Abstract
Cardiovascular disturbances are a frequent occurrence in neonatal sepsis. Preterm and term infants are particularly vulnerable due to the unique features of their cardiovascular function and reserve, compared to older children and adults. The clinical manifestations of neonatal sepsis are a product of the variable inflammatory pathways involved (warm vs. cold shock physiology), developmental state of the cardiovascular system, and hormonal responses. Targeted neonatal echocardiography has played an important role in advancing our knowledge, may help delineate specific hemodynamic phenotypes in real-time, and supports an individualized physiology-based management of sepsis-associated cardiovascular dysfunction.
Impact
Cardiovascular dysfunction is a common sequela of sepsis. This review aims to highlight the pathophysiological mechanisms involved in hemodynamic disturbance in neonatal sepsis, provide insights from targeted neonatal echocardiography-based clinical studies, and suggest its potential incorporation in day-to-day management.
Similar content being viewed by others
Sepsis is one of the most commonly encountered pathologies in the neonatal intensive care unit (NICU) and is associated with significant morbidity and mortality.1 Many infants with sepsis develop cardiovascular instability; preterm infants are particularly vulnerable due to the unique features of their cardiovascular function and reserve. The aim of this review is to highlight the pathophysiological mechanisms involved in a hemodynamic disturbance in neonatal sepsis, provide insights gained from targeted neonatal echocardiography (TNE)-based clinical studies, and suggest its potential incorporation in day-to-day management.
Cardiovascular function in sepsis
Characterization of sepsis-related cardiovascular dysfunction has been traditionally based on the clinical patterns identified by bedside physical examination, typically dichotomized as warm and cold shock physiology.2 Warm shock is defined by significant vasodilation leading to low systemic vascular resistance (SVR), producing hypotension, brisk capillary refill time (CRT), bounding peripheral pulses, and flushed skin. Conversely, cold shock is a vasoconstrictive state, causing cold or pale extremities, delayed CRT, and weak peripheral pulses. Initially, blood pressure (BP) may be falsely reassuring as it remains elevated despite inadequate cardiac output due to marked peripheral vasoconstriction.3 Hypotension is often a late finding in cold shock and is a consequence of the significant reduction of left ventricular systolic performance from prolonged exposure to high afterload.
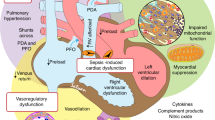
Changes in SVR associated with sepsis can have significant implications on cardiac mechanics. Peripheral vasodilation may result in lower systemic venous return and decreased right-heart preload. Tachycardia may compensate to maintain cardiac output, although neither adequately nor indefinitely. On TNE, although this generates a hyperdynamic cardiac profile, this partially compensated vasodilatory state can ultimately result in below normal range ventricular outputs, particularly in preterm neonates. Further, diminished venous return and left-heart preload may reduce left ventricular systolic performance via Frank–Starling mechanisms,4 potentially exacerbating the direct adverse impact of inflammation on myocardial contractility.5 These insults on systolic function elevate left ventricular end-diastolic pressure, further compromising its filling and may even negatively impact the right ventricle through ventricular interdependence.6 Right ventricular filling and function may also be directly compromised in the setting of elevated pulmonary vascular resistance (PVR) states that are not infrequently encountered in neonatal sepsis, such as acute pulmonary hypertension7 and hypoxic pulmonary vasoconstriction8 (a ventilation–perfusion sparing arteriolar response to diminished alveolar oxygen content).
Likewise, elevated SVR associated with cold shock physiology can have major adverse effects on the myocardium. Neonatal heart, even more so for preterm neonates, is known to be highly intolerant to acute increases in afterload,9 which can result in left ventricular systolic dysfunction, low stroke volume, and a subsequent increase in both end-systolic and end-diastolic pressures, compromising filling. Initially, the left ventricle may adapt, at least partially, in order to maintain its stroke volume by increasing inherent contractile force, tachycardia, and dilatation. However, both the contractile reserve and the ability to increase stroke volume by dilatation are limited in preterm heart,10 making these patients more vulnerable to ventricular dysfunction, low stroke volume, and, consequently, worsening hypotension and shock.
Hence, the unique clinical phenotype in neonatal sepsis is the culmination of the type of shock physiology and may evolve over the course of an illness. Understanding the specific underlying mechanisms at play is key to tailoring therapeutic interventions and providing individualized management, necessitating frequent re-evaluation and readjustment of management strategies to the changing clinical picture.
Challenges in clinical assessment and limitations of BP measurements
Although the dichotomous presentation has been useful for the establishment of clinical practice guidelines, distinguishing between “warm shock” and “cold shock” based on clinical assessment alone may be prone to error.11 Central CRT has been inconsistently shown to correlate with systemic blood flow,12,13,14 while peripheral CRT is not a reliable index for assessing the hemodynamic status of neonates.15,16,17 BP measurements, although often the primary criteria used by clinicians to diagnose shock and initiate treatments, can be particularly difficult in neonates. This relates to both the lack of established technical standards and known unreliability of selecting appropriate cuff sizes,18 as well as the lack of research to inform robust interpretation. Although a number of studies have reported on normative values19,20,21 (albeit mostly using data from older cohorts during the initial days after birth, prior to widespread integration of strategies such as delayed cord clamping and antenatal corticosteroids), data regarding specific thresholds to define “hypotension” beyond the transitional period associated with adverse clinical outcomes, or in acute conditions such as sepsis remain limited.22 Further, noninvasive BP recordings are known to be higher in preterm neonates compared to invasive readings.23,24 In practice, mean BP below corrected gestational age, referred to as the British Association of Perinatal Medicine rule,25 is often used by clinicians to guide interventions, presumably driven by lack of other data and ease of use. However, this consensus-driven rule was suggested in the context of transitional hypotension in extremely preterm neonates, and its extrapolation to acute complications later in the postnatal course may be ill-informed and cannot be recommended. Given these challenges, pending further research, a more thoughtful and thorough appraisal of the circulatory system is warranted to find the balance between avoiding both unnecessary and indiscriminate use of drugs and delaying treatment for neonates in circulatory compromise. One potential interim clinical strategy may be to compare BP recordings during the sepsis episode to the patient’s own pre-illness baseline values and corroborate changes with any evidence of end-organ dysfunction, in order to detect deviations that may be clinically relevant for individual patients.
Mechanisms of cardiovascular dysfunction
Inflammation
Inflammatory pathways activated in sepsis may directly impact the vascular tone and cardiac function, and play a critical role in defining the clinical hemodynamic presentation. The host response to sepsis induces immune system activation and an endothelial response. In its native state, the endothelium and its components have multiple roles including barrier function and vasomotor regulation.26 The release of inflammatory mediators in sepsis can compromise endothelial wall integrity through the destruction of gap junctions;26 subsequent endothelial leakiness causes fluid shifts to extravascular spaces, lowering circulating blood volume, and subsequently cardiac preload. Inflammation can also compromise endothelial glycocalyx, releasing nitric oxide (NO) and endothelin, two predominant mediators of vascular tone, resulting in alterations in blood flow to organs.27 In adults with sepsis, NO in its inducible form plays a role in arteriolar vasodilatation and microvascular dysfunction,28,29,30 and activation of NO signaling pathways has been found to decrease cardiac myocyte responsiveness to beta-adrenergic agonists,31 modulate pulmonary vascular tone,32 and cause vascular hyporeactivity.33 Elevated levels of endothelin, a potent vasoconstrictor, have been correlated with illness severity and myocardial dysfunction in sepsis.34,35,36 Among neonates, bacteremic infants have also been found to have higher levels of both NO and endothelin compared to noninfected patients,37,38 indicating a potentially similar pathophysiological role. The relative expression of these mediators may dictate the vasoactive phenotype and its severity in patients with sepsis. Further, when pro-inflammatory pathways in sepsis are not appropriately counteracted by anti-inflammatory mechanisms, dysregulated inflammation or systemic inflammatory response syndrome (SIRS) may occur, causing additional compromise to microcirculatory perfusion and oxygen delivery.39,40 Although neonates generate a relatively lower amount of inflammatory cytokines compared to adults,41 SIRS may complicate the clinical course for many patients. In addition, inflammation can directly impact the heart. In animal models of sepsis, various cytokines have been shown to depress cardiac contractility,42 alter coronary arterial autoregulation,43 and trigger excessive interstitial infiltration of inflammatory mediators in the myocardium.44 While specific neonatal studies are lacking, sepsis-induced myocardial dysfunction, and cardiomyopathy, directly mediated by inflammatory mediators, are well-established.
Hormonal influences
In addition to immune activation, sepsis also triggers neuroendocrine responses, leading to the release of catecholamines and the recruitment of additional hormonal pathways. Vasopressin and cortisol are the most studied adjunctive hormones that are thought to play important hemodynamic roles in sepsis. Although high-quality studies examining the use of exogenous catecholamines, vasopressin, and corticosteroids in neonatal sepsis are lacking, these agents are frequently used in the clinical setting for hypotension in sepsis,45,46,47 presumably driven by pathophysiological considerations and extrapolation of evidence from adult critical care.
Catecholamines
The release of endogenous catecholamines is induced by bacteria or bacterial products such as endotoxins, in part due to stimulation of pro-inflammatory pathways.48 In turn, catecholamines exert anti-inflammatory effects by inhibiting the production of pro-inflammatory cytokines and augmenting the release of anti-inflammatory cytokines. The catecholamine effect is predominantly beta-adrenergic, although some alpha-adrenergic activation can be seen.49
Vasopressin
Vasopressin or antidiuretic hormone, responsible for regulating circulating volume and osmolality under physiological conditions, appears to play an important role in severe sepsis and catecholamine-unresponsive sepsis-induced hypotension among older patients.50 It acts via V1a receptors to induce vascular smooth muscle vasoconstriction, providing a catecholamine receptor-independent pathway to modulate vasodilation.51 In sepsis, circulating vasopressin levels are described to be abnormally low,52 and its stores deplete quicker in shock due to sepsis vs. non-septic etiologies such as cardiogenic shock.53,54 While there are no large trials, in adult critical care, vasopressin is being increasingly used as an adjunctive therapy for catecholamine-unresponsive hypotension from sepsis.55
Cortisol
Cortisol plays many roles in maintaining cardiovascular stability, including regulating transmembrane calcium homeostasis, augmenting beta-receptor frequency and limiting their degradation, and increasing adrenergic receptor sensitivity to circulating catecholamines.56 Cortisol is critical to maintaining homeostasis especially in response to physiologic stress; preterm infants, however, are thought to have relative adrenal insufficiency related to an immature hypothalamic–pituitary axis as well as a limited ability of the adrenal gland for de novo cortisol synthesis.57 Preterm infants needing vasopressor therapies demonstrate lower basal and stimulated cortisol levels,58,59 which may be due to low conversion of cortisol precursors.60 In addition, glucocorticoids are thought to have an essential function in maintaining vascular wall integrity, which may have a role in regulating sepsis-related endothelial destruction and related capillary leak.61
Extracardiac physiologic contributions to hemodynamic instability
Renal
Adequate renal function is critical to managing shifts in fluid balance that can occur during sepsis from factors such as volume resuscitation, cardiac pump function, and “third spacing” due to loss of endothelial integrity. In sepsis, renal injury can occur through both altered perfusion and direct tubular injury. Studies in adults have demonstrated that despite a hyperdynamic state with globally increased cardiac output, sepsis is often associated with loss of renal autoregulation.62 Microcirculatory changes within the renal cortex or medulla can also occur in sepsis, independent of normal or even increased renal blood flow,63 largely stemming from the inflammation-mediated local release of excessive NO.26 Renal damage through tubular injury is thought to be related to pro-inflammatory cytokines filtered through the glomerulus, directly impacting the proximal tubules.64,65 Given the challenges and often lack of feasibility of renal replacement therapy in neonatal patients, clinicians must remain vigilant in anticipating the potential consequences of associated renal failure and avoid, as much as possible, fluid overload from overzealous use of volume resuscitation. Mortality in neonates who develop renal failure in sepsis has recently been noted to be higher than in those without renal failure,66 although exact mechanisms and therapeutic targets remain to be elucidated.
Respiratory
Respiratory failure is a common manifestation of neonatal sepsis and may originate from either ventilation–perfusion mismatch from acute pulmonary hypertension, or impaired oxygen transport at the alveolar or interstitial level, referred to its most severe form as acute respiratory distress syndrome (ARDS). The distinction is crucial to optimizing management; strategies to lower PVR are the mainstay for an acute pulmonary hypertension crisis, while for ARDS, typical strategies include an exogenous surfactant, prone positioning, and trial of conventional ventilation.67 Sepsis-related acute pulmonary hypertension is thought to result from the excessive production of vasoconstrictor mediators such as endothelins.68,69 Early use of echocardiography for neonates with sepsis-associated hypoxic respiratory failure to assess the presence of pulmonary hypertension may aid timely diagnosis and appropriate titration of therapies. Pathophysiologically, ARDS results from pro-inflammatory plasma proteins leaking in the alveolar space causing damage to the surfactant biofilm. Lysis of the surfactant phospholipid releases free fatty acids, triggering the release of more inflammatory mediators.70 In adult critical care, ARDS is well studied with established diagnostic criteria, manifesting as significant respiratory failure with new hazy infiltrates on chest x-ray.71 In neonatal medicine, however, ARDS is generally underappreciated and likely underdiagnosed, perhaps because of a lack of established diagnostic criteria, difficulty in clinically distinguishing from acute pulmonary hypertension, and a decreased awareness among neonatal clinicians of ARDS as a potential etiology of severe hypoxic respiratory failure. Recently, an international group published a consensus-based operational definition for neonatal ARDS,71 although its clinical validity, utilization, and impact need further evaluation. It is essential that clinicians looking after neonates with sepsis maintain a high index of suspicion for ARDS.
Additional cardiovascular considerations in premature neonates with sepsis
In addition to the organ-specific physiological factors highlighted above, there are developmentally regulated cardiovascular structural and functional considerations that may govern the overall hemodynamic phenotype and ability to accommodate altered physiologic states in premature neonates (Fig. 1). Structurally, the immature heart has less lower mass, fewer and less organized myofibrils, fewer mitochondria (amounting to lower inherent inotropy and contractile reserve), fewer L-type calcium channels, and shallower T-tubules, resulting in decreased ability to facilitate the release of calcium from sarcoplasmic stores (amounting to lower ability to generate contractile force and active relaxation), higher overall collagen content as well as a higher ratio of collagen rigidity-increasing type I to elasticity-increasing type III (amounting to lower compliance and higher stiffness), and less adrenergic innervation and adrenoreceptor density.72,73,74,75,76 Functionally, these anatomic differences translate to lower functional reserve in response to altered loading conditions and stresses,77 lower diastolic performance, less ability to increase stroke volume in response to increases in preload, and a greater tendency for systolic dysfunction and lower stroke volume in face of acute increases in afterload.78,79,80
While individual variations may occur, the predominant physiological response to sepsis in the vascular system also appears to be developmentally regulated. In adult patients, the predominant physiology is known to be that of warm shock,81 whereas in children, sepsis tends to produce primarily cold shock physiology.82,83 Physiological studies in preterm infants, similar to adults, have demonstrated warm shock physiology to be the predominant phenotype.39,84 This is postulated to be due to an impaired ability to regulate vascular tone during shock,39 in part driven by an inherent imbalance of the autonomic nervous system characterized by a relatively higher parasympathetic drive.85 Relative adrenal insufficiency, by decreasing SVR, may also be a contributing factor in preterm infants.86 Further, preterm neonates have a baseline elevated PVR by virtue of a lower capacity vascular bed, which, in combination with pre-existing lung disease often seen in these patients, may place them at higher risk of pulmonary vascular complications such as acute pulmonary hypertension and pulmonary edema.87
TNE in neonatal sepsis
TNE or neonatologist-performed echocardiography refers to the bedside use of functional echocardiography by neonatologists extensively trained in cardiovascular physiology and hemodynamic assessment of critically sick neonates.88 Clinical integration of the comprehensive hemodynamic data obtained through TNE allows the definition of underlying cardiovascular physiology in real-time, titration of therapeutic interventions and dose-targeting, and sequential assessments to monitor disease progression and treatment response. Recently, a large number of NICUs across the globe have adopted an in-house TNE program, allowing for its increasing incorporation in day-to-day assessment and management of hemodynamic complications.89 The widespread availability of appropriate equipment and development of local expertise have also opened the doors for hemodynamic researchers to undertake physiological explorations in this vulnerable and high-risk patient population.
Although large definitive TNE-based studies are still needed, over the past 10 years, several physiological studies have been published providing important clues to help advance our understanding of hemodynamic complications of neonatal sepsis.90,91,92,93,94,95,96,97,98,99,100,101 The salient features of these TNE-based studies are summarized in Table 1. In summary, these studies have consistently demonstrated that the predominant phenotype associated with sepsis in neonates is of warm shock physiology, characterized by lower venous return (higher inferior vena cava collapsibility), lower calculated SVR, and higher cardiac outputs compared to controls or published normative values.90,93,97,100,101 Septic patients also consistently demonstrate inferior diastolic and global cardiac performance (myocardial performance index or Tei index) for both the right and left ventricle.91,92,95,96,98 The findings in relation to systolic performance are more equivocal. One study reported mildly lower conventional markers of systolic function (ejection fraction, fractional shortening) during sepsis vs. controls, albeit still within normal range;96 another found no difference.93 Systolic function using tissue Doppler velocities in one study found lower velocities in sepsis;95 however, this marker is highly load-dependent and lower velocity may be a reflection of lower preload instead of contractility. Myocardial deformation parameters such as strain and strain rate have not been assessed during sepsis in neonates. Similarly, while acute pulmonary hypertension has been reported as a complication of neonatal sepsis,98,99,102,103 its systematic evaluation using echocardiography outside of the transitional period and the impact of pulmonary vasodilator therapies have not been described. The few studies reporting on pulmonary arterial pressures have found a significant but mild increase in TNE-measured pulmonary arterial pressures during sepsis compared to controls; however, the actual pressures were still too low to be clinically relevant.95,98,99 Echocardiographic findings in patients presenting with hypoxic respiratory failure in sepsis are not described. Few small studies have also examined the prognostic significance of TNE-derived variables. Although these data at present are weak and not definitive, they suggest that some TNE variables may have prognostic value for clinical outcomes. Sepsis survivors were found to have a relatively lower myocardial performance index (better global performance) and slightly higher left ventricular fractional shortening (systolic function).91,92,98 Ultimately, systematic, prospective, and adequately powered investigations are needed to establish the prognostic value of TNE.
Management of hemodynamic dysfunction in neonates with sepsis and role for TNE
General principles and monitoring
Early and adequate hemodynamic stabilization is a key component of critical care in sepsis. Prompt identification of patients with progression of sepsis severity may be accomplished through frequent clinical assessments and monitoring of changes to physiologic indices (heart rate, BP, urine output, CRT, and neurologic status) and biochemical variables (blood gas, lactate, electrolytes, and creatinine). Continuous physiologic monitoring of invasively acquired resuscitative endpoints such as central venous oxygen saturation is typically not feasible in neonates; clinicians are therefore reliant on serial bedside assessments and intermittent blood sampling to monitor disease progression and response to therapeutic interventions. Clues to the underlying initial hemodynamic physiology may be derived from the type of hypotension exhibited. Diastolic BP is more reflective of SVR;104 therefore, hypotension driven mainly by lower diastolic BP may indicate a vasodilatory state. On the other hand, an exclusively lower systolic BP may be more indicative of low left ventricular output.105 In many cases, both diastolic and systolic BPs may be low. Careful appraisal of the BP component that decreased first may prove to be helpful. In our experience, particularly in preterm neonates with warm shock, hypotension often starts as low diastolic BP and progresses to both low diastolic and systolic BP. These cases may represent initial cardiac compensation to maintain stroke volume (normal initial systolic BP), which, after a variable time-period, fails to keep up with ongoing decreases in preload, resulting in a drop in cardiac output and ultimately lowering of both BP components. The primary physiology in these cases is of low SVR.
Although BP is the most commonly used bedside hemodynamic variable in this population, it alone may not adequately reflect organ perfusion and must be interpreted in the context of other markers of organ function obtained through physical examination and laboratory investigations. Invasive BP monitoring is frequently not feasible in preterm infants due to technical challenges. Regular cycling of noninvasive BP is often the only option, although it may overestimate BP.23,24 Near-infrared spectroscopy (NIRS) and continuous cardiac output monitoring are promising modalities that may provide valuable data on tissue perfusion and changes in pump function; however, their routine use at present is limited by resource availability and lack of operational thresholds to direct interventions. At this time, evaluation of physiologic changes in sepsis remains primarily through serial patient assessments. Clinicians must stay vigilant and practice a high index of suspicion recognizing the limitations and nonspecific nature of physical exam findings in this patient population, which may miss key nuances in identifying underlying cardiovascular physiology.
Choice of vasoactive medications
When hypotension or deceased organ perfusion is noted, strategies to improve systemic blood flow must be considered. Initial resuscitation is typically through fluid administration, although this must be done judiciously, given preterm infants’ decreased ability to adjust to sudden changes in loading conditions. If low organ perfusion persists, vasoactive medications are initiated. Given the predominant phenotype of warm shock physiology, vasopressors are usually started first-line; although no strong evidence currently exists to guide primary drug choice among infants. Dopamine is the most commonly used vasopressor in the NICU and has been shown to be safe and effective in increasing BP during transitional hypotension;106 however, its use has not been studied rigorously in late-onset sepsis. Among adults, recent meta-analyses have shown that dopamine was associated with higher mortality and an increased frequency of arrhythmias compared to norepinephrine.107,108 Clinical practice guidelines for sepsis management in adults, the Surviving Sepsis guidelines, have integrated these findings; norepinephrine is now recommended as the first-line vasopressor for sepsis-induced hypotension in adults (strong recommendation, moderate quality of evidence).109 Norepinephrine use has been shown to increase BP, cardiac output, and regional blood flow,110,111,112 and may also improve outcomes among adult patients with septic shock.113 Similarly, the pediatric iteration of the Surviving Sepsis guidelines recommends norepinephrine, rather than dopamine, as a first-line vasopressor (weak recommendation, very low quality of evidence).114 Although this guideline does include neonates, the recommendations are limited to term infants with sepsis in the transitional period. Small cohort studies have found that norepinephrine may have a role in neonatal septic shock.115,116 Dopamine has been compared with epinephrine in one trial of 40 neonates with fluid-refractory septic shock, finding a similar rate of shock reversal.117 In this study, among infants <30 6/7 weeks gestation, hemodynamic stability was more frequently seen in the epinephrine group, which may reflect preterm infants’ postulated diminished ability to convert dopamine to epinephrine, thereby losing the additional benefit of dopamine effect on non-dopaminergic receptors.118 Presumably driven by findings in older populations, many NICUs, including ours, have changed practice to using norepinephrine as the primary vasopressor in sepsis. At present, no firm recommendations can be made for first-line therapy in neonates; early recognition and prompt evaluation of treatment efficacy in an individual patient is perhaps more important than the actual drug choice.
Particularly in catecholamine-unresponsive warm shock, alternate hormonal pathways can be considered to improve hypotension. Although vasopressin is being used increasingly in NICUs for persistent hypotension, there remains little data to guide its use,119,120,121 and it cannot be recommended as primary therapy. However, vasopressin may be considered as a second-line agent in catecholamine-resistant warm shock, to target BP improvement through non-catecholamine receptor pathways. Hydrocortisone has also been found to be effective in treating refractory hypotension in preterm infants and decreasing the need for inotropic support,122,123,124 although data on its use specifically in sepsis-related hypotension are lacking.
Clinical incorporation of TNE in sepsis management
The use of echocardiography to guide hemodynamic management in sepsis has been shown to be associated with improved outcomes in adults;125 neonatal studies are still needed. Ideally, all patients demonstrating hemodynamic compromise from sepsis would have a TNE to delineate underlying pathophysiological derangements and direct individualized therapeutic interventions. However, this has significant resource implications. Round-the-clock availability of TNE may not be feasible in most centers. Identification of the hemodynamic pattern by clinical assessment, as highlighted above, is therefore important for the timely initiation of treatment. While TNE should be obtained as early as feasible in these patients, in our opinion, an urgent evaluation should be obtained if patients demonstrate unanticipated clinical characteristics. These include clinical features suggestive of cold shock physiology (which has cardiovascular implications that may be challenging to appreciate clinically), severe hypoxic respiratory failure (which may be associated with pulmonary hypertension and can be difficult to differentiate from ARDS), and when there is a suspicion of change in underlying physiology (because of unexpected deterioration after initial stabilization or failure of expected response to initial treatments). When performed, TNE clinicians must interpret echocardiography findings in the context of the clinical picture to appropriately identify the pathophysiology and necessary therapeutic interventions. An overview of the key principles of TNE evaluation in neonatal sepsis and associated interpretation is summarized in Table 2.
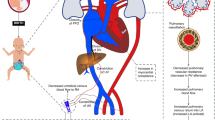
In older populations, the Surviving Sepsis guidelines provide an approach to the identification and management of sepsis-related hemodynamic dysfunction.2,109 High-quality evidence to establish similar guidelines in neonates is still needed; however, at our institution, we developed and implemented a physiology-driven consensus-based algorithm for the management of hemodynamic dysfunction in neonatal sepsis, including the role of TNE (Fig. 2). While we aspire to evaluate the impact of this policy on clinical outcomes in the future, at present this can be considered as our opinion based on physiology and currently available evidence.
Future directions
Much of the current management in neonates with sepsis-related hemodynamic complications is driven by historical practices or extrapolation of evidence from adult studies. The developmental differences in neonates, however, have important implications, necessitating dedicated research. Studies to date have proposed a potential role for TNE, while the utility of other noninvasive modalities such as NIRS and noninvasive cardiac output monitoring remains insufficiently explored. Nonetheless, large-scale studies are still needed to establish the relevance of both static and dynamic hemodynamic variables, as well as to directly compare commonly used therapeutic options. Adequately powered clinical trials would be ideal to answer some of these outstanding questions. However, clinical trials pose significant operational challenges in neonates, highlighted by the difficulties in recruitment encountered by recent investigators despite dedicating a large volume of resources.126 Further, due to their highly controlled nature, traditional trials may not accurately reflect routine clinical practice or variability of practices across units. This highlights the need for alternative strategies to develop evidence in this patient population. Potential solutions that may be explored include establishing multicenter prospective data registries to collect highly granular data including TNE variables, innovative research methods such as comparative effectiveness research, and predictive machine learning.
Conclusions
Cardiovascular dysfunction is a common sequala sequela to sepsis. Among neonates, the diverse hemodynamic manifestations are a product of variable inflammatory pathways, cardiac immaturity, and hormonal responses. The classic assessment of warm vs. cold shock provides many clinical clues about the underlying cardiovascular derangements; although these are often thought of as two dichotomous entities, they are mediated by the same complex inflammatory cascade. As such, the clinical picture can evolve during the sepsis course, likely reflecting the relative dominant physiologic pathway at the time of clinical assessment. In the absence of high-quality evidence to support the initiation of therapeutic strategies, management of hemodynamic instability in neonatal sepsis should be done after careful consideration of the underlying pathophysiology and individualized to the patient. The use of TNE may help corroborate clinical hemodynamic variables, add to our existing knowledge regarding mechanisms of action of common therapies used for hemodynamic instability, and support our understanding of short- and long-term outcomes in infants with abnormal cardiovascular health states.
References
Shane, A. L., Sánchez, P. J. & Stoll, B. J. Neonatal sepsis. Lancet 390, 1770–1780 (2017).
Weiss, S. L. et al. Surviving Sepsis Campaign International Guidelines for the Management of Septic Shock and Sepsis-Associated Organ Dysfunction in Children. Intens. Care Med. 46, 10–67 (2020).
Ceneviva, G., Paschall, J. A., Maffei, F. & Carcillo, J. A. Hemodynamic support in fluid-refractory pediatric septic shock. Pediatrics 102, e19 (1998).
Peverill, R. E. Understanding preload and preload reserve within the conceptual framework of a limited range of possible left ventricular end-diastolic volumes. Adv. Physiol. Educ. 44, 414–422 (2020).
Drosatos, K. et al. Pathophysiology of sepsis-related cardiac dysfunction: driven by inflammation, energy mismanagement, or both? Curr. Heart Fail. Rep. 12, 130–140 (2015).
Greer, J. Pathophysiology of cardiovascular dysfunction in sepsis. BJA Educ. 15, 316–321 (2015).
Giesinger, R. E. et al. Controversies in the identification and management of acute pulmonary hypertension in preterm neonates. Pediatr. Res. 82, 901–914 (2017).
Sorensen, G. K., & Redding, G. J. (1984, October). Cardiopulmonary effects of sepsis in the newborn. In Seminars in Respiratory Medicine Vol. 6 141–147. (Thieme Medical Publishers, Inc., 1984).
Noori, S., Friedlich, P., Seri, I. & Wong, P. Changes in myocardial function and hemodynamics after ligation of the ductus arteriosus in preterm infants. J. Pediatr. 150, 597–602 (2007).
Wolf, A. R. & Humphry, A. T. Limitations and vulnerabilities of the neonatal cardiovascular system: considerations for anesthetic management. Pediatr. Anesth. 24, 5–9 (2014).
Tibby, S. M., Hatherill, M., Marsh, M. J. & Murdoch, I. A. Clinicians’ abilities to estimate cardiac index in ventilated children and infants. Arch. Dis. Child. 77, 516–518 (1997).
LeFlore, J. L. & Engle, W. D. Capillary refill time is an unreliable indicator of cardiovascular status in term neonates. Adv. Neonatal Care 5, 147–154 (2005).
Osborn, D., Evans, N. & Kluckow, M. Clinical detection of low upper body blood flow in very premature infants using blood pressure, capillary refill time, and central-peripheral temperature difference. Arch. Dis. Child. Fetal Neonatal Ed. 89, F168–F173 (2004).
Miletin, J., Pichova, K. & Dempsey, E. Bedside detection of low systemic flow in the very low birth weight infant on day 1 of life. Eur. J. Pediatr. 168, 809 (2009).
Strozik, K. S., Pieper, C. H. & Cools, F. Capillary refilling time in newborns-optimal pressing time, sites of testing and normal values. Acta Paediatr. 87, 310–312 (1998).
Strozik, K. S., Pieper, C. H. & Roller, J. Capillary refilling time in newborn babies: normal values. Arch. Dis. Child Fetal Neonatal Ed. 76, F193–F196 (1997).
Raju, N. V., Maisels, M. J., Kring, E. & Schwarz-Warner, L. Capillary refill time in the hands and feet of normal newborn infants. Clin. Pediatr. 38, 139–144 (1999).
Devinck, A., Keukelier, H., De Savoye, I., Desmet, L. & Smets, K. Neonatal blood pressure monitoring: visual assessment is an unreliable method for selecting cuff sizes. Acta Paediatr. 102, 961–964 (2013).
Kent, A. L., Kecskes, Z., Shadbolt, B. & Falk, M. C. Normative blood pressure data in the early neonatal period. Pediatr. Nephrol. 22, 1335–1341 (2007).
Kent, A. L., Meskell, S., Falk, M. C. & Shadbolt, B. Normative blood pressure data in non-ventilated premature neonates from 28–36 weeks gestation. Pediatr. Nephrol. 24, 141–146 (2009).
Pejovic, B., Peco-Antic, A. & Marinkovic-Eric, J. Blood pressure in non-critically ill preterm and full-term neonates. Pediatr. Nephrol. 22, 249–257 (2007).
Baczynski, M. et al. Bloodstream Infections in Preterm Neonates and Mortality-Associated Risk Factors. J. Pediatr. 237, 206–212.e1. https://doi.org/10.1016/j.jpeds.2021.06.031 (2021).
Takci, S., Yigit, S., Korkmaz, A. & Yurdakök, M. Comparison between oscillometric and invasive blood pressure measurements in critically ill premature infants. Acta Paediatr. 101, 132–135 (2012).
Briassoulis, G. Arterial pressure measurement in preterm infants. Crit. Care Med. 14, 735–738 (1986).
Levene, M. et al. Development of audit measures and guidelines for good practice in the management of neonatal respiratory distress syndrome. Arch. Dis. Child. 67, 1221–1227 (1992).
Ince, C. et al. The endothelium in sepsis. Shock 45, 259 (2016).
Dugas, B., Mossalayi, M. D., Damais, C. & Kolb, J.-P. Nitric oxide production by human monocytes: evidence for a role of Cd23. Immunol. Today 16, 574–580 (1995).
Sciorati, C. et al. Generation of nitric oxide by the inducible nitric oxide synthase protects Γδ T cells from Mycobacterium tuberculosis-induced apoptosis. J. Immunol. 163, 1570–1576 (1999).
Nathan, C. Inducible nitric oxide synthase: what difference does it make?. J. Clin. Investig. 100, 2417–2423 (1997).
Matejovic, M. et al. Selective inducible nitric oxide synthase inhibition during long-term hyperdynamic porcine bacteremia. Shock 21, 458–465 (2004).
Ungureanu-Longrois, D., Balligand, J.-L., Kelly, R. A. & Smith, T. W. Myocardial contractile dysfunction in the systematic inflammatory response syndrome: role of a cytokine-inducible nitric oxide synthase in cardiac myocytes. J. Mol. Cell. Cardiol. 27, 155–167 (1995).
Lorente, J. A. et al. Role of nitric oxide in the hemodynamic changes of sepsis. Crit. Care Med. 21, 759–767 (1993).
Kirkebøen, K. & Strand, Ø. The role of nitric oxide in sepsis—an overview. Acta Anaesthesiol. Scand. 43, 275–288 (1999).
Tschaikowsky, K., Sdner, S., Lehnert, N., Kaul, M. & Ritter, J. Endothelin in septic patients: effects on cardiovascular and renal function and its relationship to proinflammatory cytokines. Crit. Care Med. 28, 1854–1860 (2000).
Freeman, B. D., Machado, F. S., Tanowitz, H. B. & Desruisseaux, M. S. Endothelin-1 and its role in the pathogenesis of infectious diseases. Life Sci. 118, 110–119 (2014).
Goto, T. et al. Endothelin receptor antagonist attenuates inflammatory response and prolongs the survival time in a neonatal sepsis model. Intens. Care Med. 36, 2132–2139 (2010).
Marom, D., Yuhas, Y., Sirota, L., Livni, G. & Ashkenazi, S. Nitric oxide levels in preterm and term infants and in premature infants with bacteremia. Neonatology 86, 160–164 (2004).
Figueras-Aloy, J. et al. Plasma endothelin-1 and clinical manifestations of neonatal sepsis. J. Perinat Med. 32, 522–526. https://doi.org/10.1515/JPM.2004.126 (2004).
Wynn, J. L. & Wong, H. R. Pathophysiology and treatment of septic shock in neonates. Clin. Perinatol. 37, 439–479 (2010).
Virág, M., Leiner, T., Rottler, M., Ocskay, K. & Molnar, Z. Individualized hemodynamic management in sepsis. J. Pers. Med. 11, 157 (2021).
Bessler, H. et al. Cd14 receptor expression and lipopolysaccharide-induced cytokine production in preterm and term neonates. Neonatology 80, 186–192 (2001).
Kumar, A., Parrillo, J. E. & Kumar, A. Clinical review: myocardial depression in sepsis and septic shock. Crit. Care 6, 1–9 (2002).
Cunnion, R. E., Schaer, G. L., Parker, M. M., Natanson, C. & Parrillo, J. E. The coronary circulation in human septic shock. Circulation 73, 637–644 (1986).
Cuenca, J., Martín-Sanz, P., Álvarez-Barrientos, A. M., Boscá, L. & Goren, N. Infiltration of inflammatory cells plays an important role in matrix metalloproteinase expression and activation in the heart during sepsis. Am. J. Pathol. 169, 1567–1576 (2006).
Altit, G., Vigny-Pau, M., Barrington, K., Dorval, V. & Lapointe, A. 174: corticosteroid therapy in neonatal management of shock. Paediatr. Child Health 20, e96–e96 (2015).
Alanee, A., H. & Azeez, S. Effect of hydrocortisone therapy on the outcome of neonatal sepsis. Med. J. Tikrit Univ. 17, 177–186 (2011).
Ni, M. et al. Use of vasopressin in neonatal intensive care unit patients with hypotension. J. Pediatr. Pharmacol. Ther. 22, 430–435 (2017).
Frayn, K. N. Hormonal control of metabolism in trauma and sepsis. Clin Endocrinol (Oxf). 24, 577–599 (1986).
van der Poll, T. Effects of catecholamines on the inflammatory response. Sepsis 4, 159–167 (2001).
Mitra, J. K., Roy, J. & Sengupta, S. Vasopressin: its current role in anesthetic practice. Indian J. Crit. Care Med. 15, 71 (2011).
Demiselle, J., Fage, N., Radermacher, P. & Asfar, P. Vasopressin and its analogues in shock states: a review. Ann. Intens. Care 10, 1–7 (2020).
Mutlu, G. M. & Factor, P. Role of vasopressin in the management of septic shock. Intens. Care Med. 30, 1276–1291 (2004).
Landry, D. W. et al. Vasopressin deficiency contributes to the vasodilation of septic shock. Circulation 95, 1122–1125 (1997).
Aradhya, A. S. et al. Low vasopressin and progression of neonatal sepsis to septic shock: a prospective cohort study. Eur. J. Pediatr. 179, 1147–1155 (2020).
Patel, A. et al. Vasopressin for septic shock in a medical-surgical intensive care unit. Can. J. Hosp. Pharm. 73, 209 (2020).
Prigent, H., Maxime, V. & Annane, D. Clinical review: corticotherapy in sepsis. Crit. Care 8, 1–8 (2003).
Fernandez, E. & Watterberg, K. Relative adrenal insufficiency in the preterm and term infant. J. Perinatol. 29, S44–S49 (2009).
Ng, P. et al. Refractory hypotension in preterm infants with adrenocortical insufficiency. Arch. Dis. Child. Fetal Neonatal Ed. 84, F122–F124 (2001).
Scott, S. M. & Watterberg, K. L. Effect of gestational age, postnatal age, and illness on plasma cortisol concentrations in premature infants. Pediatr. Res. 37, 112–116 (1995).
Khashana, A., Ojaniemi, M., Leskinen, M., Saarela, T. & Hallman, M. Term neonates with infection and shock display high cortisol precursors despite low levels of normal cortisol. Acta Paediatr. 105, 154–158 (2016).
Zielińska, K. A., Van Moortel, L., Opdenakker, G., De Bosscher, K. & Van den Steen, P. E. Endothelial response to glucocorticoids in inflammatory diseases. Front. Immunol. 7, 592 (2016).
Bersten, A. D. & Holt, A. W. Vasoactive drugs and the importance of renal perfusion pressure. N. Horiz. 3, 650–661 (1995).
Bezemer, R. et al. Real-time assessment of renal cortical microvascular perfusion heterogeneities using near-infrared laser speckle imaging. Opt. Express 18, 15054–15061 (2010).
Zarbock, A., Gomez, H. & Kellum, J. A. Sepsis-induced AKI revisited: pathophysiology, prevention and future therapies. Curr. Opin. Crit. Care 20, 588 (2014).
Zarbock, A., Gomez, H. & Kellum, J. A. Sepsis-induced acute kidney injury revisited: pathophysiology, prevention and future therapies. Curr. Opin. Crit. Care 20, 588–595 (2014).
Mathur, N., Agarwal, H. S. & Maria, A. Acute renal failure in neonatal sepsis. Indian J. Pediatr. 73, 499–502 (2006).
Peck, T. J. & Hibbert, K. A. Recent advances in the understanding and management of ARDS. F1000Research 8, F1000 Faculty Rev–1959 (2019).
de Jong, H. K., van der Poll, T. & Wiersinga, W. J. The systemic pro-inflammatory response in sepsis. J. Innate Immun. 2, 422–430 (2010).
Vincent, J.-L., Zhang, H., Szabo, C. & Preiser, J.-C. Effects of nitric oxide in septic shock. Am. J. Respir. Crit. Care Med. 161, 1781–1785 (2000).
Fujishima, S. Pathophysiology and biomarkers of acute respiratory distress syndrome. J. Intens. Care 2, 1–6 (2014).
De Luca, D. et al. The Montreux definition of neonatal ARDS: biological and clinical background behind the description of a new entity. Lancet Respir. Med. 5, 657–666 (2017).
Joyce, J. J. et al. Normal right and left ventricular mass development during early infancy. Am. J. Cardiol. 93, 797–801 (2004).
Marijianowski, M. M., van der Loos, C. M., Mohrschladt, M. F. & Becker, A. E. The neonatal heart has a relatively high content of total collagen and type I collagen, a condition that may explain the less compliant state. J. Am. Coll. Cardiol. 23, 1204–1208 (1994).
Archie, J. G., Collins, J. S. & Lebel, R. R. Quantitative standards for fetal and neonatal autopsy. Am. J. Clin. Pathol. 126, 256–265 (2006).
Anderson, P. A. Fetal and neonatal physiology and pharmacology. Curr. Opin. Cardiol. 5, 3–16 (1990).
Kane, C., Couch, L. & Terracciano, C. Excitation–contraction coupling of human induced pluripotent stem cell-derived cardiomyocytes. Front. Cell Dev. Biol. 3, 59 (2015).
Bensley, J. G., Moore, L., De Matteo, R., Harding, R. & Black, M. J. Impact of preterm birth on the developing myocardium of the neonate. Pediatr. Res. 83, 880–888 (2018).
Friedman, W. F. & George, B. L. Treatment of congestive heart failure by altering loading conditions of the heart. J. Pediatr. 106, 697–706 (1985).
Romero, T., Covell, J. & Friedman, W. F. A comparison of pressure-volume relations of the fetal, newborn, and adult heart. Am. J. Physiol. 222, 1285–1290 (1972).
Rowland, D. G. & Gutgesell, H. P. Noninvasive assessment of myocardial contractility, preload, and afterload in healthy newborn infants. Am. J. Cardiol. 75, 818–821 (1995).
Parrillo, J. E. Pathogenetic mechanisms of septic shock. N. Engl. J. Med. 328, 1471–1477 (1993).
Aneja, R. K. & Carcillo, J. A. Differences between adult and pediatric septic shock. Miner. Anestesiol. 77, 986–992 (2011).
McKiernan, C. A. & Lieberman, M. Circulatory shock in children. Pediatr. Rev. 26, 451 (2005).
Singh, Y., Katheria, A. C. & Vora, F. Advances in diagnosis and management of hemodynamic instability in neonatal shock. Front. Pediatr. 6, 2 (2018).
Wright, I. M. & Dyson, R. M. in Microcirculation Revisited—From Molecules to Clinical Practice. (ed. Lenasi, H.) (IntechOpen, 2016).
Noori, S. et al. Hemodynamic changes after low-dosage hydrocortisone administration in vasopressor-treated preterm and term neonates. Pediatrics 118, 1456–1466 (2006).
Alvira, C. M. Aberrant pulmonary vascular growth and remodeling in bronchopulmonary dysplasia. Front. Med. 3, 21 (2016).
Mertens, L. et al. Targeted neonatal echocardiography in the neonatal intensive care unit: practice guidelines and recommendations for training: Writing Group of the American Society of Echocardiography (Ase) in Collaboration with the European Association of Echocardiography (Eae) and the Association for European Pediatric Cardiologists (Aepc). Eur. J. Echocardiogr. 12, 715–736 (2011).
Kharrat, A., McNamara, P. J., Weisz, D. & Jain, A. Merits and perils of targeted neonatal echocardiography-based hemodynamic research: a position statement. Can. J. Physiol. Pharmacol. 97, 183–186 (2019).
de Waal, K. & Evans, N. Hemodynamics in preterm infants with late-onset sepsis. J. Pediatr. 156, 918–922.e911 (2010).
Abdel-Hady, H. E., Matter, M. K. & El-Arman, M. M. Myocardial dysfunction in neonatal sepsis: a tissue Doppler imaging study. Pediatr. Crit. Care Med. 13, 318–323 (2012).
Tomerak, R. H., El-Badawy, A. A., Hussein, G., Kamel, N. R. & Razak, A. R. Echocardiogram done early in neonatal sepsis: What does it add? J. Investig. Med. 60, 680–684 (2012).
Saini, S. S., Kumar, P. & Kumar, R. M. Hemodynamic changes in preterm neonates with septic shock: a prospective observational study*. Pediatr. Crit. Care Med. 15, 443–450 (2014).
Ramadhina, N. et al. Ventricular function and high-sensitivity cardiac troponin T in preterm infants with neonatal sepsis. Paediatr. Indones. 55, 203–208 (2015).
Awany, M., Tolba, O., Al-Biltagi, M., Al-Asy, H. & El-Mahdy, H. Cardiac functions by tissue doppler and speckle tracking echocardiography in neonatal sepsis and its correlation with sepsis markers and cardiac troponin-T. J. Pediatr. Neonatal Care 5, 184–181 (2016).
Alzahrani, A. Cardiac function affection in infants with neonatal sepsis. J. Clin. Trial 7, 329 (2017). 870.
Deshpande, S., Suryawanshi, P., Chaudhary, N. & Maheshwari, R. Cardiac output in late onset neonatal sepsis. J. Clin. Diagn. Res. 11, 25–28 (2017).
Fahmey, S. S., Hodeib, M., Refaat, K. & Mohammed, W. Evaluation of myocardial function in neonatal sepsis using tissue doppler imaging. J. Matern. Fetal Neonatal Med. 33, 3752–3756 (2020).
Deshpande, S. et al. Pulmonary hypertension in late onset neonatal sepsis using functional echocardiography: a prospective study. J. Ultrasound (2021). Epub ahead of print.
Saini, S. S., Sundaram, V., Kumar, P. & Rohit, M. K. Functional echocardiographic preload markers in neonatal septic shock. J. Matern. Fetal Neonatal Med. 1–8 (2021). Online ahead of print.
Yengkhom, R. et al. Point of care neonatal ultrasound in late-onset neonatal sepsis. J. Neonatol. 09732179211007599 (2021).
Verma, B., Daga, S. R. & Mahapankar, A. Persistent pulmonary hypertension among neonates with sepsis. Indian J. Pediatr. 73, 250–251 (2006).
Abdel Mohsen, A. H. & Amin, A. S. Risk factors and outcomes of persistent pulmonary hypertension of the newborn in neonatal intensive care unit of Al-Minya University Hospital in Egypt. J. Clin. Neonatol. 2, 78–82 (2013).
De Simone, G. & Pasanisi, F. Systolic, diastolic and pulse pressure: pathophysiology. Ital. Heart J. Suppl. 2, 359–362 (2001).
Kharrat, A. et al. The relationship between blood pressure parameters and left ventricular output in neonates. J. Perinatol. 39, 619–625 (2019).
Sassano-Higgins, S., Friedlich, P. & Seri, I. A meta-analysis of dopamine use in hypotensive preterm infants: blood pressure and cerebral hemodynamics. J. Perinatol. 31, 647–655 (2011).
De Backer, D., Aldecoa, C., Njimi, H. & Vincent, J.-L. Dopamine versus norepinephrine in the treatment of septic shock: a meta-analysis. Crit. Care Med. 40, 725–730 (2012).
Avni, T. et al. Vasopressors for the treatment of septic shock: systematic review and meta-analysis. PLoS ONE 10, e0129305 (2015).
Rhodes, A. et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intens. Care Med. 43, 304–377 (2017).
Martin, C., Papazian, L., Perrin, G., Saux, P. & Gouin, F. Norepinephrine or dopamine for the treatment of hyperdynamic septic shock? Chest 103, 1826–1831 (1993).
Martin, C., Viviand, X., Arnaud, S., Vialet, R. & Rougnon, T. Effects of norepinephrine plus dobutamine or norepinephrine alone on left ventricular performance of septic shock patients. Crit. Care Med. 27, 1708–1713 (1999).
De Backer, D., Creteur, J., Silva, E. & Vincent, J.-L. Effects of dopamine, norepinephrine, and epinephrine on the splanchnic circulation in septic shock: Which is best? Crit. Care Med. 31, 1659–1667 (2003).
Martin, C., Viviand, X., Leone, M. & Thirion, X. Effect of norepinephrine on the outcome of septic shock. Crit. Care Med. 28, 2758–2765 (2000).
Martin, K. & Weiss, S. L. Initial resuscitation and management of pediatric septic shock. Miner. Pediatr. 67, 141 (2015).
Tourneux, P., Rakza, T., Abazine, A., Krim, G. & Storme, L. Noradrenaline for management of septic shock refractory to fluid loading and dopamine or dobutamine in full‐term newborn infants. Acta Paediatr. 97, 177–180 (2008).
Rizk, M., Lapointe, A., Lefebvre, F. & Barrington, K. Norepinephrine infusion improves haemodynamics in the preterm infants during septic shock. Acta Paediatr. 107, 408–413 (2018).
Baske, K., Saini, S. S., Dutta, S. & Sundaram, V. Epinephrine versus dopamine in neonatal septic shock: a double-blind randomized controlled trial. Eur. J. Pediatr. 177, 1335–1342 (2018).
Ezaki, S. et al. Levels of catecholamines, arginine vasopressin and atrial natriuretic peptide in hypotensive extremely low birth weight infants in the first 24 h after birth. Neonatology 95, 248–255 (2009).
Matok, I. et al. Terlipressin as rescue therapy for intractable hypotension during neonatal septic shock. Pediatr. Crit. Care Med. 5, 116–118 (2004).
Meyer, S., Gottschling, S., Baghai, A., Wurm, D. & Gortner, L. Arginine-vasopressin in catecholamine-refractory septic versus non-septic shock in extremely low birth weight infants with acute renal injury. Crit. Care 10, 1–6 (2006).
Meyer, S., Löffler, G., Polcher, T., Gottschling, S. & Gortner, L. Vasopressin in catecholamine‐resistant septic and cardiogenic shock in very‐low‐birthweight infants. Acta Paediatr. 95, 1309–1312 (2006).
Altit, G., Vigny-Pau, M., Barrington, K., Dorval, V. G. & Lapointe, A. Corticosteroid therapy in neonatal septic shock—Do we prevent death? Am. J. Perinatol. 35, 146–151 (2018).
Baker, C. et al. Hydrocortisone administration for the treatment of refractory hypotension in critically ill newborns. J. Perinatol. 28, 412–419 (2008).
Johnson, P. J. Hydrocortisone for treatment of hypotension in the newborn. Neonatal Netw. 34, 46–51 (2015).
Feng, M. et al. Transthoracic echocardiography and mortality in sepsis: analysis of the MIMIC-III database. Intens. Care Med. 44, 884–892 (2018).
Dempsey, E. M. et al. Hypotension in Preterm Infants (Hip) Randomised Trial. Arch. Dis. Child. Fetal Neonatal Ed. 106, 398–403 (2021).
Author information
Authors and Affiliations
Contributions
A.K. and A.J. both conceived and designed the review. A.K. drafted the manuscript and A.J. revised it critically. Both A.K. and A.J. approve the final version as submitted.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kharrat, A., Jain, A. Hemodynamic dysfunction in neonatal sepsis. Pediatr Res 91, 413–424 (2022). https://doi.org/10.1038/s41390-021-01855-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-021-01855-2
This article is cited by
-
Assessment of hemodynamic dysfunction in septic newborns by functional echocardiography: a systematic review
Pediatric Research (2024)
-
Neonatal sepsis and cardiovascular dysfunction I: mechanisms and pathophysiology
Pediatric Research (2024)
-
Predicting the effectiveness of drugs used for treating cardiovascular conditions in newborn infants
Pediatric Research (2024)
-
Organ dysfunction and mortality in preterm neonates with late-onset bloodstream infection
Pediatric Research (2023)
-
The association between patterns of early respiratory disease and diastolic dysfunction in preterm infants
Journal of Perinatology (2023)