Abstract
Background
Thrombelastometry, allowing timely assessment of global hemostatic function, is increasingly used to guide hemostatic interventions in bleeding patients. Reference values are available for adults and children, including infants but not neonates immediately after birth.
Methods
Neonates were grouped as preterm (30 + 0 to 36 + 6 weeks/days) and term (37 + 0 to 39 + 6 weeks/days). Blood samples were drawn from the umbilical cord immediately after cesarean section and analyzed by thrombelastometry. Reference ranges were determined for the extrinsic and intrinsic coagulation pathways, fibrin polymerization, and hyperfibrinolysis detection.
Results
All extrinsically activated test parameters, but maximum lysis (P = 0.139) differed significantly between both groups (P ≤ 0.001). Maximum clot firmness in the fibrin polymerization test was comparable (P = 0.141). All intrinsically activated test parameters other than coagulation time (P = 0.537) and maximum lysis (P = 0.888) differed significantly (P < 0.001), and so did all aprotinin-related test parameters (P ≤ 0.001) but maximum lysis (P = 0.851).
Conclusions
This is the first study to identify reference ranges for thrombelastometry in preterm and term neonates immediately after birth. We also report differences in clot initiation and clot strength in neonates born <37 versus ≤40 weeks of gestation, mirroring developmental hemostasis.
Impact
-
Impact: This prospective observational study is the first to present reference ranges in preterm and term infants for all types of commercially available tests of thrombelastometry, notably also including the fibrin polymerization test.
-
Importance: Viscoelastic coagulation assays such as thrombelastometry have become integral to the management of perioperative bleeding by present-day standards. Reference values are available for adults, children, and infants but not for neonates.
-
Key message: Clot initiation and formation was faster and clot strength higher in the term than in the preterm group. Parameters of thrombelastometry obtained from cord blood do not apply interchangeably to preterm and term neonates.
Similar content being viewed by others
Introduction
Rapid progress in specialized medical care has improved the survival rates of premature infants, associated with a rise in comorbidities.1,2,3 Major surgical procedures to deal with congenital malformations and typical complications related to preterm birth (e.g. necrotizing enterocolitis) will often entail bleeding complications. As blood volumes are extremely low in neonates, it is crucial to ensure fast and target-oriented replacement of blood components and coagulation factors in these perioperative settings.4,5,6
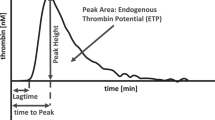
Viscoelastic coagulation assays such as thrombelastometry (ROTEM) have become integral to the management of perioperative bleeding by present-day standards.7,8 Thrombelastometry is a point-of-care technique performed on whole blood enabling a global assessment of hemostatic function. The test results are obtained with short turnaround times and reflect initial fibrin formation, platelet–fibrin interaction, platelet aggregation, clot strengthening, fibrin cross-linking, and, if present, early clot lysis.9 Of note, blood volumes to perform ROTEM are low, which is important to minimize iatrogenic blood loss.
Thrombelastometry tests to determine extrinsically activated clotting (EXTEM) and fibrin polymerization (FIBTEM) are of great interest for the provision of targeted substitution in bleeding patients. Reference values have been determined for adults and children—including infants but only a few isolated reports on EXTEM data, and no reports on FIBTEM data, in preterm and term neonates immediately after birth.10,11,12,13,14
Against this background, we designed a prospective observational study with the primary objective of determining reference ranges for all types of commercially available thrombelastometry tests in preterm and term neonates. As a secondary objective, we aimed to compare both of these gestational age groups with regard to the values obtained for the various thrombelastometry parameters.
Methods
Ethics approval, registration, and conduct
Prior to patient enrollment, the study protocol was approved by the institutional review board (EK 1132/2012) and registered in the German Clinical Trials Register (DRKS00003972; 12 July 2012). Written informed consent was obtained prior to delivery from a parent or legal guardian of each neonate to be included. The study was conducted at our center (Department of Anesthesia, Critical Care and Pain Medicine at Medical University of Vienna, Austria) between June 2013 and December 2016. All procedures were performed in accordance with the standards laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Patient population and exclusion criteria
We prospectively enrolled 142 consecutive neonates. All had been delivered in-house (Obstetrics and Gynaecology, Medical University of Vienna) by cesarean section for obstetric indications and were assigned to one of two gestational age groups: preterm group (30 weeks + 0 days to 36 weeks + 6 days) or term group (37 weeks + 0 days to 39 weeks + 6 days). A positive bleeding history before pregnancy, perinatal low platelet count or altered conventional coagulation testing, or ASA (American Society of Anesthesiologists) score ≥ III of the parturient led to exclusion from the study.
Blood sampling, tests, and endpoints
Right after cesarean delivery, blood samples of 3 ml were drawn from the placental umbilical cord, transferred to 1 ml trisodium citrate (9:1 v/v) tubes (Vacuette®; Greiner Bio-One, Kremsmünster, Austria), and subsequently used for thrombelastometry tests, conventional coagulation assays, and coagulation factor XIII (FXIII) determination as follows:
(a) Thrombelastometry tests: These were performed in citrated whole blood, by medical assistants and anesthetists trained in point-of-care diagnostics, as per the manufacturer’s instructions (ROTEM® delta; TEM Innovations, Munich, Germany). For details, the reader is referred to previous communications.9,14 The assays included extrinsically and intrinsically activated thrombelastometry (EXTEM, INTEM) tests, the FIBTEM test to detect fibrin polymerization, and the APTEM test to detect early hyperfibrinolysis. The main endpoints analyzed were coagulation time (CT in s), clot formation time (CFT in s), α-angle (α in °), amplitude at 10 min (A10 in mm), maximum clot firmness (MCF in mm), and maximum lysis (ML in %).
(b) Conventional coagulation assays and coagulation factor XIII determination: We further determined prothrombin time (PT; reference range: 40–115%), activated partial thromboplastin time (aPTT; reference range: 30–45 s), antithrombin (AT; reference range: 60–90%), fibrinogen levels using the Clauss method (reference range: 200–400 mg dl−1), and coagulation FXIII (reference range: 70–140%). Routine coagulation parameters were assessed with a STA R Max 2® coagulometer (Diagnostica Stago SAS, Asnières-sur-Seine, France) and coagulation FXIII using a Sysmex® CS-5100 coagulation analyzer (Siemens Healthcare Diagnostics, Vienna, Austria). Age-dependent reference ranges for FXIII were provided by our in-house central laboratory.
Data handling and statistical analysis
All patients were pseudonymized by unique identification numbers and their data stored on a restricted computer in a password-protected file at our division (Anesthesia, General Intensive Care and Pain Medicine). Computerized processing was enabled by transferring the original data from paper-based case report forms.
Distributions of continuous variables were visually inspected by box plots and quantile–quantile plots. Robust measures for central tendency and dispersion were derived, as most variables comprised extreme observations. In accordance with guidelines of the Clinical and Laboratory Standards Institute (formerly NCCLS), we used 2.5th and 97.5th percentiles (derived by quantile algorithm type 7 as reported by Hyndman and Fan) as reference ranges for the thrombelastometry parameters.15,16
Mann–Whitney U tests were used to assess differences in value distribution between the gestational age groups and sexes. Relationships between parameters were analyzed by Spearman’s rank correlation coefficient, including hypothesis testing. All tests were two-sided and P values < 0.05 considered statistically significant. All statistical analyses were performed with R software (v. 3.6.1; R Project for Statistical Computing, Vienna, Austria).17
Results
Table 1 lists pertinent patient data. Table 2 and Fig. 1 summarize and illustrate the median values and reference ranges (2.5th and 97.5th percentiles) obtained for the thrombelastometry parameters. The distribution of findings differed significantly between the preterm and term groups. No significant difference was found between females and males. Interquartile ranges decreased with gestational age from preterm to term, the mean difference being −42.5% with a minimum and maximum difference over all parameters of −20.0% and −66.9%, respectively.
In accordance with NCCLS guidelines,15 the references ranges we identified for commercially available test applications (a–d) are expressed as 2.5th and 97.5th percentiles, here presented in the form of translucent gray bars. Red bars indicate the preterm group (30 weeks + 0 days to 36 weeks + 6 days) and green bars the term group (37 weeks + 0 days to 39 weeks + 6 days). The following parameters are shown: amplitude at 10 min (A10), clot formation time (CFT), coagulation time (CT), maximum clot firmness (MCF), and maximum lysis (ML).
EXTEM
The only EXTEM parameter to reveal no significant difference between the preterm and term groups was ML (P = 0.139). All other differences were significant (P ≤ 0.001): median CTs of 56 (IQR: 48–66) vs. 48 (IQR: 44–55) s; CFTs of 130 (IQR: 99–177) vs. 96 (IQR: 83–109) s; α-angles of 65 (IQR: 58–71) vs. 71 (IQR: 68–73)°; amplitudes at 10 min of 44 (IQR: 38–49) vs. 51 (IQR: 48–55) mm; and MCF of 53 (IQR: 45–58) vs. 58 (IQR: 55–61) mm. We observed no significant differences between females and males.
FIBTEM
Parameters relevant to interpreting FIBTEM assays, used to detect fibrin polymerization, notably include amplitudes at 10 min, here yielding medians of 9 (IQR: 6–12) vs. 10 (IQR: 8–12) mm in the preterm vs. the term group, and maximum clot firmness, here yielding medians of 10 (IQR: 6–13) vs. 11 (IQR: 9–13) mm. No significant impact on the test results was seen between the groups (amplitude at 10 min: P = 0.053; maximum clot firmness: P = 0.141) or the sexes (P = 0.494 and 0.418). Maximum clot firmness was found to correlate significantly with fibrinogen levels in the preterm (ρ = 0.66; P < 0.001) and the term (ρ = 0.65; P < 0.001) groups (Fig. 2).
INTEM
Significant intergroup differences were also observable for all INTEM parameters but CT (P = 0.537) and ML (P = 0.888). All the rest involved significant differences (P < 0.001) between the preterm and term groups, with median CFTs of 93 (IQR: 70–126) vs. 65 (IQR: 54–80) s; α-angles of 72 (IQR: 67–76) vs. 77 (IQR: 74–79)°; amplitudes at 10 min of 48 (IQR: 42–53) vs. 55 (IQR: 51–59) mm; and maximum clot firmness of 52 (IQR: 47–58) vs. 60 (IQR: 56–64) mm. Again, no significant differences emerged between the sexes.
APTEM
All intergroup differences obtained with APTEM were significant (P ≤ 0.001), again with the exception of ML (P = 0.851) and again with comparable results for females and males. In daily clinical practice, APTEM tests are directly compared to the EXTEM ones for differential diagnosis of hyperfibrinolysis, early hyperfibrinolysis being defined by a >25% decrease in APTEM from EXTEM clotting time or a >25% increase in APTEM from EXTEM maximum clot firmness, whereas hyperfibrinolysis requiring therapeutic intervention is commonly defined as EXTEM ML >15%. By these criteria, early hyperfibrinolysis was present in 4 (8%) preterm and 12 (5.9%) term neonates and hyperfibrinolysis requiring intervention in 1 (2.1%) or 3 (3.2%) cases. These findings were not, however, associated with any immediate perinatal bleeding complications.
Conventional coagulation assays
Table 3 lists the median values and reference ranges of these tests. We observed significant intergroup differences for PT with medians of 45% (IQR: 37–52%) in the preterm vs. 51% (IQR: 47–56%) in the term group (P = 0.001), and for AT III with 40% (IQR: 32–50%) vs. 51% (IQR: 44–58%), respectively (P < 0.001). No significant differences were seen for aPTT with median values of 55 (IQR: 47–59) in the preterm vs. 51 (IQR: 48–56) s in the term group (P = 0.145), or for fibrinogen concentrations with 171 (IQR: 153–200) or 190 (IQR: 165–211) mg dl−1, respectively (P = 0.061). None of these parameters involved a significant difference between females and males (PT: P = 0.433; AT III: P = 0.593; aPTT: P = 0.145; fibrinogen: P = 0.353).
Coagulation FXIII
The findings for FXIII are also compiled in Table 3. Its median values were 61% (IQR: 53–71%) in the preterm and 59% (IQR: 54–66%) in the term group, both falling short of the 70–140% reference range. No significant difference emerged between the groups (P = 0.656) or sexes (P = 0.595). Of note, we observed no significant difference in EXTEM maximum clot firmness (P = 0.315), EXTEM ML (P = 0.051), or APTEM ML (P = 0.112) in neonates who showed FXIII levels below the normal range.
Discussion
This is the first study to present reference ranges in preterm and term neonates for all types of commercially available thrombelastometry tests, notably also including the FIBTEM test. We found significant differences between both groups, with faster clot initiation and formation as well as higher clot strength in the term group. FIBTEM yielded no significant intergroup difference in fibrin polymerization. APTEM did suggest a few cases of early hyperfibrinolysis. We found no significant differences between female and male neonates, and the results were generally more variable in the preterm group.
Current literature recommends a targeted approach of algorithm-based substitution to deal with perioperative bleeding in children, using coagulation factor concentrates and platelets.7,18,19 Results can be had in a matter of minutes with point-of-care tests like thrombelastometry, allowing for prompt decisions about hemostatic interventions that may be crucial to avoid dilutional coagulopathy and exsanguination.20 Oswald et al.14 reported INTEM, EXTEM, and FIBTEM reference ranges based on a cohort of 51 children (age: 0–3 months) with an intravenous access for elective surgery. Compared to our results, their INTEM and EXTEM findings indicated faster clotting and higher clot strengths. Fibrin polymerization also seemed to be enhanced. Table 2 with its side-by-side listing of thrombelastometry results for preterms, terms, and 0–3 months old may be assumed to represent the physiological maturation trend of the hemostatic system, also known as “developmental hemostasis.”21 Supplementary Fig. 1 illustrates the day-to-day developments of clot initiation, clot strength, and fibrin polymerization during the gestational period reflected by our study population.
Three studies are available on thrombelastometry findings in neonates.10,11,12 Cvirn et al.10 published results for clot strength. Analyzing maximum clot firmness in cord blood from 20 healthy term neonates born after 38–42 weeks of gestation, they reported median results of 55.3 ± 3.8 mm by EXTEM and 11.6 ± 2.3 mm by FIBTEM, which is consistent with our own findings in the term group. They also found significant differences in clot strength compared to adults, attributing this finding to weaker fibrin polymerization. Strauss et al.11 discussed EXTEM results in neonates. Using a low tissue-factor (lowTF) variant of the test, they analyzed cord blood from 47 preterm and 187 term neonates and blood from healthy adults, finding that the clot strength was significantly lower among the preterms than terms and that clot initiation and strength correlated with gestational age. Their lowTF EXTEM test also indicated that clot initiation and formation was significantly faster in neonates than adults. Given the modified TF level in that study, its results cannot be directly compared to ours.
Sokou et al.12 reported EXTEM data for 282 healthy neonates, including 84 preterm and 198 term neonates. These data were based on arterial blood drawn between days 2 and 7 after birth. Unlike in our study, both groups revealed no significant differences in CT, CFT, amplitudes at 10 min, and maximum clot firmness. Also, the former two of these parameters showed higher, and the latter two lower, median values in both of our gestational age groups. While it is well documented that fibrin polymerization adds to EXTEM CT, CFT, and maximum clot firmness, it remains speculative if enhanced fibrin polymerization might account for the generally more pronounced data for clot strength and initiation reported by those authors because they reported neither FIBTEM data nor plasma fibrinogen levels.9,12,14 Also, their EXTEM median values for maximum clot firmness of 64 mm (preterms) and 66 mm (terms) exceed not only ours but clearly also those reported by Cvirn et al.10
In the same study, lysis index scores at 60 min (LI60) differed significantly between both groups and were inversely correlated with gestational age and birth weight in the preterm group.12 The authors suggested that this higher fibrinolytic activity may be due to physiologically low levels of fibrinolysis inhibitors in preterms. By comparison, we analyzed not LI60 but ML, and while the term group did include fewer patients with signs of early hyperfibrinolysis (5.9% vs. 8%), the prevalence of ML >15% was also slightly more prevalent in this group (3.2% vs. 2.1%). What this implies is unclear, as we noted no clinically relevant cases of perinatal bleeding, and coagulation factor XIII levels did not significantly contribute to ML >15% (P = 0.051).
Our study is the first to report FIBTEM values in preterm neonates. Fibrinogen being the first coagulation factor that gets consumed and rapidly declines in the presence of active bleeding, prompt and targeted substitution is essential to prevent the coagulopathy from exacerbating further.5,18,22 Maximum clot firmness as determined by FIBTEM indicates fibrin polymerization and correlates highly with fibrinogen in conventional coagulation assays.8,20 Median values of 16 mm have been reported in adults, compared to 14 mm in children aged 0–3 months and ~12 mm in cord blood of term infants ≥38 weeks of gestational age.10,13,14 Our own median results for FIBTEM maximum clot firmness of 10 mm in the preterm and 11 mm in the term group did not significantly differ. Yet, fibrinogen function remains to be fully elucidated in our specific patients, and, perhaps more importantly, our results are based on non-bleeding neonates.
Another factor contributing to clot strength, alongside fibrinogen, is coagulation factor XIII. We found its median levels to fall short of the reference ranges provided by our in-house central laboratory in 72.9% of preterm and 82.4% of term neonates, which did not, however, seem to affect EXTEM maximum clot firmness in a significant way. This observation is supported by data available from Haas et al.,23 who reported that thrombelastometry parameters were mostly unchanged despite low levels of coagulation factor XIII at the outset of major surgical procedures in children.
Two limitations of our study should be mentioned. First of all, while blood sampling from the umbilical cord is well established for neonatal research,24,25,26 access may be restricted in preterm neonates by the current practice of delayed cord clamping or cord milking for placental transfusion, which has been shown to reduce transfusion requirements in the hospital and to improve neonatal outcomes.11,12,27,28 Thus, the residual volumes of blood available to us did not always suffice to perform all of the planned tests (Supplementary Table 1 lists the exact patient base for each of our findings by thrombelastometry, conventional coagulation, and coagulation factor XIII assays). For the same reason, we chose not to analyze platelet counts, as these have been discussed in the literature and standard thrombelastometry parameters do not reflect platelet function.21,29,30,31
The second limitation concerns our observation of outliers that noticeably affected the reference ranges, especially in the preterm group. At the same time, however, these outliers are reliable data not explainable by measurement errors.
In summary, the present investigation into thrombelastometry reference values for preterm and term neonates does reveal significant differences in clot initiation and clot strength, mirroring developmental hemostasis, in cord blood obtained ≥30 to <37 vs. ≥37 to ≤40 weeks of gestation.
References
Helenius, K., Gissler, M. & Lehtonen, L. Trends in centralization of very preterm deliveries and neonatal survival in Finland in 1987–2017. Transl. Pediatr. 8, 227–232 (2019).
Santhakumaran, S. et al. Survival of very preterm infants admitted to neonatal care in England 2008–2014: time trends and regional variation. Arch. Dis. Child Fetal Neonatal Ed. 103, F208–F215 (2018).
Patel, R. M. Short- and long-term outcomes for extremely preterm infants. Am. J. Perinatol. 33, 318–328 (2016).
Crighton, G. L., New, H. V., Liley, H. G. & Stanworth, S. J. Patient blood management, what does this actually mean for neonates and infants? Transfus. Med. 28, 117–131 (2018).
Goel, R., Cushing, M. M. & Tobian, A. A. Pediatric patient blood management programs: not just transfusing little adults. Transfus. Med. Rev. 30, 235–241 (2016).
Kahl, L. K. & Hughes, H. K. (eds). in Harriet Lane Handbook 21st edn (Elsevier, Philadelphia, 2018).
Goobie, S. M. & Haas, T. Bleeding management for pediatric craniotomies and craniofacial surgery. Paediatr. Anaesth. 24, 678–689 (2014).
Haas, T. et al. Comparison of thrombelastometry (ROTEM®) with standard plasmatic coagulation testing in paediatric surgery. Br. J. Anaesth. 108, 36–41 (2012).
Hans, G. A. & Besser, M. W. The place of viscoelastic testing in clinical practice. Br. J. Haematol. 173, 37–48 (2016).
Cvirn, G. et al. Clot strength: a comparison between cord and adult blood by means of thrombelastometry. J. Pediatr. Hematol. Oncol. 30, 210–213 (2008).
Strauss, T. et al. Clot formation of neonates tested by thromboelastography correlates with gestational age. Thromb. Haemost. 103, 344–350 (2010).
Sokou, R. et al. Reference ranges of thrombelastometry in healthy full-term and preterm neonates. Clin. Chem. Lab. Med. 55, 1592–1597 (2017).
Lang, T. et al. Multi-centre investigation on reference ranges for ROTEM thrombelastometry. Blood Coagul. Fibrinolysis 16, 301–310 (2005).
Oswald, E. et al. Thrombelastometry (ROTEM) in children: age-related reference ranges and correlations with standard coagulation tests. Br. J. Anaesth. 105, 827–835 (2010).
Clinical Laboratory and Standards Institute. How to Define and Determine Reference Intervals in the Clinical Laboratory; Approved Guideline - Second Edition. CLSI document C28-A2 (Clinical Laboratory and Standards Institute, Wayne, 2000).
Hyndman, R. J. & Fan, Y. Sample quantiles in statistical packages. Am Stat. 50 361–365 (1996).
R Core Team (2019). R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing V, Austria, 2020).
Goobie, S. M. & Haas, T. Perioperative bleeding management in pediatric patients. Curr. Opin. Anaesthesiol. 29, 352–358 (2016).
Haas, T., Goobie, S., Spielmann, N., Weiss, M. & Schmugge, M. Improvements in patient blood management for pediatric craniosynostosis surgery using a ROTEM®-assisted strategy - feasibility and costs. Paediatr. Anaesth. 24, 774–780 (2014).
Theusinger, O. M., Stein, P. & Levy, J. H. Point of care and factor concentrate-based coagulation algorithms. Transfus. Med. Hemother. 42, 115–121 (2015).
Kenet, G., Barg, A. A. & Nowak-Gottl, U. Hemostasis in the very young. Semin. Thromb. Hemost. 44, 617–623 (2018).
Haas, T. et al. Higher fibrinogen concentrations for reduction of transfusion requirements during major paediatric surgery: a prospective randomised controlled trial. Br. J. Anaesth. 115, 234–243 (2015).
Haas, T. et al. Perioperative course of FXIII in children undergoing major surgery. Paediatr. Anaesth. 22, 641–646 (2012).
Neary, E. et al. Coagulation indices in very preterm infants from cord blood and postnatal samples. J. Thromb. Haemost. 13, 2021–2030 (2015).
Christensen, R. D. et al. Reference intervals for common coagulation tests of preterm infants (CME). Transfusion 54, 627–632 (2014). quiz 626.
Del Vecchio, A., Motta, M. & Romagnoli, C. Neonatal platelet function. Clin. Perinatol. 42, 625–638 (2015).
Rabe, H., Gyte, G. M., Diaz-Rossello, J. L. & Duley, L. Effect of timing of umbilical cord clamping and other strategies to influence placental transfusion at preterm birth on maternal and infant outcomes. Cochrane Database Syst. Rev. 9, CD003248 (2019).
Balasubramanian, H. et al. Effect of umbilical cord blood sampling versus admission blood sampling on requirement of blood transfusion in extremely preterm infants: a randomized controlled trial. J. Pediatr. 211, 39–45.e2 (2019).
Chakravorty, S. & Roberts, I. How I manage neonatal thrombocytopenia. Br. J. Haematol. 156, 155–162 (2012).
Pearson, K., Jensen, H., Kander, T. & Schott, U. Desmopressin in vitro effects on platelet function, monitored with Multiplate, ROTEM and Sonoclot. Scand. J. Clin. Lab. Invest. 76, 282–290 (2016).
Solomon, C., Ranucci, M., Hochleitner, G., Schochl, H. & Schlimp, C. J. Assessing the methodology for calculating platelet contribution to clot strength (platelet component) in thrombelastometry and thrombelastography. Anesth. Analg. 121, 868–878 (2015).
Andrew, M. et al. Development of the human coagulation system in the healthy premature infant. Blood 72, 1651–1657 (1988).
Andrew, M. et al. Development of the human coagulation system in the full-term infant. Blood 70, 165–172 (1987).
Acknowledgements
This work was supported by departmental funds. Tem Innovations GmbH sponsored part of the disposables used, but had no influence whatsoever on the content of this article.
Author information
Authors and Affiliations
Contributions
All authors have met the Pediatric Research authorship requirements. M.W. had full access to all of the data in the study and takes responsibility for the integrity of the data. Concept and design: O.K., P.M., L.T., H.W. Acquisition, analysis, or interpretation of data: M.W., E.S., A.B., O.K., L.T. Drafting of the manuscript: M.W. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: A.B., O.K. Supervision: E.S. Christine Hlozanek, medical assistant, performed thrombelastometry tests.
Corresponding author
Ethics declarations
Competing interests
M.W. received travel reimbursement and speaker’s fees from Ekomed GmbH, Haemonetics Corporation, and CSL Behring. All other authors declare no competing interests.
Statement of consent
Written informed consent was obtained prior to delivery from a parent or legal guardian of each neonate to be included.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wiegele, M., Kimberger, O., Schaden, E. et al. Establishing reference ranges of cord blood: point-of-care hemostatic function assessment in preterm and term neonates. Pediatr Res 90, 452–458 (2021). https://doi.org/10.1038/s41390-020-01310-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-01310-8