Abstract
Dietary habits represent the main determinant of health. Although extensive research has been conducted to modify unhealthy dietary behaviors across the lifespan, obesity and obesity-associated comorbidities are increasingly observed worldwide. Individually tailored interventions are nowadays considered a promising frontier for nutritional research. In this narrative review, the technologies of importance in a pediatric clinical setting are discussed. The first determinant of the dietary balance is represented by energy intakes matching individual needs. Most emerging studies highlight the opportunity to reconsider the widely used prediction equations of resting energy expenditure. Artificial Neural Network approaches may help to disentangle the role of single contributors to energy expenditure. Artificial intelligence is also useful in the prediction of the glycemic response, based on the individual microbiome. Other factors further concurring to define individually tailored nutritional needs are metabolomics and nutrigenomic. Since most available data come from studies in adult groups, new efforts should now be addressed to integrate all these aspects to develop comprehensive and—above all—effective interventions for children.
Impact
-
Personalized dietary advice, specific to individuals, should be more effective in the prevention of chronic diseases than general recommendations about diet.
-
Artificial Neural Networks algorithms are technologies of importance in a pediatric setting that may help practitioners to provide personalized nutrition.
-
Other approaches to personalized nutrition, while promising in adults and for basic research, are still far from practical application in pediatrics.
Similar content being viewed by others
Introduction
Obesity and obesity-associated comorbidities are increasingly observed worldwide.1 In parallel, non-communicable diseases, such as cardiovascular and metabolic diseases, are still the major killers globally.2 It has been estimated that up to one every eighth case of cardiometabolic diseases and one every third case of cancer would be prevented by changing lifestyle.3 In 2017, 11 millions of deaths and 255 million disability-adjusted life-years (DALYs) were attributable to the dietary habits. Specifically, a high intake of sodium and low intake of whole grains and fruits are the leading dietary risk factors for deaths and DALYs, globally.4 It is also well known that prevention begins early in life. Despite a growing body of evidence on the determinants of childhood obesity is being accumulated in the latest years on, the prevalence of this condition is increasing at an alarming rate both in developed countries and in developing countries. Respectively, 23.8% of boys and 22.6% of girls and 12.9% of boys and 13.4% of girls were overweight or obese in 2013.5 Teenagers affected with chronic conditions will enter adulthood with several years of disease duration, resistance to treatment, and greater risk of early complications.6,7 However, interventions to modify dietary attitudes and to increase health and well-being across the lifespan have shown to exert effect only in a small percentage of people.8 Recent evidences suggest that one-size-fits-all is not a good enough approach. One of the reasons underlying these issues is that the individual responses to dietary interventions are heterogeneous among the single individuals and most trials did not comprehensively consider the complex relationship of differing individual characteristics, such as the genome, microbiome, and environmental exposure. These innovative evidences have generated the idea that a better understanding of those individual characteristics may improve the definition of nutritional interventions, tailoring them on the specific needs of a subject, or a group sharing the same features. Consequently, numerous questions are being raised about how the interventions of personalized nutrition might be constructed.
Definition: There is not yet a widely agreed definition of personalized nutrition.8 In the pediatric settings, we are hereby describing that personalized nutrition is often used interchangeably with the term: precision nutrition. Precision nutrition meant a more intricate approach in the definition of personalized nutrition. It considers all the relationships between individual’s characteristics, phenotype, and health status, to adapt to nutrition interventions. Special importance among these characteristics is those based on omics. To that end, precision nutrition approach, in addition to genetics, is comprehensive of metabolic status, gut microbiota, metabolome, physiologic status, and environmental exposures (included dietary habits and food behavior) of any individual.9 Many tools are available to improve dietary habits for the prevention (or treatment) of chronic diseases and to improve public health. The nutrigenetics comprehends the phenotypic responses to a diet, depending on the genotype of the individual; the nutrigenomics involves the characterization of all gene products affected by nutrients; the exposome is the collection of environmental factors that may affect health. As one moves further through the levels of characterization, a major complexity is needed to achieve the desired goal of tailoring intervention. The metabolomics is the analysis of the metabolites produced by a cell, tissue, or organism and the microbiomics is the study of the totality of microbes in a specific environment.8,9
This narrative review aims to summarize the recent technologies that can help providing indications towards precision nutrition, such as Artificial Neural Networks (ANNs) in the field of metabolism, together with metabolomics, microbiomics, and nutrigenomics. The present review is not intended to be a comprehensive discussion of medical-oriented technologies, since it is limited to those applicable within pediatric clinical settings.
Machine learning algorithms approach
The prediction of individual resting energy expenditure
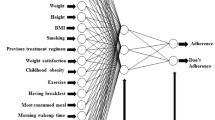
Children are a vulnerable population. Their adequate growth depends on balanced conjunction between good nutrition and regular physical activity.10 The accurate estimate of energy requirements is the first step to achieve a good nutritional status and it is mainly based on the assessment of resting energy expenditure (REE). In the literature, many predictive equations have been created to estimate REE, but none has been demonstrated to be accurate enough, particularly when applied to ill populations.11 Total daily energy expenditure (TDEE), expended over 24 h, is the sum of three components: REE, diet-induced thermogenesis (DIT) and energy expenditure of physical activity. In children, the energy spent on growth is another fundamental factor that accounts for TDEE.12 REE is the energy required to sustain biochemical systems of the body at rest and accounts for ~70% of TDEE in sedentary individuals.13 The gold standard technique for its measurement is the indirect calorimetry (IC). Fat-free mass is the greatest determinant of REE, accounting for ~70% of its variance. Sex, age, and fat mass are some of the remaining significant contributors.14 Energy cost of physical activity is the energy consumed in muscular work during voluntary exercise, and is the most variable component of TDEE, accounting from ~15% in sedentary individuals to ~ 50% in highly active individuals.13 Lastly, DIT increases REE in response to food ingestion and accounts for ~10%. When energy expenditure equals energy intake, the energy balance is zero and the organism meets the energy equilibrium. Metabolic adaptation to weight changes relates to body weight control, obesity, and malnutrition. The energy requirement is the amount of energy from food needed to maintain body composition and a level of physical activity consistent with long-term good health. In children, the energy requirements also include the energy needed for the synthesis and deposition of new tissues.12,15 The approach of “prediction equations” has been recently placed under discussion, even as misleading.16 The accuracy of different predictive formulae has been challenged11 and, accordingly, all the formulae considered show a low level of accuracy at an individual level.17,18 Across all equations, the absolute bias is highly inaccurate in the youngest and most vulnerable children.19 An external cross-validation study of ten equations to estimate REE in obese and non-obese children has recently shown that Schofield and Harris-Benedict equations are the less accurate to estimate REE within 10% of measured (by IC) REE.20 The approach of ANN algorithms may be a useful tool to check the predictive value of REE. ANNs are computerized algorithms resembling interactive processes of the human brain allowing for a more comprehensive approach to very complex non-linear phenomena such as biological systems.21 Accordingly, they have been successfully implemented in a population of 561 healthy children.22 The dataset used for ANN modeling consisted of demographic and anthropometric variables (such as age, gender, body mass index, and other bioindicators). ANNs have inference at an individual level rather than at a group level. They seem to be a valid alternative both to IC and predictive equations, being strongly correlated to directly measured REE. Analyzing the semantic connectivity maps (the main components of the trees building up the algorithms), the hypothesis emerges that the classic equations actually cannot account for the rapid evolutionary changes connected to a shift towards a major representation of fat mass in the pediatric population during the past century, representing a determinant of “unpredictable and incalculable” modifications of energy requirements.23 In the subgroup of obese children, the performance of ANNs was even better, and the grade of imprecision was lower (just mildly >5%).24 Literature from various medical fields presents the development and validation of algorithms. Hirose et al.25 successfully implemented a model to predict the 6-year incidence of metabolic syndrome using ANN based on clinical factors. Furthermore, a study on 853 obese patients validated an ANN model for the prediction of REE, demonstrating accurate higher precision than established REE predictive equations, independently from BMI subgroups.23 The development of the ANN approach seems to be particularly promising even in emergency conditions for pediatric patients in PICUs, taking into account the potential impact of the variability of blood gases (CO2, O2) to redefine energy and substrate needs in critical condition and preventing glycemic unbalances.26
The microbiomics
While the new ANN approach may have the potential to develop personalized dietary interventions, both for preventive (obesity and related clinical complications) and therapeutic (children suffering from either acute or chronic disorders) purposes, machine-learning algorithms have included other potential biomarkers, possibly connected with personalized nutrition in a cause–effect relationship. An elegant study investigated the possible role of anthropometrics, dietary habits, physical activity, blood parameters, and gut microbiota in a cohort of 800 Israeli subjects applying machine-learning algorithms to predict personalized postprandial glycemic response to real-life meals.27 Blood glucose levels are largely influenced by diet, but, while foods with low glycemic index are associated with positive changes in many metabolic indicators (e.g., glycated proteins) and inflammatory markers,28,29 different subjects, even eating identical meals, may present with high variability in post-meal blood glucose response.30 The variability in glycemic response might depend on several factors, some modifiable (e.g., lifestyle and insulin sensitivity) and other unmodifiable (e.g., genetics).31 Zeevi et al.27 added that clinical and microbiome profiles were able to accurately predict the glycemic response and, conversely, that the individual approach to improve the glycemic profile of the diet was able to support the selection of bacterial strains in the gut more favorably modulating, in turn, the glycemic curves. Further studies confirmed the effects of this approach within US populations.32,33 Of note, no study so far has been published investigating this model in children. Some efforts aimed at developing personalized nutritional tools in children are emerging. The first international pilot study conducted by the Schneider Children’s Medical Center and the Weizmann Institute of Science of Israel together with the Department of Translational Medical Sciences of the University of Naples “Federico II” in Italy started in 2018. This study is focused on personalization of the Mediterranean Diet in southern Italy and Israel.34 These observations in adults suggest that gut microbiome might play a key role in developing personalized dietary interventions, since it actively interacts with the human body and affects the capabilities of nutrients and energy harvesting from food.35 The potential of the microbiome to allow for a more precise development towards models of personalized nutrition might be summarized as follows: (1) microbiome profile is partially able to predict the metabolic consequences of nutritional interventions (e.g., the consumption of non-caloric artificial sweetener paradoxically tends to increase the development of glucose-intolerance by promoting dysbiosis in some individuals) and (2) the microbiome characterization might contribute to identify children who are especially at risk of developing diet-associated conditions, such as obesity or atopy.36,37 Therefore, gut microbiome profiling may represent a further promising approach in tailoring personalized nutritional interventions38 to be extended to the pediatric population. So far, most available studies in children report associations between specific microbiome profiles and defined clinical conditions,39,40 out of the otherwise healthy pediatric population. In evaluating cost/benefit ratios, one should also consider the costs of microbiome profiles’ investigation, potentially limiting its use on a large-scale population.
Metabolomics approach to individual needs
Metabolomics represents a recent approach aimed at the quantitative analysis of the byproducts of cellular activity, derived from metabolic pathways characterizing living systems, and allowing for an “individually tailored” evaluation of changes in gene expression.41 The epigenetics consists of those modifications (methylation, histone modification, microRNAs) that modify the expression and function of the genetic material of an organism.8 These modifications may follow environmental exposures during pregnancy, infancy, and childhood, thus altering the offspring’s growth and development with inter-generational modifications of diseases.42 Therefore, understanding how these mechanisms may contribute to transgenerational transmission and long-term metabolic modifications are crucial for the development of novel early detection and prevention strategies.43,44 To understand the high impact of epigenetics, it is helpful to mention the observations derived from the Dutch (1944–1945) and Chinese (1959–1961) famine,45,46,47 where children of mothers who were exposed to the calorie restriction in utero and during the first years of life because of the food shortage gave birth to newborns with larger birth size as compared to offspring from mothers not exposed to the famine. Accordingly, epigenetics may trigger the maternal accrual of adipose tissue while activating genes controlling for lipogenesis and low-grade inflammation in early pregnancy. These metabolic alterations may occur prior to any changes in maternal phenotype.47
Some metabolic alterations may occur also later in childhood. For instance, patterns of metabolites related to the gut microbiota led to observe metabolic phenotype differences in autistic children, providing novel insights into the role of the gut–brain axis in the etiology of several diseases.48 Moreover, Perng et al.49 explored metabolomics profiles of obesity risk in children aged 6 to 10 years, identifying metabolites involved in lipid, amino acid, and carbohydrate metabolism as correlates of a metabolic syndrome risk score,49 with changes in glycemia and lipid biomarkers during the adolescent transition.50
A growing body of evidence has been accumulating in the latest years regarding the impact of single foods and dietary patterns on the long-term health status. The inconsistency of studies results, even if the enrolled population is stratified for a known single-gene haplotype, suggests the existence of a robust inter-individual variance51 as a result of a complex interplay between the network of several genes and the quality of quantity of macro- and micronutrients in the diet.52 In addition, the gene response to foods may, in turn, be individually shaped by social determinants, such as education and socio-economics status.53 Thus, each individual is likely to be the owner of a personal, well-individualized risk profile that has been built not only by himself during the lifespan, but also during the mother’s childbearing age by her dietary and social behaviors. Nowadays, the challenge is to associate epigenetic modifications and their metabolomic expressions through the measurement of specific and sensitive serum biomarkers able to predict a personal poly-factorial score of metabolic risk.54
Nutrigenomics approach to individual needs
Genetic and nutrition interactions play a strong influence on the individual’s phenotype. Nutritional genomics studies these relationships, with the goal to unravel the interaction between genetics and dietary intake and bringing together emerging branches of biology such as bioinformatics, nutrition, molecular biology, genetics, genomics, epidemiology, and molecular medicine.55 The different responses to lifestyle interventions, especially those modulating diet, because of genetic variants, may affect indeed how dietary components are absorbed, metabolized, and utilized.56 One application of nutrigenomics concerns personalized dietary advice, according to the particular genotype of a given individual. This approach might be more effective in the prevention of chronic diseases, compared to population-based general recommendations on recommended dietary intakes.57 Research activities in the field of nutrigenomics have two major foci: (1) identifying genes responsive to dietary changes and (2) studying the interactions between dietary changes and metabolic homeostasis.58 Nutrients are dietary signals that are detected by the cellular sensory systems influencing gene and protein expression and, subsequently, metabolic indices.58 The effect of gene–diet interaction on risk of childhood obesity are evident either in animals and in humans.59 The connection between birth weight and adult weight suggests that there are enduring effects of the in utero environment on later obesity risk.60 Conversely, maternal nutrient deprivation during late fetal development could result in the reduction of offspring birth weight, leading, as well, to glucose intolerance and insulin resistance later in life.61 This suggests that gene variants may determine an increase of energy deposition as fat over time, to maintain the reproductive function and enhance survival in stress conditions, according to “thrifty genotype hypothesis.”62 While this area of interest is progressively expanding, more data on related bioindicators are needed to improve the approach to a personalized diet in the pediatric age based on nutrigenomics.
Conclusion
Personalized nutrition is one of the fascinating frontiers of medicine. Individually tailored interventions have been claimed by many scientists as the turning point for nutritional research. Available data, while suggesting that a tailored nutritional intervention might be effective in preventing several conditions, including obesity and its consequences, paralleling the concept of precision medicine, show also the practical difficulties in applying this approach to the pediatric age.
Indeed, while the use of artificial intelligence may be relevant to improve precision and accuracy of predictions in clinical settings, gene–diet interactions have been widely reported, but data on direct interventions are still lacking, particularly for the pediatric age. Moreover, evidence on the effectiveness of personalized nutrition is currently limited to adult populations and it is still unknown how children could respond to such interventions.
New efforts from the pediatric scientific communities are required to investigate the impact of this approach on children, comprehensive of all the aspects surrounding nutritional status, metabolism, and nutritional requirements. Accounting for the great number of variables and their implications, emphasis should be given, and efforts directed to the implementation of highly effective nutrition-specific and nutrition-sensitive studies, starting from the earliest ages of life, with long-term observations, with affordable costs, and identifying easy-to-use biomarkers for a more widespread application.
References
Kumar, S. & Kelly, A. S. Review of childhood obesity: from epidemiology, etiology, and comorbidities to clinical assessment and treatment. Mayo Clin. Proc. 92, 251–265 (2017).
Martinez, R. et al. Trends in premature avertable mortality from non-communicable diseases for 195 countries and territories, 1990-2017: a population-based study. Lancet Glob. Health 8, e511–e523 (2020).
World Health Organization. The World Health Report 2008: Primary Health Care: Now More Than Ever (World Health Organization, Geneva, 2008).
Afshin, A. & et al. Health effects of dietary risks in 195 countries, 1990–2013;2017: a systematic analysis for the Global Burden of Disease Study. Lancet 393, 1958–1972 (2017).
Ng, M. et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 384, 766–781 (2014).
Mayer-Davis, E. J. et al. Incidence trends of type 1 and type 2 diabetes among youths, 2002–2012. N. Engl. J. Med. 376, 1419–1429 (2017).
Wu, Y., Perng, W. & Peterson, K. E. Precision nutrition and childhood obesity: a scoping review. Metabolites 10, 235 (2020).
Ordovas, J. M., Ferguson, L. R., Tai, E. S. & Mathers, J. C. Personalised nutrition and health. BMJ 361, bmj.k2173 (2018).
Gibney, M., Walsh, M. & Goosens, J. in Personalized Nutrition: Paving the Way to Better Population Health, Good Nutrition: Perspectives for the 21st Century (eds Eggersdorfer, M. et al.) 235–248 (Karger Publishers, Basel, 2016).
Boreham, C. & Riddoch, C. The physical activity, fitness and health of children. J. Sports Sci. 19, 915–929 (2001).
Siervo, M. et al. Accuracy of predictive equations for the measurement of resting energy expenditure in older subjects. Clin. Nutr. 33, 613–619 (2014).
EFSA Panel on Dietetic Products, NaAN. Scientific opinion on dietary reference values for energy. EFSA J. https://doi.org/10.2903/j.efsa.2013.3418 (2013).
Lam, Y. Y. & Ravussin, E. Analysis of energy metabolism in humans: a review of methodologies. Mol. Metab. 5, 1057–1071 (2016).
Weyer, C., Snitker, S., Rising, R., Bogardus, C. & Ravussin, E. Determinants of energy expenditure and fuel utilization in man: effects of body composition, age, sex, ethnicity and glucose tolerance in 916 subjects. Int. J. Obes. Relat. Metab. Disord. 23, 715–722 (1999).
Hall, K. D. et al. Energy balance and its components: implications for body weight regulation. Am. J. Clin. Nutr. 95, 989–994 (2012).
Bottà, G. et al. Evaluating human basal metabolism: the erroneous and misleading use of so-called “prediction equations”. Int. J. Food Sci. Nutr. 71, 249–255 (2020).
Agostoni, C. et al. Accuracy of prediction formulae for the assessment of resting energy expenditure in hospitalized children. J. Pediatr. Gastroenterol. Nutr. 63, 708–712 (2016).
Hofsteenge, G. H., Chinapaw, M. J., Delemarre-van de Waal, H. A. & Weijs, P. J. Validation of predictive equations for resting energy expenditure in obese adolescents. Am. J. Clin. Nutr. 91, 1244–1254 (2010).
Smallwood, C. D. & Mehta, N. M. Estimating energy expenditure in critically Ill children: still shooting in the dark?. J. Pediatr. 184, 10–12 (2017).
Bedogni, G. et al. External validation of equations to estimate resting energy expenditure in 2037 children and adolescents with and 389 without obesity: a cross-sectional study. Nutrients 12, 1421 (2020).
Grossi, E. & Buscema, M. Introduction to artificial neural networks. Eur. J. Gastroenterol. Hepatol. 19, 1046–1054 (2007).
De Cosmi, V. et al. Prediction of resting energy expenditure in children: may artificial neural networks improve our accuracy? J. Clin. Med. 9, 1026 (2020).
Disse, E. et al. An artificial neural network to predict resting energy expenditure in obesity. Clin. Nutr. 37, 1661–1669 (2018).
Mehta, N. M., Bechard, L. J., Leavitt, K. & Duggan, C. Cumulative energy imbalance in the pediatric intensive care unit: role of targeted indirect calorimetry. J. Parenter. Enteral. Nutr. 33, 336–344 (2009).
Hirose, H., Takayama, T., Hozawa, S., Hibi, T. & Saito, I. Prediction of metabolic syndrome using artificial neural network system based on clinical data including insulin resistance index and serum adiponectin. Comput. Biol. Med. 41, 1051–1056 (2011).
Van den Berghe, G. Intensive insulin therapy in the ICU-reconciling the evidence. Nat. Rev. Endocrinol. 8, 374–378 (2012).
Zeevi, D. et al. Personalized nutrition by prediction of glycemic responses. Cell 163, 1079–1094 (2015).
Livesey, G., Taylor, R., Hulshof, T. & Howlett, J. Glycemic response and health-a systematic review and meta-analysis: relations between dietary glycemic properties and health outcomes. Am. J. Clin. Nutr. 87, 258S–268S (2008).
Schwingshackl, L. & Hoffmann, G. Long-term effects of low glycemic index/load vs. high glycemic index/load diets on parameters of obesity and obesity-associated risks: a systematic review and meta-analysis. Nutr. Metab. Cardiovasc. Dis. 23, 699–706 (2013).
Vrolix, R. & Mensink, R. P. Variability of the glycemic response to single food products in healthy subjects. Contemp. Clin. Trials 31, 5–11 (2010).
Augustin, L. S. et al. Glycemic index, glycemic load and glycemic response: An International Scientific Consensus Summit from the International Carbohydrate Quality Consortium (ICQC). Nutr. Metab. Cardiovasc. Dis. 25, 795–815 (2015).
Mendes-Soares, H. et al. Model of personalized postprandial glycemic response to food developed for an Israeli cohort predicts responses in Midwestern American individuals. Am. J. Clin. Nutr. 110, 63–75 (2019).
Mendes-Soares, H. et al. Assessment of a personalized approach to predicting postprandial glycemic responses to food among individuals without diabetes. JAMA Netw. Open 2, e188102 (2019).
EFA. Mediterranean diet, an Italian-Israeli research project. https://www.efanews.eu/item/3478-mediterranean-diet-an-italian-israeli-research-project.html (2018).
Zmora, N., Zeevi, D., Korem, T., Segal, E. & Elinav, E. Taking it personally: personalized utilization of the human microbiome in health and disease. Cell Host Microbe 19, 12–20 (2016).
Koleva, P. T., Bridgman, S. L. & Kozyrskyj, A. L. The infant gut microbiome: evidence for obesity risk and dietary intervention. Nutrients 7, 2237–2260 (2015).
Bisgaard, H. et al. Reduced diversity of the intestinal microbiota during infancy is associated with increased risk of allergic disease at school age. J. Allergy Clin. Immunol. 128, 646–652.e641–645 (2011).
de Toro-Martín, J., Arsenault, B. J., Després, J. P. & Vohl, M. C. Precision nutrition: a review of personalized nutritional approaches for the prevention and management of metabolic syndrome. Nutrients 9, 913 (2017).
Del Chierico, F. et al. Gut microbiota markers in obese adolescent and adult patients: age-dependent differential patterns. Front. Microbiol. 9, 1210 (2018).
Bashiardes, S., Abdeen, S. K. & Elinav, E. Personalized nutrition: are we there yet? J. Pediatr. Gastroenterol. Nutr. 69, 633–638 (2019).
Nicholson, J. K., Lindon, J. C. & Holmes, E. ‘Metabonomics’: understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica 29, 1181–1189 (1999).
Koletzko, B. et al. Growth, development and differentiation: a functional food science approach. Br. J. Nutr. 80, S5–S45 (1998).
Arifeen, S. E. et al. Infant growth patterns in the slums of Dhaka in relation to birth weight, intrauterine growth retardation, and prematurity. Am. J. Clin. Nutr. 72, 1010–1017 (2000).
Martinovic, M. et al. Prevalence of and contributing factors for overweight and obesity among Montenegrin schoolchildren. Eur. J. Public Health 25, 833–839 (2015).
Huang, C., Li, Z., Narayan, K. M., Williamson, D. F. & Martorell, R. Bigger babies born to women survivors of the 1959-1961 Chinese famine: a puzzle due to survival selection? J. Dev. Orig. Health Dis. 1, 412–418 (2010).
Lumey, L. H. Decreased birthweights in infants after maternal in utero exposure to the Dutch famine of 1944-1945. Paediatr. Perinat. Epidemiol. 6, 240–253 (1992).
Lumey, L. H., Stein, A. D. & Ravelli, A. C. Timing of prenatal starvation in women and birth weight in their first and second born offspring: the Dutch Famine Birth Cohort Study. Eur. J. Obstet. Gynecol. Reprod. Biol. 61, 23–30 (1995).
Segata, N. Gut microbiome: westernization and the disappearance of intestinal diversity. Curr. Biol. 25, R611–R613 (2015).
Perng, W., Rifas-Shiman, S. L., Hivert, M.-F., Chavarro, J. E. & Oken, E. Branched chain amino acids, androgen hormones, and metabolic risk across early adolescence: a prospective study in Project Viva. Obesity 26, 916–926 (2018).
Perng, W. et al. Metabolomic profiles and development of metabolic risk during the pubertal transition: a prospective study in the ELEMENT Project. Pediatr. Res. 85, 262–268 (2019).
Gardner, C. D. et al. Effect of low-fat vs low-carbohydrate diet on 12-month weight loss in overweight adults and the association with genotype pattern or insulin secretion: the DIETFITS Randomized Clinical Trial. JAMA 319, 667–679 (2018).
Xie, G., Li, X., Li, H. & Jia, W. Toward personalized nutrition: comprehensive phytoprofiling and metabotyping. J. Proteome Res. 12, 1547–1559 (2013).
Havranek, E. P. et al. and on behalf of the American Heart Association Council on Quality of Care and Outcomes Research CoEaP, Council on Cardiovascular and Stroke Nursing, Council on Lifestyle and Cardiometabolic Health, and Stroke Council. Social determinants of risk and outcomes for cardiovascular disease: a scientific statement from the American Heart Association. Circulation 132, 873–898 (2015).
Figueroa, J. F., Frakt, A. B. & Jha, A. K. Addressing social determinants of health: time for a polysocial risk score. JAMA 323, 1553–1554 (2020).
Neeha, V. S. & Kinth, P. Nutrigenomics research: a review. J. Food Sci. Technol. 50, 415–428 (2013).
Hesketh, J. Personalised nutrition: how far has nutrigenomics progressed? Eur. J. Clin. Nutr. 67, 430–435 (2013).
Nielsen, D. E. & El-Sohemy, A. A randomized trial of genetic information for personalized nutrition. Genes Nutr. 7, 559–566 (2012).
Müller, M. & Kersten, S. Nutrigenomics: goals and strategies. Nat. Rev. Genet. 4, 315–322 (2003).
Budge, H. et al. Maternal nutritional programming of fetal adipose tissue development: long-term consequences for later obesity. Birth Defects Res. C 75, 193–199 (2005).
Whitaker, R. C. & Dietz, W. H. Role of the prenatal environment in the development of obesity. J. Pediatr. 132, 768–776 (1998).
Ravelli, A. C. J. et al. Glucose tolerance in adults after prenatal exposure to famine. Lancet 351, 173–177 (1998).
Hinney, A., Vogel, C. I. & Hebebrand, J. From monogenic to polygenic obesity: recent advances. Eur. Child Adolesc. Psychiatry 19, 297–310 (2010).
Acknowledgements
Research during work for this review was supported by a contribution from the Italian Ministry of Health (IRCCS grant).
Author information
Authors and Affiliations
Contributions
G.P.M., V.D.C, A.M., C.A., and M.S. drafted the manuscript, proofread, sorted the references, and made a final revision of the draft. C.A. and M.S. critically reviewed the paper. S.B. reviewed and edited the manuscript. All the authors agreed on the manuscript in its current form. Each author has met the Pediatric Research authorship requirements.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Milani, G.P., Silano, M., Mazzocchi, A. et al. Personalized nutrition approach in pediatrics: a narrative review. Pediatr Res 89, 384–388 (2021). https://doi.org/10.1038/s41390-020-01291-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-01291-8