Abstract
Background
The engagement of the complement regulatory proteins CD46 and CD3 in human CD4+ T cells induces the type 1 regulatory T cells (Tr1) and interleukin-10 (IL-10) secretion. This study aimed to elucidate the molecular changes of Tr1 cells through CD46 cytoplasmic Cyt1 tail in lupus nephritis (LN) respond to intravenous methylprednisolone (ivMP) therapy.
Methods
We enrolled 40 pediatric patients with LN and 30 healthy controls. Clinical characteristics and peripheral blood mononuclear cells were collected before and 3 days after the administration of ivMP. Kidney specimens were taken from five LN and five minimal-change nephrotic syndrome patients.
Results
We found that defective CD46-mediated T-helper type 1 contraction (IL-10 switching) is present in active LN patients. The ivMP therapy enhanced LN remission, restored the production of IL-10, increased the CD46-Cyt1/Cyt2 ratio, AKT, and cAMP-responsive element-binding protein phosphorylation, and induced migration with the expression of chemokine receptor molecules CCR4, CCR6, and CCR7 of CD3/CD46-activated Tr1 cells.
Conclusions
Pharmacologic interventions that alter the patterns of CD46-Cyt1/Cyt2 expression and the secretion of IL-10 by CD3/CD46-activated Tr1 cells can be used in patients with active LN.
Impact
-
In patients with LN, ivMP was associated with increased IL-10 production and increased CD46-Cyt1/Cyt2 ratio and AKT phosphorylation by Tr1 cells, with enhanced potential to migration in response to CCL17.
-
These results suggest that expression levels of CD46 isoforms Cyt1 and Cyt2 in CD4 + CD46 + Tr1 cells differ in patients with active LN but can be corrected by corticosteroid treatment.
-
Enhancing the expression of functional CD4 + CD46 + Tr1 cells may be a useful therapeutic approach for LN.
Similar content being viewed by others
Introduction
Lupus nephritis (LN) is the major cause of morbidity and mortality in systemic lupus erythematosus (SLE). Dysregulated cytokine production in SLE contributes to immune dysfunction and tissue inflammation.1 The inappropriate activation of T-helper type 1 (Th1) cells, which leads to the production of pro-inflammatory cytokine interferon-gamma (IFN-g) in response to self-antigens or innocuous antigens, has been associated with the development and progression of SLE.2,3,4 To establish effective therapies for deregulated complement hyperactivation, researchers have shown interest in understanding the importance of reducing glomerular inflammation in LN.5,6,7 Interleukin-10 (IL-10)-secreting type 1 regulatory T cells (Tr1) play a role in the regulation of inflammation-dependent adaptive immunity and the reduction of tissue damage, and these Tr1 cells may be important in maintaining immune tolerance in LN.8,9,10,11,12,13,14
To prevent autologous complement-mediated lysis of cells in an inflammatory environment, the complement regulatory protein CD46 must be upregulated in activated leukocyte subsets.15 CD46 expression in resting T cells maintains T cell homeostasis, and the engagement of CD46 proteins in TCR-stimulated CD4+ T cells can contribute to the production of IL-10 and granzyme B, a phenotype shared with Tr1 cells.16,17,18,19 CD46 can help IL-2-dependent CD3-activated CD4+ T cells to switch from nonsuppressive IFN-g+IL-10− Th1 cells to suppressive IFN-g−IL-10+ Th1 cells.20 The CD46 and IL-2-induced IFN-g to IL-10 switching is defective in autoimmune diseases, such as rheumatoid arthritis15, multiple sclerosis,18 and allergic asthma.19,21
Alegretti et al. have shown that lymphocytes have a decreased CD46 expression in individuals with SLE, which is correlated to disease activity.22 One study has reported that CD46 mutations increase the risk of developing early-onset LN.23 There are two CD46 cytoplasmic isoforms (Cyt1 and Cyt2) in CD4+ T cells with two regulatory expressed cytoplasmic tails.15 Cyt1 and Cyt2 differ in terms of amino-acid sequence, and they have motifs required for signaling. Cyt1 inhibits inflammation, whereas Cyt2 increases, inflammation.16 CD4+ T cells in a transgenic mice model express CD46 bearing the Cyt1 cytoplasmic tail, produce IL-10, and inhibit contact-hypersensitivity reaction after T cell receptor (TCR) and CD46 activation. In contrast, the activation of the Cyt2 cytoplasmic tail of CD46 had weak proliferation and low IL-10 production, but an increased contact-hypersensitivity reaction.24 To date, the rescue of the IFN-g to IL-10 switching of CD46-mediated Tr1-like cells in LN has not been comprehensively assessed.
Intravenous methylprednisolone (ivMP) pulse therapy for LN has long-term effects on immune tolerance as it inhibits inflammation.11,25,26 However, data about the effects of corticosteroids on CD46-induced Tr1-like cells in maintaining immune tolerance in individuals with active class III and IV LN are limited. Herein, we first evaluated the IFN-g to IL-10 switching in CD3/CD46-activated Tr1-like cells, the migratory capacity of CD3/CD46-activated Tr1-like cells in suppressing inflammation, and the altered expression of CD46 cytoplasmic isoforms before and after ivMP therapy in patients with active LN. These investigations could provide further evidence that can elucidate the recruitment mechanisms involving CD3/CD46-induced Tr1-like cells, not only in suppressing kidney inflammation but also in providing new therapeutic strategies for LN.
Materials and methods
For this prospective cohort study, 40 active Class III or IV LN patients with prominent proteinuria, aged 10–18 years (15.6 ± 3.1, 32 females and 8 males) fulfilling the 2019 European League Against Rheumatism and American College of Rheumatology criteria, with disease onset before 10 years of age, and nephrotic range proteinuria (>40 mg/m2/h or >1 g/day/m2) were recruited. Class III or IV LN was classified according to the 2018 revised International Society of Nephrology, and Renal Pathology Society Criteria for exclusion were previous cyclophosphamide or mycophenolate mofetil therapy, and administration of calcineurin inhibitors or rituximab. Clinical data including serum creatinine levels, glomerular filtration rate, and 24 h proteinuria were recorded. Disease activity was assessed by a SLEDAI score -2k (SLEDAI-2k), validated for use in children, and LN activity was defined by a renal score of SLEDAI-2k.27,28 All patients received ivMP pulse therapy (15 mg/kg/day and maximum with 1 g/day for 3 days), followed by oral prednisolone 1 mg/kg of body weight.29 Clinical responses with SLEDAI and SLEDAI-2k scores were calculated before, and 14 days after ivMP treatment in LN subjects.
Peripheral blood mononuclear cells (PBMCs) were obtained from LN patients before (day 0) and after (day 6) ivMP pulse therapy. Follow-up serum C3, C4, anti-dsDNA antibody levels, and 24 h proteinuria were calculated 2 weeks after ivMP pulse therapy. In addition, this study included 30 age-matched (14.0 ± 1.1 years old) healthy individuals, unrelated to the patients and without inflammatory or autoimmune diseases. All individuals enrolled in each group completed the study and written informed consent was obtained from each participant and their guardian. This study was approved by the institutional review boards of China Medical University Hospital and Changhua Christian Hospital (DMR97-IRB-259 and CCH IRB No.161208) and all methods were performed in accordance with the relevant guidelines and regulations.
Antibodies and reagents
Anti-human CD4, CD8, CD46 IL-10, IFN-g monoclonal antibodies (mAbs), and isotype-matched control mAbs conjugated with FITC, PE, or PC5, and neutralizing anti-IL-10 mAbs were obtained from BD Biosciences (San Diego, CA). Total AKT mAb (Clone:9272, Cell signaling), anti-phospho-AKT (Clone:9198, Cell Signaling), total CREB mAb (Clone:9197, Cell Signaling), anti-phospho-CREB (Clone:9271, Cell Signaling), and β-actin were obtained for western blot analysis (Abcam, Cambridge, UK).
Cell isolation and cell culture
Fresh PBMCs were isolated by Ficoll-Hypaque (Pharmacia, Uppsala, Sweden) gradient centrifugation after obtaining the blood specimens. PBMCs (1 × 106) were cultured in RPMI-1640 medium with 10% heat-inactivated FCS, at 37 °C, 5% CO2 in 96-well plates. CD4+ T cells were purified from PBMCs with microbeads, as per the manufacturer’s (BD Biosciences) protocol within 24 h. The purity of the isolated CD4+ T population was analyzed by flow cytometry as ≧95%. CD4+ T cells (1 × 106) were cultured with IL-2 (10 U/ml), anti-human CD3 mAb (Clone: BD555336) (2 μg/ml), anti-human CD28 mAb (Clone: BD556620) (5 μg/ml), or anti-human CD46 mAb (Clone: BD555948) (5 μg/ml) (BD Biosciences) for 5 days.18
Confocal immunofluorescence and immunohistochemistry
Kidney specimens were taken from class III or IV LN (n = 5) and minimal-change nephrotic syndrome patients (14.6 ± 1.1 years old) as control subjects (n = 5). Kidneys were embedded and frozen in OCT compound (Ted Pella, Redding, CA) and snap-frozen in liquid nitrogen. Frozen tissue sections were incubated with anti-CD4 FITC antibody, anti-CD46 PE antibody (BD Biosciences), and 4′,6‐diamidino‐2‐phenylindole (nuclear stain), and observed with a confocal laser scanning microscope (Leica TCS SP2). Formalin-fixed and paraffin-embedded blocked renal biopsy specimens were treated according to the protocol described previously.29 Immunohistochemistry for CD4+CD46+ T cells in kidney specimens according to the protocol described previously. Rabbit monoclonal anti-CD46 antibody (Genetex, Irvine, CA) and anti-CD4 antibody (Abcam, Cambridge, MA) were diluted 1:500 with antibody diluent (Lab Vision, Fremont, CA). The kidney specimens were incubated with the primary antibodies overnight at 4 °C, followed by a second reaction with anti-rabbit antibody conjugated with envision polymer (Thermo Fisher Scientific) for 30 min. Finally, a diaminobenzidine reaction was performed on the section using a kit (TL-060-QHD; Thermo Fisher Scientific). Three randomly chosen high-power fields at ×400 magnification per individual participant were evaluated by two experienced researchers in a blinded manner.
Flow cytometry and cell proliferation assay
PBMCs were stained with fluorescein-conjugated mAbs targeting CD46 (FITC) and CD4 (PE-Cy5) for surface expression, and then the harvested cells were incubated with propidium iodide to estimate the viability of the cells (BD Biosciences). The percentage of CD4+CD46+ T cells from total lymphocytes in SLE patients (n = 30) and controls (n = 30) subjects analyzed by flow cytometry. Chemokine receptors CCR4 and CCR7 staining of CD3/CD46-activated CD4+ T cells from LN patients (n = 20) and healthy controls (n = 20) was determined by flow cytometry (FC500, Beckman Coulter, Fullerton, CA). For intracytoplasmic staining for IL-10 (Human PE Clone: JES3-19F1, BD Biosciences) and IFN-g cytokines (Human FITC Clone: B27, BD Biosciences) staining in independent normal (n = 30) and LN subjects (n = 30), CD4+ T cells were stimulated with phorbol myristate acetate (10 ng/mL) plus ionomycin (1 μg/mL) for 5 h, and brefeldin A (10 μg/mL) was added for the final hour. IL-10 and IFN-g were measured using the standard procedures (eBioscience, San Diego, CA)21 and then analyzed by FACS using a scanning flow cytometer (FC500, Beckman Coulter, Fullerton, CA) by acquiring data for 10,000 events. To rate, CD3/CD46-activated CD4+ T cell suppression from 30 paired LN subjects, CD4+CD25− T cells were labeled with 5 μM carboxyfluorescein diacetate succinimidyl ester (CFSE) (Invitrogen, Carlsbad, CA) for 15 min at 37 °C, washed twice, and stimulated with IL-2 and anti-CD3 mAb, and cultured with or without CD3/CD46-activated CD4+ T cells at a 10:1 ratio for 5 days, as previously described.21 After culture, the proliferation of CD4+ T cells was calculated according to CFSE fluorescence and flow cytometry.
Enzyme-linked immunosorbent assay (ELISA)
IL-10 and IFN-g concentrations of cell supernatants were examined by commercially available ELISA-based assay systems (R&D Systems, London, UK). Assays were performed as per the manufacturer’s protocol.
Real-time PCR
Reverse transcription-quantitative real-time PCR (RT-qPCR) analysis of Cyt1 mRNA and Cyt2 mRNA in CD4+ T cells by CD3/CD46 stimulation in LN patients (n = 40) before and after ivMP therapy and in control subjects (n = 30). Real-time PCR was conducted with a Cepheid SmartCycler thermocycler using SYBR Green in the reaction mixtures. Specific primers used were: Cyt1—sense, 5′-CTAACTGATGAGACCCACAGAG AAGT-3′ and antisense, 5′-TCAGCTCCACCATC TGCTTTC-3′; Cyt2—sense, 5′-GAAG AAAGGGAAAG CAGATGGT-3′ and antisense, 5′-CCTCTCTGCTCTGCTGGAGTG-3′. β-Actin—sense, 5′-TTCTGTGGCATCACG AAACT-3′ and antisense 5′-GAAGCATT TGCGGTGGACGAT-3′, as previously published.18 mRNA expression levels (ΔCT) were calculated as fold change using the formula \(2^{{\Delta}C_{{{{{\rm{T}}}}}}}\), ΔCT = ΔCT (control) − ΔCT (target).
Chemotaxis assay
Chemotaxis assays were performed in Transwell chambers with 5 μM pore size (Costar, Cambridge, MA), and the bottom chamber of each well was filled with CCR4 ligand, TARC (CCL17, R&D Systems, Minneapolis, MN) 100 ng/ml, and carefully overlaid with a polycarbonate membrane. CD3/CD46-activated CD4+ T lymphocytes from 20 paired LN subjects (1 × 106 cells/ml) loaded into the upper chamber were left to transmigrate for 2 h at 37 °C, 5% CO2, and cells migrating into the lower chambers were counted in duplicate samples.
Western blot analysis
Cellular proteins from CD3/CD46-activated CD4+ T cells in six paired LN patients were separated on 10% SDS-polyacrylamide gels. After electrophoresis, proteins were transferred onto PVDF membranes and blotted with antibodies against nuclear phospho-CREB and phospho-AKT, as previously described.19
Statistical analysis
Demographic data of LN patients are shown as means ± SD. The Mann–Whitney U tests were used to test the difference between the two study groups. Wilcoxon’s signed-rank test was used to assess the difference in serum cytokine levels in LN patients during the ivMP therapy. Groups of data sets were compared using Kruskal–Wallis tests, followed by the Duncan test. A p value < 0.05 was considered statistically significant.
Results
Clinical response to ivMP therapy among patients with LN
Table 1 shows the clinical characteristics of 40 participants with class III or IV active LN. All patients completed the protocol, and no mortality cases were recorded. All patients with LN who received ivMP therapy had better SLE disease activity index (SLEDAI) and SLEDAI-2k scores and complement levels and decreased daily protein levels (p < 0.05).
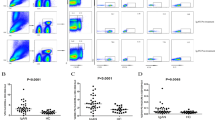
Decrease in the number of CD46+CD4+ T cells in patients with active LN before ivMP therapy
The confocal microscopic analysis of kidney tissues revealed that the number of CD4+CD46+ T cells was lower in participants with class III or IV active LN (n = 5) than in controls with minimal changes in the disease (n = 5) (Fig. 1a–c). The percentage of blood circulating CD4+CD46+ cells significantly decreased before ivMP therapy in patients with active LN compared with healthy participants (p < 0.05) (Fig. 1d, e).
a Confocal microscopic analysis of kidney tissue biopsies in Class IV LN subjects (n = 5), after immunofluorescent antibody staining, shows decrease numbers of CD4+ and CD46+ cells in the cortical interstitium as compared to minimal-change nephrotic syndrome controls (n = 5). Representative images (b) and quantification (c) of immunohistochemistry (CD4+ T cells indicated by the red arrow; original magnification ×400). d Representative gate strategy of live lymphocytes for CD4+CD46+ T cells in blood by flow cytometry after stimulation with anti-CD3 and anti-CD46. e The percentage of CD4+CD46+ T cells from circulating lymphocytes in SLE patients (n = 30) and controls (n = 30) subjects analyzed by flow cytometry (*p < 0.05).
Defective IFN-g to IL-10 switching in CD3/CD46-mediated Tr1 cells in patients with active LN and rescue with iVMP therapy
Based on the association between the CD46 costimulatory pathway and induction of Tr1 cells, we analyzed the number of CD3/CD46-mediated Tr1 cells in patients with LN during flare-ups and after the administration of ivMP compared with healthy controls. To assess human CD4+ T cell proliferation, the purified CD4+ T cells were labeled with carboxyfluorescein succinimidyl ester (CFSE) and were stimulated with CD3, CD3/CD28, and CD3/CD46 mAbs in the presence of IL-2. No significant difference was found between the proliferation of CD3, CD3/CD28, and CD3/CD46 mAb-activated CD4+ T cells between healthy controls and patients with active LN during ivMP therapy (Fig. 2a). Then, the experiments were performed to examine the expression of IL-10 and IFN-g in CD3/CD46-activated Tr1 cells. Increased IFN-g (24.4 ± 5.2 vs 6.2 ± 3.5%, p < 0.05) and decreased IL-10 expression (4.4 ± 3.2 vs 13.2 ± 4.5%, p < 0.05) in CD3/CD46-mediated Tr1 cells were observed in the acute phase in patients with LN (n = 30) compared with healthy controls (n = 30) (Fig. 2b). A significant increase in IL-10 expression cells (9.4 ± 3.4 vs 4.4 ± 3.2%, p < 0.05), accompanied by a decrease in IFN-g expression cells (13.4 ± 4.4 vs 24.4 ± 5.2%, p < 0.05) among CD3/CD46-mediated Tr1 cells was observed after ivMP therapy (Fig. 2c, d). When cytokine secretion was quantified using ELISA, a striking defect involving CD3/CD46- and CD3/CD28-mediated IL-10, but not IFN-g, was observed in patients with active LN compared with controls (p < 0.05) (Table 2). Notably, the switching defect of IFN-g to IL-10 secretion in CD3/CD46-mediated Tr1 cells was reversed after ivMP therapy (Table 2).
a Purified, CFSE-labeled purified CD4+ T cells from LN subjects (n = 10) or healthy controls (n = 10) were pretreated with IL-2, anti-CD3 and/or anti-CD28, and anti-CD46 mAb for 5 days and then analyzed by flow cytometry. b Representative figures of intracellular IL-10 and IFN-gamma levels in CD3/CD46-activated CD4+ T cells from LN subjects (n = 30) and healthy controls (n = 30). Increased proportions of IL-10+ (c) and decreased proportions of IFN-gamma+ (d) cells were found after ivMP treatment (#p < 0.05 compared to controls and *p < 0.05 compared after ivMP administration).
Increased suppressive activity of CD3/CD46-mediated CD4+ Tr1 cells via IL-10 after ivMP therapy
Before ivMP therapy, the CD25-depleted CD4+ T cells in patients with LN and controls were stimulated with anti-CD3 mAb and IL-2 and then labeled with CFSE. Before and after ivMP therapy, purified non-CFSE-labeled CD3/CD46-activated CD4+ Tr1 cells were added to the CD25-depleted CD4+ T cells, and cell proliferation was assessed. In a representative study, the addition of CD3/CD46-mediated CD4+ Tr1 cells significantly inhibited CD4+ T cell proliferation (Fig. 3a). Figure 3b depicts the data of 30 patients with LN: CD3/CD46-mediated CD4+ Tr1 cells had less suppression of CD25-depleted CD4+ T cell proliferation in patients with active LN, and the administration of ivMP increased the CD3/CD46-mediated CD4+ Tr1 cell suppression of CD25-depleted CD4+ T cell proliferation. Notably, there was an increase in CD25-depleted CD4+ T cell proliferation in co-cultures with CD3/CD46-induced CD4+ Tr1 cells after treatment with neutralizing anti-IL-10 mAb (Fig. 3b).
a CFSE-labeled cells (CD4+CD25− T cells) stimulated with IL-2 and anti-CD3 mAb, and cultured with or without CD3/CD46-activated CD4+ T cells at a ratio of 10:1 and with/without neutralizing anti-IL-10 mAb for 5 days, and proliferation of CD4+ T cells analyzed by flow cytometry; representative histograms are shown. b A summary from 30 paired samples showed a significant difference in suppression of CD4+ T proliferation in the presence of CD3/CD46-activated CD4+ T cells (#p < 0.05 compared to controls and *p < 0.05 compared after ivMP treatment).
Altered expression of CD46 cytoplasmic isoform after ivMP therapy
To further investigate the mechanism involving the loss of IL-10 secretion by CD3/CD46-activated CD4+ Tr1 cells in patients with active LN, we examined the expression of Cyt1 and Cyt2 via a quantitative real-time quantitative polymerase chain reaction analysis. Decreased CD46-Cyt1 isoform mRNA (Fig. 4a) and increased CD46-Cyt2 isoform mRNA (Fig. 4b) expressions in CD3/CD46-induced Tr1 cells were observed in patients with active LN, but not in healthy controls (p < 0.05). There was a reciprocal change in the increase in CD46-induced Cyt1 mRNA expression (Fig. 4a) associated with a decreased CD46-induced Cyt2 mRNA expression (Fig. 4b) after ivMP therapy. Thus, the expression of these intracytoplasmic isoforms might regulate the outcome of the response initiated by CD46 engagement.
ivMP therapy enhanced the adhesion and migration of CD3/CD46-activated CD4+ Tr1 cells in patients with LN
The specific expression of the adhesion receptors CCR4, CCR6, and CCR7 in T-regulatory (Treg) cells might allow migration toward the sites of inflammation in the kidney and inhibition of the responding cells. The percentage of CCR4 and CCR7 in CD3/CD46-mediated CD4+ Tr1 cells significantly decreased in patients with active LN (n = 20) before ivMP therapy compared with healthy controls (n = 20) (p < 0.05) (Fig. 5a and Supplementary Fig. 1). ivMP pulse therapy increased the expression of CCR4, CCR7, and CCR6 by CD3/CD46-mediated CD4+ Tr1 cells in patients with LN (p < 0.05) (Fig. 5a and Supplementary Fig. 1). To identify the functional chemotactic activity of CD3/CD46-costimulated CD4+ Tr1 cells after ivMP therapy, the migration capacity of CD3/CD46-activated CD4+CD46+ T lymphocytes activated by CCL17 was assessed in vitro. The ratio of migrating CD3/CD46-mediated CD4+CD46+ T cells activated by CCL17 decreased in patients with active LN before ivMP compared with controls (p < 0.05) (Fig. 5b). Then, the experiments were performed on 20 paired samples. Results showed that ivMP therapy increased the CCL17-mediated migration of CD3/CD46-activated CD4+CD46+ Tr1 cells in patients with LN (p < 0.05) (Fig. 5b).
a Expression of homing chemokine receptors on CD3/CD46-induced CD4+ T cells in LN patients and healthy controls were tested by flow cytometry. Experiments were performed for 20 paired samples. b ivMP pulse therapy increased CCL17-mediated migration of CD3/CD46-activated CD4+ T cells in 20 paired LN subjects. c AKT phosphorylation and d cAMP-responsive element-binding protein (CREB) in CD46-activated CD4+ T cells in the presence of CCL17 was examined. Increased phosphorylation of AKT and CREB in CD3/CD46-activated CD4+ T cells after ivMP pulse therapy was noted in six LN patients (#p < 0.05 compared to controls and *p < 0.05 compared after ivMP administration).
Increased phosphorylation of AKT and cAMP-responsive element-binding protein (CREB) in CD3/CD46-induced CD4+ Tr1 cells after ivMP therapy in patients with LN
The AKT and CREB signaling pathways might be responsible for the functional defects involving chemotactic activity in peripheral CD46-induced Tr1 cells in patients with LN. In the presence of CCL17, the phosphorylation of AKT (Fig. 5c) and CREB (Fig. 5d) in CD3/CD46-activated CD4+ Tr1 cells after ivMP therapy was higher in patients with LN than in healthy controls.
Discussion
Several abnormalities in B and T cell compartments have been reported in individuals with active LN, and these include uncontrolled autoantibody production, presence of self-antigens that induce immune complex nephritis, hyperactive secretion of Th1 pro-inflammatory cytokines by the effector T cells, and defects in the numbers and/or function of natural Treg.2,4 The downregulation of the effector T cell response via the development of IL-10-producing Tr1 cells might represent a novel role in the decreased immune response.30 The current study showed that a major defect in human active LN is associated with the CD46 costimulation pathway in CD4+ Tr1 cells. Both the Cyt1 and Cyt2 cytoplasmic domains of CD46 contain signaling motifs in CD4+ T cells: CD46-Cyt1 inhibits inflammatory reactions, whereas CD46-Cyt2 increases inflammation.15,24,31,32,33,34 We observed that patients with class III or IV LN have impaired IFN-g to IL-10 switching in CD3/CD46-costimulated Tr1 cells, accompanied by an increased expression of the Cyt2 isoform. ivMP therapy improved clinical symptoms, including proteinuria, and restored CD3/CD46-mediated Tr1 cells with IL-10 production correlated to Cyt1 expression. Our study first showed an altered pattern of expression in these CD46 isoforms and the development of remission in patients with active LN after ivMP treatment.
The complement regulatory factor CD46 appears in renal tissues; its chief function is to protect autologous cells from complement attack via C3 inactivation, thereby protecting kidney cells from an unwanted deposition of activated complement fragments.30 SLE patients with lymphopenia and neutropenia have a decreased expression of CD46, which is correlated to disease activity and/or the activation of the complement system.35,36 Moreover, studies have shown the role of complement regulatory proteins in glomerular injury in individuals with lupus. In particular, abnormalities involving CD46 may lead to the development of complement-mediated thrombotic microangiopathy, with progressive life-threatening thrombocytopenia, microangiopathic hemolytic anemia, and progressive renal failure, in LN, similar to the atypical hemolytic uremic syndrome.37 In accordance with the abovementioned findings, our study revealed a significantly lower percentage of CD4+CD46+ cells in the blood and kidney tissues in patients with active class III or IV LN than in healthy controls.
The specific expression of chemokine receptors in Treg cells may allow their migration toward inflammatory sites, leading to the suppression of responding T cells and defective chemotactic activity of Tregs at the sites of inflammation. Moreover, it may be involved in the pathogenesis of SLE.10,38 The decreased CCR4 expression in CD4+CD25+ Treg cells has been observed in patients with active lupus,11 and the inappropriate distribution of CCR4+CD4+ T cells may be involved in the pathogenesis of LN.10,39 In T cells, the activation of CD46 induces the activation of numerous adhesion molecules, such as CCR4 and CCR7, and CCL17 is involved in Tr1 cell migration.19,40 In previous studies, decreased CCR4 expression in CD3/CD46-costimulated Tr1 cells was observed in patients with allergic asthma.19,41 This study showed that decreased CCR4 and CCR7 expressions in CD3/CD46-costimulated Tr1 cells might be important in the recruitment of Tr1 cells, which inhibits inflammation, in patients with LN.
Treg cells can be upregulated with the use of appropriate doses of IL-2 and dexamethasone by activating the AKT signaling pathway, thereby inhibiting airway inflammation.19,42 Indeed, AKT phosphorylation is involved in CCL17 signaling in CD3/CD46-activated CD4+ Tr1 cells. Our study first presented the functional defects involving chemotactic activity in peripheral CD46-induced Tr1 cells in patients with LN via the AKT and CREB signaling pathways. This functional dysregulation of CD4-mediated Tr1 cells might be rescued by ivMP therapy.
Female children and adolescents develop LN more commonly. All patients in our cohort were first diagnosed with lupus under 10 years old who received hydroxychloroquine without immunosuppressive drugs because of no obvious proteinuria. The mean age of active LN patients at the study entry was 15.6 years old possibly due to the hormonal changes of puberty. Although there are some limitations about how Tr1 cells are isolated and adaptive therapy in clinical practice, further study was warranted to investigate the mechanism of Tr1 cells differentiation during the natural history of lupus development.
In summary, the number of CD3/CD46-mediated Tr1s decreased and was dysregulated in patients with active LN. ivMP therapy targeting CD46-mediated Tr1 cells and inhibiting inflammatory activity could induce remission. Blockade CD46 shedding via matrix metalloproteinases (MMP-9) during T cell activation led to restoring the impaired CD46-mediated IL-10 switching and contraction of Th1 cells in lupus patients.30 Thus, pharmacologic interventions that alter the patterns of CD46-Cyt1/Cyt2 expression by inducing IL-10 secretion by CD3/CD46-mediated Tr1 cells could be effective in promoting tolerance and inhibiting inflammation in patients with LN.
References
Cope, A., Le Friec, G., Cardone, J. & Kemper, C. The Th1 life cycle: molecular control of IFN- g to IL-10 swiching. Trends Immunol. 32, 278–286 (2011).
Ohl, K. & Tenbrock, K. Inflammatory cytokines in systemic lupus erythematosus. J. Biomed. Biotechnol. 2011, 432595 (2011).
Abeler-Dörner, L. et al. Interferon-α abrogates the suppressive effect of apoptotic cells on dendritic cells in an in vitro model of systemic lupus erythematosus pathogenesis. J. Rheumatol. 40, 1683–1696 (2013).
Yan, B. et al. Dysfunctional CD4+CD25+ regulatory T cells in untreated active systemic lupus erythematosus secondary to interferon-alpha-producing antigen-presenting cells. Arthritis Rheum. 58, 801–812 (2008).
Trouw, L. A., Pickering, M. C. & Blom, A. M. The complement system as a potential therapeutic target in rheumatic disease. Nat. Rev. Rheumatol. 13, 538–547 (2017).
Alvarado-Sanchez, B. et al. Regulatory T cells in patients with systemic lupus erythematosus. J. Autoimmun. 27, 110–118 (2006).
Kuhn, A., Beissert, S. & Krammer, P. H. CD4(+)CD25 (+) regulatory T cells in human lupus erythematosus. Arch. Dermatol Res. 301, 71–81 (2009).
Saraiva, M. & Garra, O. A. The regulation of IL-10 production by immune cells. Net. Rev. Immunol. 10, 170–181 (2010).
Horwitz, D. A. Identity of mysterious CD4+CD25-Foxp3+ cells in SLE. Arthritis Res Ther. 12, 101 (2010).
Bonelli, M., Smolen, J. S. & Scheinecker, C. Treg and lupus. Ann. Rheum. Dis. 69, i65–i66 (2010).
Lee, H. Y., Hong, Y. K., Yun, H. J., Kim, Y. M. & Yoo, W. H. Altered frequency and migration capacity of CD4+CD25+ regulatory T cells in systemic lupus erythematosus. Rheumatology 47, 789–794 (2008).
Sawla, P., Hossain, A., Hahn, B. H. & Singh, R. P. Regulatory T cells in systemic lupus erythematosus (SLE); role of peptide tolerance. Autoimmun. Rev. 11, 611–614 (2012).
Groux, H. et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature 389, 737–742 (1997).
Roncarolo, M. G., Gregori, S., Bacchetta, R., Battaglia, M. & Gagliani, N. The biology of T regulatory type 1 cells and their therapeutic application in immune-mediated diseases. Immunity 49, 1004–1019 (2018).
Cardone, J. et al. Complement regulator CD46 temporally regulates cytokine production by conventional and unconventional T cells. Nat. Immunol. 11, 862–871 (2010).
Le Friec, G. et al. The CD46-Jagged1 interaction is critical for human TH1 immunity. Nat. Immunol. 13, 1213–1221 (2012).
Kolev, M. et al. Complement regulates nutrient influx and metabolic reprogramming during Th1 cell responses. Immunity 42, 1033–1047 (2015).
Astier, A. L. T-cell regulation by CD46 and its relevance in multiple sclerosis. Immunology 124, 149–154 (2008).
Tsai, Y. G. et al. Functional defects of CD46-induced regulatory T cells to suppress airway inflammation in mite allergic asthma. Lab Invest. 92, 1260–1269 (2012).
Kemper, C. et al. Activation of human CD4+ cells with CD3 and CD46 induces a T-regulatory cell 1 phenotype. Nature 421, 388–392 (2003).
Tsai, Y. G. et al. Enhanced CD46-induced regulatory T cells suppress allergic inflammation after Dermatophagoides pteronyssinus-specific immunotherapy. J. Allergy Clin. Immunol. 134, 1206–1209 (2014).
Alegretti, A. P. et al. Diminished expression of complement regulatory proteins on peripheral blood cells from systemic lupus erythematosus patients. Clin. Dev. Immunol. 2012, 725684 (2012).
Jonsen, A. et al. Mutations in genes encoding complement inhibitors CD46 and CFH affect the age at nephritis onset in patients with systemic lupus erythematosus. Arthritis Res. Ther. 13, R206 (2011).
Marie, J. C. et al. Linking innate and acquired immunity: divergent role of CD46 induces a T-regulatory cell 1 phenotype. Nature 3, 659–666 (2005).
Suárez, A., López, p, Gómez, J. & Gutiérrez, C. Enrichment of CD4+CD25high T cell population in patients with systemic lupus erythematosus treated with glucocorticoids. Ann. Rheum. Dis. 65, 1512–1517 (2006).
Cepika, A. M., Marinic, i, Morovic-vergles, J., Soldo-Juresa, D. & Gagro, A. Effect of steroids on the frequency of regulatory T cells and expression of FOXP3 in a patient with systemic lupus erythematosus: a two-year follow-up. Lupus 16, 374–377 (2007).
Brunner, H. I., Feldman, B. M., Bombardier, C. & Silverman, E. D. Sensitivity of the systemic lupus erythematosus disease activity index, British Isles Lupus Assessment Group Index, and systemic lupus activity measure in the evaluation of clinical change in childhood-onset systemic lupus erythematosus. Arthritis Rheum. 42, 1354–1360 (1999).
Gladman, D. D., Ibanez, D. & Urowitz, M. B. Systemic lupus erythematosus disease activity index 2000. J. Rheumatol. 29, 288–291 (2002).
Tsai, Y. G., Lee, C. Y., Lin, T. Y. & Lin, C. Y. CD8+ Treg cells associated with decreasing disease activity after intravenous methylprednisolone pulse therapy in lupus nephritis with heavy proteinuria. PLoS ONE 9, e81344 (2014).
Ellinghaus, U. et al. Dysregulated CD46 shedding inferes with Th1-contraction in systemic lupus erythematosus. Eur. J. Immunol. 47, 1200–1210 (2017).
Liszewski, M. K. et al. Intracellular complement activation sustains T cell homeostasis and mediates effector differentation. Immunity 39, 1143–1157 (2013).
Lee, S. W. et al. CD46 is phosphorylated at tyrosine 354 upon infection of epithelial cells by Neisseria gonorrboeae. J. Cell Biol. 156, 951–957 (2002).
Wang, G., Liszewski, M., Chan, A. & Atkinson, J. P. Membrane cofactor protein (MCP; CD46): isoform-specific tyrosine phosphorylation. J. Immunol. 164, 1839–1846 (2000).
Ni Choileain, S. et al. The dynamic processing of CD46 intracellular domains provides a molecular rheostat for T cell activation. PLoS ONE 6, e16287 (2011).
Nangaku, M. Complement regulatory proteins in glomerular diseases. Kidney Int. 54, 1419–1428 (1998).
Tseng, M. H. et al. Serum complement factor I is associated with disease activity of systemic lupus erythematosus. Oncotarget 9, 8502–8511 (2018).
Park, M. H., Caselman, N., Ulmer, S. & Weitz, I. C. Complement-mediated thrombotic microangiopathy associated with lupus nephritis. Blood Adv. 2, 2090–2094 (2018).
Lopez, P., Gomez, J., Prado, C., Gutierrez, C. & Suarez, A. Influence of functional interleukin 10/tumor necrosis factor-alpha polymorphisms on interferon-alpha, IL-10, and regulatory T cell population in patients with systemic lupus erythematosus receiving antimalarial treatment. J. Rheumatol. 35, 1559–1566 (2008).
Yamada, M. et al. Selective accumulation of CCR4+ T lymphocytes into renal tissue of patients with lupus nephritis. Arthritis Rheum. 46, 735–740 (2002).
Alford, S. K., Longmore, G. D., Stenson, W. F. & Kemper, C. CD46-induced immunomodulatory CD4+ T cells express the adhesion molecule and chemokine receptor pattern of intestinal T cells. J. Immunol. 181, 2544–2555 (2008).
Lin, C. Y. & Tsai, Y. G. Complements and allergic asthma. Pediatr. Respirol. Crit. Care Med 3, 3–7 (2019).
Meiffren, G., Flacher, M., Azocar, O., Rabourdin-Combe, C. & Faure, M. Cutting edge: abortive proliferation of CD46-induced Tr1-like cells due to a defective Akt/Survivin signaling pathway. J. Immunol. 177, 4957–4961 (2006).
Funding
This work was partially supported by grants from the Ministry of Science and Technology, Taiwan (MOST 106-2314-B-371-008 and MOST 107-2314-B-371-011-MY2), from Changhua Christian Hospital, Taiwan (110-CCH-IRP-030, 110-CCH-ICO-152, 106-CCH-IRP-079, 108-CCH-IRP-051; (Y_105_0246) (Y_105_0023) (Y_105_0050)), and from China Medical University Hospital, Taiwan (DMR-100-180), and Academia Sinica, Taiwan (AS-SS-111-02-1).
Author information
Authors and Affiliations
Contributions
Y.-G.T., J.-W.C., K.-H.H., and C.-Y.L.: conceptualized and designed the study, performed the experiments, drafted the initial manuscript, and approved the final manuscript as submitted. Y.-M.C., T.-C.S., and P.-F.C.: carried out the initial analyses, reviewed and revised the manuscript, and approved the final manuscript as submitted.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent statement
Written informed consent was obtained from each participant and their guardian.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Tsai, YG., Chien, JW., Chiu, YM. et al. Lupus nephritis with corticosteroid responsiveness: molecular changes of CD46-mediated type 1 regulatory T cells. Pediatr Res 92, 1099–1107 (2022). https://doi.org/10.1038/s41390-021-01882-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-021-01882-z