifugation over Histopaque®-1077 (Sigma 10771). RPMI 1640 medium supplemented with 10% heat-inactivated FBS and 100× penicillin and streptomycin were added to suspend tonsil lymphocytes. After assessment of cell density using a counting chamber, the tonsil lymphocyte suspension was transferred into four 6-round well plates and 4/6 wells of each plate were divided into control (1 μl dimethyl sulfoxide (DMSO)), 10 ng/ml-CHI3L1, 100 ng/ml-CHI3L1, and 1000 ng/ml-CHI3L1 groups. In a 5% CO2 incubator at 37 °C for 6, 12, 24, and 48 h, cell proliferation was determined by Cell Counting Kit-8 (CCK-8) kits.
Cell proliferation assay
CCK-8 assay
CCK-8 (E606335-0100 Sangon Biotech) assay was used to value cell proliferation according to the manufacturer’s instructions. The concentration of 1 × 106 viable cells and volume of 100 μl were transferred into 96-well plates after different time points of culture and treatment with different concentrations of CHI3L1, 10 μl of CCK-8 solution (WST-8) were added to each well and then incubated for 1 h at 37 °C in the incubator. The absorbance of dissolved yellow formazan was measured at a test wavelength of 450 nm with a microplate reader (SpectraMax.i3x) and each sample was repeated for 3 wells.
Flow cytometry assay
Every sample (100 μl PTLCs) was washed and suspended with PBS at a cell density of 1 × 106 cells/ml. Then every sample was added to 20 μl Ab mixture (Anti-Human-CD19-APC 5 μl, Anti-Human-CD45-PE-Cy7 5 μl, Anti-Human-CD8a-PE 5 μl, Anti-Human-CD4-FITC 5 μl) at 4 °C for 2 h. The samples were analyzed by C6 flow cytometer. Data were analyzed using the BD Accuri C6 software.
ERK1/2 activation in PTLCs
Tonsil lymphocytes suspension was transferred into 6-round bottom well plates and 4/6 wells were divided into control (1 μl DMSO), treated with 100 ng/ml-CHI3L1, PMA (100 nM) for 48 h, and the fourth well was pretreated with AG126 for 1 h and then exposed to CHI3L1 (100 ng/ml) for 48 h. Tonsil lymphocytes were collected and the protein of cells was extracted in RIPA lysis buffer with PMSF. The procedure of concentration determination and western blotting were according to the procedure described in “Western blotting of tonsil tissues.” Antibodies of ERK1/2 (1:1000), p-ERK1/2 (1:1000), and β-actin (1:2000) were incubated overnight at 4 °C.
Statistical analysis
Data are reported as mean ± standard deviation (SD). T test and one-way analysis of variance tests were used to determine the statistical significance of two groups and the differences between groups. Shapiro–Wilk normality test was used to test the normality. Chi-square was used to evaluate the rates between the two groups. Spearman coefficient was used to analyze the correlation between the relative expression of CHI3L1 and PCNA. Statistical analyses were performed with GraphPad Prism 7.0. P value < 0.05 was considered to be statistically significant.
Results
Demographics in children with PS and OSAS
Sixty-four children undergoing tonsillectomy for PS and OSAS were recruited for this study. All of the children underwent overnight polysomnography (PSG). Demographic characteristics and major parameters of PSG for pediatric patients are presented in Table 1. The control group (PS) consisted of pediatric patients with OAHI ≤ 1/h and the study group (OSAS) consisted of patients with OAHI > 1/h. There were no significant differences in ages, sex, body mass index (BMI), and adenoid’s size. Significant differences were found in AHI, oxygen saturation nadir, and tonsils’ size between the two groups (Table 1).
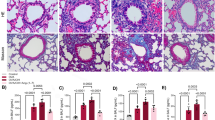
CHI3L1 and PCNA are overexpressed in the tonsils of children with OSAS (tonsils IHC)
For each section, five photos were taken in different visual fields. The CHI3L1-positive cells were stained with brown-yellow in cytoplasm and perinucleus of lymphatic epithelium lymphocytes of the germinal center (Fig. 1). Nuclei PCNA-positive cells were stained with brown in tonsil lymphocytes (Fig. 2). The proportion of positive cells in the total cells was analyzed by the ImageJ v1 8.0 program (NIH). The expression levels of CHI3L1 and PCNA protein were described by integral optical density. The expression level of CHI3L1 in the OSAS group (3.123 ± 1.251) was higher than that in the PS group (0.672 ± 0.464). The expression level of PCNA in the OSAS group (30.25 ± 7.598) was higher than that in the PS group (9.566 ± 5.62). Significant differences (P < 0.001) were noted between the two groups. The expression levels of CHI3L1 are significantly related to PCNA (r = 0.76, P < 0.001, Fig. 2f).
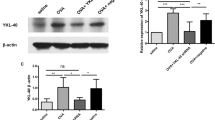
The mRNA and protein of CHI3 L1 are overexpressed in tonsils of children with OSAS
The relative mRNA expression of CHI3L1 was higher in the OSAS group (8.63 ± 10.0) (upper and lower quartiles 1.723–12.546) than that in the PS group (2.89 ± 3.1) (upper and lower quartiles 0.513–3.248). A significant difference (P < 0.05) was noted between the two groups (Fig. 3a). The relative protein expression was compared to the levels of β-actin according to intergrated density changes by western blotting. The protein expression of CHI3L1 was higher in the OSAS group (2.21 ± 2.11) than that in the PS group (1.13 ± 0.562). A significant difference (P < 0.05) was noted between the two groups (Fig. 3b).
PTLC proliferation is examined by CCK-8 and flow cytometry assay
CCK-8 was performed to examine the effect of CHI3L1 on children PTLCs. The cells were stimulated with different concentrations of CHI3L1 for different time points. The proliferation of PTLCs could be stimulated with CHI3L1 in a concentration- and time-dependent condition. The proliferation of PTLCs reached a peak when the concentration was increased to 100 ng/ml and at a time point of 24 h (Fig. 4a).
a CHI3L1 increased the cell viability in a concentration- and time-dependent manner in PTLCs. CHI3L1 with 100 ng/ml and 24 h promoted cell viability to a peak. b Cell viability in PTLCs cultured for 24 h with DMSO (Control, Con), CHI3L1 (100 ng/ml), PMA (100 nM, positive control), AG126 (20 μM for 1 h), and then CHI3L1 (100 ng/ml). c Cell viability in PTLCs cultured for 48 h in the Control, CHI3L1 (100 ng/ml), PMA, and AG126 groups. (ns P > 0.05, *P < 0.05, **P < 0.01,***P < 0.001).
The effects of CHI3L1 on the proliferation of CD4+, CD8+ T cells, and B cells in PTLCs were assessed after stimulation with 100 ng/ml CHI3L1 for 24 h. The percentage of CD4+ cells in the CHI3L1 group (49.17 ± 7.27) was higher than that in the control (37.23 ± 10.25) and a significant difference was noted (Fig. 5a). Percentage of CD8+ cells in the CHI3L1 group (5.20 ± 1.63) vs. Control (4.27 ± 1.6), percentage of CD19 B cells in the CHI3L1group (61.41 ± 5.50) vs. Control (58.77 ± 5.71), and no significant difference were noted in the groups (Fig. 5b, c). CHI3L1 could promote the proliferation of CD4+, CD8+ T cells, and CD19+ B cells relative to control. The appreciation rate of CD4+ T cells was 41.37 ± 37.99, of CD8+ T cells 25.58 ± 23.67, and that of CD19+ B cells 4.62 ± 4.03. There was no significant difference between CD4+ and CD8+ T cells and between CD8+ T cells and CD19+ B cells. A significant difference was noted between CD4+ T cells and CD19+ B cells (Fig. 5d).
a Percentage of CD4+ cells in Control (DMSO for 24 h) and CHI3L1 (100 ng/ml for 24 h), b percentage of CD8+ cells in Control (DMSO for 24 h) and CHI3L1 (100 ng/ml for 24 h), c percentage of CD19 B cells in Control (DMSO for 24 h) and CHI3L1 (100 ng/ml for 24 h), d the appreciation rate of CD4+ T, CD8+ T cell, and CD19 B cell (CHI3L1 compared to the Control group) (n = 6, ns P > 0.05, **P < 0.01).
ERK1/2 is activated and PTLC proliferation is induced by CHI3L1
The MAPK (mitogen-activated protein kinase pathways) family is well known to play a key role in mediating cell proliferation. ERK1/2 activation of MAPK pathways was verified in PTLC proliferation (Fig. 6a). CHI3L1 (100 ng/ml for 24 h) significantly increased the phosphorylation of ERK1/2, which could be stimulated by ERK1/2 activator (PMA 100 nM for 24 h). EKR1/2 phosphorylation was blocked by pretreatment with ERK1/2 inhibitor (AG126 20 μM for 1 h) and then CHI3L1 (100 ng/ml for 24 h, Fig. 6b). CHI3L1 (100 ng/ml for 24 h) and PMA (100 nM for 24 h) significantly induced PTLC proliferation (n = 6, Fig. 4b). AG126 (20 μM for 1 h) could restrain the proliferation of PTLCs. When PTLCs were cultured for 48 h with the same concentration of CHI3L1 and PMA, the proliferation slowed and had no difference compared with the control groups (n = 6, Fig. 4c).
a Representative immunoblots for P-ERK1/2, ERK1/2, and the corresponding β-actin control in PTLCs are shown. PTLCs were incubated for 24 h with DMSO (Control, Con), CHI3L1 (100 ng/ml), PMA (100 nM, positive control), AG126 (20 μM for 1 h), and then CHI3L1 (100 ng/ml for 24 h) using western blotting analyses with specific antibodies. b The relative P-ERK1/2 expression in the four groups (n = 6, ns P > 0.05, *P < 0.05, **P < 0.01, ***P < 0.001).
Discussion
A recent systematic literature search was performed to identify eligible studies involving the relationship between serum CHI3L1/YKL40 level and OSAS in adults. This study indicates that OSAS adults have a higher serum CHI3L1/YKL40 level, which may serve as a potential biomarker for OSAS diagnosis and monitoring.8 But the expression of CHI3L1 in childhood OSAS and mechanistic studies about the role of CHI3L1/YKL40 in the pathogenesis of OSAS have not been reported. In the previous study, we found that there was a higher CHI3L1 level in serum of children with OSAS, and CHI3L1 level was related to AHI of OSAS (to be published). To further clarify the relevance of CHI3L1/YKL40 with childhood OSAS, we confirmed the increased expression of CHI3L1/YKL40 in children’s OSAS by IHC, quantitative PCR, and western blotting. To explore the mechanism of CHI3L1/YKL40 to childhood OSAS, PTLCs were cultured10 and recombinant human CHI3L1 protein was used to stimulate PTLCs. Our study showed that CHI3L1 could promote the proliferation of tonsil lymphocytes via ERK1/2 signaling pathway. These findings indicate that CHI3L1/YKL40 possibly plays a regulatory role in the pathogenesis of OSAS in children.
YKL40 is a chitinase-like glycoprotein expressed and secreted by a variety of cell types.2 IHC results in this study showed that CHI3L1 is located in endochylema of tonsil lymphatic epithelium cells and lymphocytes. CHI3L1 staining in tonsil lymphatic epithelium cells and lymphocytes of patients with OSAS was stronger than in PS children. PCNA is a good indicator of reflecting the cell proliferation state. PCNA staining in lymphatic cells, especially in the germinal center, in the tonsils of patients with OSAS was stronger than that in PS patients. Moreover, there was a significant relationship between the expression of CHI3L1 and PCNA. So, we speculate that lymphocyte proliferation is induced by the action of CHI3L1, which is secreted by tonsil lymphangioepithelial paracrine action. The result was consistent with previous studies, namely, Serpero et al. study showed that the proliferative rates in tonsillar cell cultures from children with OSA were increased compared to recurrent tonsillitis (RI) group.10 Differential proliferative responses of lymphocytes in tonsils, which are the largest lymphatic tissues in the upper airway, cause different diseases such as OSAS and PS. In this study, we also find that the relative mRNA and protein expression are higher in the tonsil tissues in the OSAS group than in the PS one. And this suggests that YKL40 may increase tonsils’ size and cause tonsil hypertrophy. Adenoidal and tonsil hypertrophy are the risk factors for OSAS in children. So CHI3L1 may be one of the risks of OSAS and the level of CHI3L1 expression is positively associated with the severity of OSAS.
So far, there are a few studies on the proliferation mechanism of tonsil lymphocytes. López-González et al. speculated that an increase of basophils in children with tonsillar hypertrophy results in increased release of interleukin-4, which could prevent lymphoid apoptosis and lead to cell proliferation in tonsillar tissue.11 Gozal and his team have done a lot of work to find candidates for tonsil-proliferative genes in OSA. They have reported phosphatases as novel targets for ATH of pediatric obstructive sleep apnea. They revealed that it was more abundantly expressed in tonsils of children with OSA, and pharmacological inhibition of phosphoserine phosphatase led to reductions in lymphocyte cell proliferation and increased apoptosis.12 They also found that substance P and neurokinin 1 receptors increase in adenotonsillar tissues of children with OSA and antagonists of them could decrease the proliferation of mixed lymphoid cell cultures.13 Shu et al. reported that leukotriene D4 could promote T cell proliferation in adenoid tissues via upregulation of the ERK1/2 pathway in children with OSAS.14 Unfortunately, there is no specific medications that can control or reduce the size of tonsils.
CHI3L1/YKL40 as a special inflammatory factor is related to various inflammatory diseases and some tumors. Previous studies showed that YKL40 could affect the cell proliferation of cancer and the inflammatory process around the tumor. YKL40 could also induce inflammatory cell proliferation, such as bronchial smooth muscle proliferation and human bronchial epithelial cells.15,16,17 Suzuki et al. assessed the proliferative effect of YKL40 on epidermal keratinocytes and found that 100 ng/ml YKL40 significantly increased cell numbers of HaCaT cells.18 YKL40-induced BSM cell proliferation occurred in a concentration-dependent manner with a maximal effect for the concentration of 300 ng/ml.19 YKL40 could induce bronchial smooth muscle proliferation and migration in a concentration-dependent manner and the peak stimulus concentration was 1000 μg/ml.16 In our study, recombinant human CHI3L1 protein/YKL40 was used to stimulate PTLCs in concentration- and time-dependent mode and the maximal effect time was 24 h and concentration was 100 ng/ml. The different effects of concentration may be the absence of a concentration gradient between 100 and 1000 ng/ml.
Jinkwan Kim reported that CD3+ T cells were significantly higher in OSA children compared to RI children with flow cytometry; furthermore, CD4+ and CD8+ mRNA were highly expressed in children with OSA compared to RI and CD4+ being the predominant cell type.20 We also found that CHI3L1 could promote the proliferation of CD4+, CD8+ T cells, and B cells in PTLCs by flow cytometry. The proliferation of CD4+ T cells was more pronounced, but there was no statistical difference between CD8+ T cells and B cells compared to the Control group. This suggests that CD4+ T cells may be the main effector cell causing tonsil proliferation.
The mechanism that YKL40 promotes proliferation involves MAPK (c-Jun NH2-terminal kinase (JNK) and ERK), TGF-1/Smads, Akt, and NF-kB pathways.16,17,21 The pathways that promote the proliferation of lymphocytes in tonsils remain unclear. MAPKs are important signaling molecules that influence a broad range of cellular processes such as proliferation, differentiation, migration, and apoptosis22. MAPKs include ERK1/2, JNKs, p38, and ERK5. ERK1/2 is the most studied signal pathway about cell proliferation. We found that CHI3L1 (100 ng/ml for 24 h) significantly increased the phosphorylation of ERK1/2, which could be stimulated by ERK1/2 activator and be inhibited by ERK1/2 inhibitor AG126 in the study. When stimulated with 100 ng/ml CHI3L1-treated PTLCs for 48 h, the proliferation rates of PTLCs decreased and were not different in different groups. This suggests that ERK1/2 activation is time-dependent.
In conclusion, our current study shows that CHI3L1/YKL40 is located in endochylema of tonsil lymphatic epithelium cells and lymphocytes. Relative CHI3L1 expression was increased in mRNA and protein levels in OSAS children. Moreover, we first found that CHI3L1/YKL40 could promote the proliferation of tonsil lymphocytes via ERK1/2 signaling pathway. These findings implicate that CHI3L1/YKL40 may be involved in the proliferation of tonsil lymphocytes and CHI3L1/YKL40 may contribute to hypertrophy of tonsils in children who subsequently develop OSAS. Inhibition of CHI3L1/YKL40 or ERK1/2 signaling is an attractive potential therapeutic target for CHI3L1/YKL40-induced OSAS. The deficiency of this study is that there are no certified tonsil lymphocyte lines. Although PTLCs are close to the reality of the OSAS disease, these have individual differences and limit homogeneous repeat experiments.
References
Marcus, C. L. et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics 130, 576–584 (2012).
Zhao, T., Su, Z., Li, Y., Zhang, X. & You, Q. Chitinase-3 like-protein-1 function and its role in diseases. Signal Transduct. Target. Ther. 5, 201 (2020).
Johansen, J. S., Jensen, B. V., Roslind, A., Nielsen, D. & Price, P. A. Serum YKL-40, a new prognostic biomarker in cancer patients? Cancer Epidemiol. Biomark. Prev. 15, 194–202 (2006).
Knihtilä, H. et al. Serum chitinase‐like protein YKL‐40 is linked to small airway function in children with asthmatic symptoms. Pediatr. Allergy Immunol. 30, 803–809 (2019).
Ma, Y., Zheng, C. & Shi, L. The role of YKL40 in the pathogenesis of CRS with nasal polyps. Eur. Arch. Otorhinolaryngol. 275, 431–438 (2018).
Pirayesh, A. et al. Local expression of mucosal YKL-40; correlation of YKL-40 with clinical manifestations and immunopathogenesis of moderate/severe persistent allergic rhinitis patients. Immunol. Investig. 49, 46–57 (2019).
Mutlu, L. C. et al. Serum YKL-40 level is correlated with apnea hypopnea index in patients with obstructive sleep apnea syndrome. Eur. Rev. Med. Pharmacol. Sci. 21, 4161–4166 (2017).
Zhang, Y., Su, X., Pan, P. & Hu, C. The serum YKL-40 level is a potential biomarker for OSAHS: a systematic review and meta-analysis. Sleep. Breath. 24, 923–929 (2020).
Working Group of Chinese Guideline for the Diagnosis and Treatment of Childhood OSA; Subspecialty Group of Pediatrics, Society of Otorhinolaryngology Head and Neck Surgery, Chinese Medical Association; Chinese guideline for the diagnosis and treatment of childhood obstructive sleep apnea (2020). Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi Actions. 55, 729–747 (2020).
Serpero, L. D. et al. A mixed cell culture model for assessment of proliferation in tonsillar tissues from children with obstructive sleep apnea or recurrent tonsillitis. Laryngoscope 119, 1005–1010 (2009).
López-González, M. A., Díaz, P., Delgado, F. & Lucas, M. Lack of lymphoid cell apoptosis in the pathogenesis of tonsillar hypertrophy as compared to recurrent tonsillitis. Eur. J. Pediatr. 158, 469–473 (1999).
Khalyfa, A. et al. Transcriptomic analysis identifies phosphatases as novel targets for adenotonsillar hypertrophy of pediatric obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 181, 1114–1120 (2010).
Gozal, D., Kim, J., Bhattacharjee, R., Goldman, J. L. & Kheirandish-Gozal, L. Substance P and neurokinin 1 receptors as potential therapeutic targets in children with OSA. Chest 145, 1039–1045 (2014).
Shu, Y. et al. Effects of leukotriene D4 on adenoidal T cells in children with obstructive sleep apnea syndrome. Am. J. Transl. Res. 8, 4329–4337 (2016).
Sun, Y. et al. YKL-40 mediates airway remodeling in asthma via activating FAK and MAPK signaling pathway. Cell Cycle 19, 1378–1390 (2020).
Tang, H. et al. YKL-40 induces IL-8 expression from bronchial epithelium via MAPK (JNK and ERK) and NF-kappaB pathways, causing bronchial smooth muscle proliferation and migration. J. Immunol. 190, 438–446 (2013).
Guan, R. et al. Chitinase-like protein YKL-40 regulates human bronchial epithelial cells proliferation, apoptosis, and migration through TGF-beta1/Smads pathway. Hum. Exp. Toxicol. 39, 451–463 (2020).
Suzuki, H., Miyagaki, T. & Sato, S. YKL-40 augments the proliferation of HaCaT cells via the extracellular regulatory kinase pathway. J. Dermatol. 46, 551–552 (2019).
Bara, I. et al. Role of YKL-40 in bronchial smooth muscle remodeling in asthma. Am. J. Respir. Crit. Care Med. 185, 715–722 (2012).
Kim, J. et al. Increased cellular proliferation and inflammatory cytokines in tonsils derived from children with obstructive sleep apnea. Pediatr Res. 66, 423–428 (2009).
Suzuki, H. et al. YKL-40 promotes proliferation of cutaneous T-cell lymphoma tumor cells through extracellular signal-regulated kinase pathways. J. Invest. Dermatol. 140, 860.e3–868.e3 (2020).
Osaki, L. H. & Gama, P. MAPKs and signal transduction in the control of gastrointestinal epithelial cell proliferation and differentiation. Int. J. Mol. Sci. 14, 10143–10161 (2013).
Acknowledgements
The authors thank Zeng Wang, Ruiqing Chen, Jinan Lin, Junying Chen and Lengxi Fu, the central lab of the First Affiliated Hospital of Fujian Medical University for their kindness of experimental technical guidance. We thank Yupeng Chen who is in the pathology department of our hospital for the helpness of IHC. We also thank our colleague Xiaohong Lin in the sleep laboratory for her assistance with the PSG examination.
Funding
This work was supported by the Ministry of Fujian Science and Technology Department (No. 2020I0016) and the Natural Science Foundation of Fujian Province (No. 2021J01210).
Author information
Authors and Affiliations
Contributions
S.Y. and C.L. designed the research, Y.-g.W. and Y.C. performed the research, G.C. and M.H. processed and interpreted the data, Y.-g.W. wrote and revised the manuscript, S.Y. modified the manuscript and finally approved the version to be published. All the authors approved of the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The research is in compliance with ethical standards and meets the requirements of the Ethics Committee of The First Affiliated Hospital of Fujian Medical University (IEC-FOM-013-2.0). Informed consent was obtained from all the participants.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, Y., Chen, G., Lin, C. et al. Possible mechanism of CHI3L1 promoting tonsil lymphocytes proliferation in children with obstructive sleep apnea syndrome. Pediatr Res 91, 1099–1105 (2022). https://doi.org/10.1038/s41390-021-01907-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-021-01907-7