Abstract
Background
Preterm very low birth weight (VLBW) infants are at risk of gut dysbiosis and neurodevelopmental deficits. Prebiotics and probiotics may modulate gut microbiota and influence brain functions. This review synthesizes literature on effect of prebiotic and/or probiotic supplementation in preterm VLBW on their neurodevelopmental outcomes.
Methods
Search was done using PubMed and CENTRAL. Randomized controlled trials (RCTs) in preterm infants (<37 weeks gestation) and/or infants with birth weight <1500 g that evaluated the effect of prebiotic and/or probiotic supplementation on neurodevelopmental outcomes were included. Weighted mean difference in cognitive and motor scores; pooled relative risks for cognitive and motor impairment, cerebral palsy, hearing, and visual impairment were estimated. Quality of evidence was assessed using the GRADE criteria.
Results
Out of 275 articles identified, seven were included for review. All, except one, were done in preterms <33 weeks of gestation. Age of assessment of outcomes was ≥18–22 months of corrected age in five studies. Interventions did not decrease or increase the risk of cognitive and motor impairment, cerebral palsy, visual, and hearing impairment. Quality of evidence was “low” to “very low.”
Conclusions
Limited evidence from RCTs does not demonstrate a difference in neurodevelopmental outcomes between prebiotic/probiotic treated and untreated control groups.
Similar content being viewed by others
Introduction
Recent preclinical studies reveal the complex nature of the gut–brain relationship and suggests that gut microbiota has an important role in the bidirectional communications between the gut and the central nervous system.1,2 These interactions not only affect gut homeostasis but also influence higher cognitive functions, emotional behavior, and response to stress and anxiety.3,4,5 Although trials involving human subjects are limited, emerging evidence suggests that gut microbiota influence the response to stress, occurrence of depressive symptoms, processing of emotions, and immune regulation.6,7,8
Probiotics are defined as “living organisms” that confer health benefits on the host.9 They are proposed to work through manipulating intestinal microbial communities by production of anti-microbial agents/metabolic compounds or competing for receptors/binding sites with other intestinal microbes on the intestinal mucosa; through immunomodulation; stimulation of epithelial cell proliferation and differentiation; and strengthening the integrity of intestinal barrier.10 Prebiotics are defined as non-viable substrates that stimulate certain bacterial taxa or bacterial activities within gut microbiome. These include certain non-digestible oligosaccharides, soluble fermentable fibers, and human milk oligosaccharides.11,12
Probiotics as well as prebiotics are being thought to bestow mental health benefits through interaction with the gut microbiota and thereby influencing the gut–brain axis. Probiotics, particularly having bifidobacteria and lactobacilli strains, have been shown to have anxiolytic and pro-cognitive effects in both rodents and humans.13,14 Prebiotics are thought to stimulate beneficial bacteria such as bifidobacteria and lactobacilli and are considered useful in diminishing anxiety-like behavior and improve cognition in animal models.15 In human adult subjects, prebiotic supplementation led to suppression of the neuroendocrine stress response and an increase in the processing of positive versus negative attentional vigilance, suggestive of anxiolytic-like profile.16 However, these are preliminary findings and more research is needed to understand the effects of prebiotic and probiotic administration of all aspects of brain function. It is important to acknowledge that research involving young infants and children to elucidate the effect of prebiotics and probiotics on mental health outcomes is limited probably due to difficulties in ascertaining mental health outcomes such as depression, anxiety, and emotional regulation.
Globally available literature has established that preterm infants and those with very low birth weight are at a risk of neurodevelopmental impairments, and, therefore, identification of interventions that could enhance early child development in this vulnerable subset of infants is necessary.17,18 Premature infants are at a higher risk of dysbiosis of gut microbiome and necrotizing enterocolitis (NEC).19,20 Oral administration of probiotics has been shown to be effective in reducing the incidence of NEC and late-onset sepsis in preterm-born infants.21,22 This could possibly lead to improved gastrointestinal function and less feeding intolerance. This, in turn, could result in improved nutrition and growth as well as neurodevelopmental outcomes. Probiotics as well as prebiotics may also modulate the gut microbiota and consequently influence brain functions through gut–brain axis. However, there is another aspect to probiotic supplementation that should be noted and could be of potential concern. Recent studies have put forward the possibility that probiotic supplementation may trigger pro-inflammatory responses in children.23,24,25 Accumulating evidence also suggests an inverse relationship between elevated concentration of inflammatory markers and cognitive ability in children.26,27 Therefore, it is possible that while probiotic supplementation seems to have the potential to improve long-term neurodevelopmental outcomes in preterm infants, it may also have a negative effect.
Our current understanding of the effect of prebiotic and probiotic supplementation in preterm infants on their neurodevelopment is sketchy and a systematic synthesis of available literature from randomized controlled trials could give a better insight. With this rationale, the current meta-analysis was conducted with the aim to document the effect of supplementation with prebiotic and/or probiotic in preterm infants on their neurodevelopmental outcomes. The primary outcomes of interest were cognitive and motor performance, while the secondary outcomes considered were “any” neurological impairment including visual and auditory impairment and cerebral palsy.
Methods
Search strategy and selection criteria
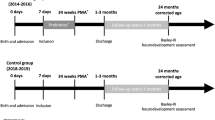
A systematic search was performed by two of the authors independently (R.P.U., R.C.) using PubMed and CENTRAL (the Cochrane Central Register of Controlled Trials, The Cochrane Library). Any discrepancy was discussed with a third author (S.T.). Search strategies used subject headings and key words with no language and time restrictions. The search strategy is presented in Supplemental Box 1 (online). There was no restriction on the initial search date. The last date of article search was 31 July 2018. To be included, the study had to be a randomized controlled trial in preterm infants (<37 weeks gestation and/or birth weight <1500 g) with intervention being probiotic and/or prebiotic supplementation and had neurodevelopmental outcomes assessed at any point of time after supplementation. After initial screening of titles and abstracts, full-text publications of possible studies were reviewed separately by two authors (R.P.U., R.C.). Discrepancies about inclusion of studies and interpretation of data were resolved by discussion with senior co-authors (S.T., T.A.S.). Manual review of reference list of articles that fulfilled our eligibility criteria was also done. The bibliographies of relevant reviews and reports were read to identify relevant primary reports. Online theses/dissertation repositories: EthOS British Library, Open Access Theses and Dissertations, Networked Digital Library of Theses and Dissertations (NDLTD), ProQuest Dissertation and Theses Global, and Open Thesis were also searched to identify unpublished literature.28,29,30,31,32 For studies with data missing or requiring clarification, we planned to contact the study investigators. PRISMA checklist was used for reporting of relevant items for this meta-analysis.33
Data analysis
Data from all studies meeting the inclusion criteria were abstracted into a tabular form. The data extraction sheet included information on first author, year of publication, study setting, inclusion criteria, details of intervention including type of probiotic, dosage and duration of intervention, sample size, age at assessment, tools used for assessment, and key findings on outcomes of interest. Two authors (R.C., R.P.U.) independently assessed risk of bias in each trial by using the Cochrane “Risk of Bias Assessment Tool.”34 Risk of bias were assessed under following domains: allocation sequence, allocation concealment, blinding of participants and outcome assessors, incomplete outcome data, selective outcome reporting, and other potential sources of bias. Potential discrepancies during the data extraction process and assessment of risk of bias were resolved by discussion and consensus among all authors.
All analyses were done using STATA 14 (Stata, College Station, TX, USA). We stratified the analysis by whether the intervention was probiotic or probiotic. The aim was to understand the respective effects of these broadly different sets of interventions on neurodevelopmental outcomes. Combining the two interventions was considered not relevant as these differ in their metabolic and physiologic effects. Considering that the risk of poor neurodevelopmental outcomes is more in those who are very preterm (28 to 32 weeks of gestation) and extremely preterm (<28 weeks of gestation) compared to moderate to late preterms (>32 to <37 weeks of gestation), we performed a sensitivity analysis after excluding studies where moderate to late preterms were also a part of the study. We also excluded studies in the sensitivity analysis where the age of assessment was ≤12 months of corrected age as outcomes assessed at this age are thought to have low reliability. We also did a subgroup analysis based on the number of probiotic strains used in the formulation (1 strain vs. >1 strain) and timing of start of probiotic supplementation after birth (within 7 days; >7 days). We could not perform the subgroup analysis based on the duration of intervention as all the included studies had similar mean duration of supplementation (i.e., 1 to 1.5 month). Test of interaction, using inverse variance method, was done to investigate whether the effect of probiotic supplementation on the outcomes varied between the subgroups.35
Effect sizes were reported as weighted mean differences (WMDs) for continuous and pooled relative risks (RRs) for categorical outcomes. All pooled estimates were reported with 95% confidence intervals (CIs). In instances where pooled RRs were required to be generated, any study where the number of events either in the intervention or a control arm was zero was excluded from the analysis.35 Heterogeneity of effects was assessed and quantified by the I2. I2 value >50% was considered to represent substantial heterogeneity.34 In cases with substantial heterogeneity, random effects model was proposed to be used. Also, meta-regression was planned to be undertaken for outcomes where heterogeneity was substantial. A P value of <0.05 was considered statistically significant for all the analyses undertaken. For each of the outcomes, publication bias was assessed using Egger’s test and visually inspected using contour-enhanced funnel plots. We plotted 1, 5, and 10% significance contours using confunnel command in STATA. Quality of the evidence generated was assessed using the GRADE criteria and categorized as “High,” “Moderate,” “Low,” or “Very low.”36,37
Results
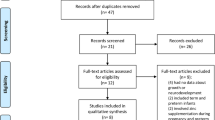
Figure 1 shows the flowchart for article selection. We obtained a total of 275 articles through electronic search (PubMed and CENTRAL). After excluding 133 duplicates, we screened title of 142 articles. Out of these, 87 were excluded based on titles and another 37 after reviewing the abstracts that appeared relevant during title screening. We assessed 18 full-text articles for eligibility and found 7 articles to be relevant for the review.38,39,40,41,42,43,44 No additional study for inclusion in the meta-analysis was identified through supplementary search strategies, that is, manual review of reference lists of included studies, bibliography of reviews and reports, and online theses/dissertation repositories. The details of the included studies have been provided in Table 1. All seven studies were done in high-income and upper-middle-income countries, classified according to World Bank classification.45 Two studies were part of the same intervention trial but assessed outcomes at different points of time, that is, at 12 and 24 months of corrected age through different assessment tools.41,42 In three studies, single probiotic strain was used,39,40,43 in two studies >1 probiotic strains were used,38,44 and in two studies (both part of the same intervention trial), a prebiotic mixture consisting of daily enteral acidic (pAOS) and neutral oligosaccharides (scGOS/IcFOS) was used.41,42 In all the studies, except one, preterms with gestational age <33 weeks were the study subjects. In the study by Romeo et al.,39 infants <37 weeks of gestation were included in the study. Five out of the total of seven studies assessed outcomes between 18 months to 5 years of age.38,40,42,43,44 The other two studies assessed outcomes at 12 months of age.39,41 Three out of a total of five studies that assessed cognitive impairment used a cut-off score of <70 on Bayley Scales of Infant Development (BSID).38,40,43 The other two studies used a cut-off score of ≤85 and <77.5 on BSID.42,44 Out of the six studies that assessed motor impairment, three used a cut-off of <70;38,40,43 one used ≤85 as the cut-off, while the other used <77.5 as the cut-off on BSID.42,44 The remaining study used Alberta Infant Motor Scale (AIMS) to assess motor functioning.41 It is worth mentioning that all the findings related to the pooling of studies pertain to probiotic supplementation, unless stated otherwise. Limited number of studies on prebiotic supplementation prevented generating pooled estimates, and, therefore, we decided to only provide a careful narrative summary of the key findings.
Effect of probiotic supplementation on cognitive outcome
The pooled mean cognitive score in children in the intervention group was 90.01 (95% CI: 80.36, 99.67) (n = 700) and in the control group was 90.05 (95% CI: 81.46, 98.64) (n = 688). Probiotic administration did not significantly affect the mean mental score (WMD 0.20; 95% CI: −1.42, 1.83) (n = 1388, I2 = 0.0%, P value for overall effect = 0.81, low-quality evidence) (Fig. 2). Supplementation with probiotic did not affect the risk of cognitive impairment (RR 0.98; 95% CI: 0.75, 1.27) (n = 1388, I2 = 0.0%, P value for overall effect = 0.86, low-quality evidence) (Fig. 3).
Effect of probiotic supplementation on motor outcome
The pooled mean motor score of children in intervention group was 91.03 (95% CI: 78.50, 103.55) (n = 700) and in the control group was 90.19 (95% CI: 78.97, 101.41) (n = 688). The difference in the mean motor scores across the intervention and control group (WMD 1.04; 95% CI: −0.43, 2.50) (n = 1388, I2 = 0.0%, P value for overall effect = 0.16, low-quality evidence) did not reach statistical significance (Fig. 2). Intervention did not affect the risk of motor impairment (RR 1.06; 95% CI: 0.79, 1.42) (n = 1388, I2 = 0.0%, P value for overall effect = 0.69, low-quality evidence) (Fig. 3).
Effect on probiotic supplementation on risk of NDI, cerebral palsy, and hearing and visual impairment
Probiotic administration did not affect the risk of neurodevelopment impairment (NDI) (RR 0.90; 95% CI: 0.75, 1.09) (n = 1637, I2 = 9.9%, P value for overall effect = 0.28, low-quality evidence) (Fig. 4). There was no substantial change in the pooled effect size for NDI (RR 0.97; 95% CI: 0.80, 1.19) (n = 1388, I2 = 0.0%, P value for overall effect = 0.77) in the sensitivity analysis wherein the study by Romeo et al.39 was excluded on account of the age of assessment being 12 months of corrected age and also because the study population comprised of moderate to late preterms. Probiotic administration also did not affect the risk of cerebral palsy (RR 1.33; 95% CI: 0.84, 2.11) (n = 1388, I2 = 0.0%, P value for overall effect = 0.22, very-low-quality evidence) and visual impairment (RR 0.34; 95% CI: 0.07. 1.64) (n = 475, I2 = 0.0%, P value for overall effect = 0.18; very-low-quality evidence) (Fig. 4). Effect on hearing impairment (RR 0.38; 95% CI: 0.14, 1.07) (n = 1139, I2 = 37.5%, P value for overall effect = 0.07; very-low-quality evidence) approached statistical significance and the pooled estimate was largely influenced by the study by Jacob et al.,44 who showed a significant reduction in risk of hearing impairment upon probiotic supplementation.
Findings of the subgroup analysis
Subgroup analysis was done based on the number of probiotic strains supplemented and the timing of the start of supplementation from birth. Findings are presented in Supplemental Table S1 (online). The test for interaction showed that the effect of probiotic supplementation on all the neurodevelopmental outcomes did not vary significantly between the subgroups. We found, however, that infants who started on probiotics within a week of birth had lower risk of hearing impairment compared to the control infants (RR 0.25; 95% CI: 0.07, 0.88) (n = 838; I2 = 15.2%; P value for overall effect = 0.03). In spite of not reaching statistical significance, we also show that infants who were started on probiotic within a week of birth and those who were supplemented with more than one probiotic strains had higher mean motor score (WMD 1.42; 95% CI: −0.15, 3.00) (n = 1087; I2 = 0.0%; P value for overall effect = 0.07) and lesser risk of hearing impairment (RR 0.33; 95% CI: 0.11, 1.02) (n = 965; I2 = 62.8%; P value for overall effect = 0.05), respectively, compared to the control group infants.
Effect of prebiotic supplementation on neurodevelopmental outcomes
Two studies (LeCouffe et al.41 and van den Berg et al.42) reported the effect of prebiotic supplementation on neurodevelopmental outcomes (Table 1). The study by LeCouffe et al.41 was nested within the study by van den Berg et al.,42 and these studies differed in the age and method of assessments. Very preterm infants with gestational age <32 weeks and/or birth weight <1500 g, admitted to the level III neonatal intensive care unit formed the study population. The intervention was provision of daily enteral 80% neutral oligosaccharides scGOS/lcFOS and 20% acidic pAOS added to breast milk of preterm formula from days 3 to 30 after birth in a dose of maximal 1.5 g/kg per day (Table 1). The control group infants received placebo powder (maltodextrin) in breast milk or preterm formula. The study by LeCouffe et al.41 performed motor and neurological assessments at 12 months of corrected age and found no significant differences among the intervention and control group infants. van den Berg et al.42 assessed preterm infants at 24 months of corrected age using Bayley Scales of Infant and Toddler Development—2nd and 3rd edition. This study reported statistically significant decrease in mean cognitive scores among intervention group children (WMD −5.00; 95% CI: −9.81, −0.19) (n = 76; P value for overall effect = 0.042) compared to those in the control group. No statistically significant differences in the mean motor scores was observed (WMD 3.00; 95% CI: −4.58, 10.58) (n = 68; P value for overall effect = 0.438). No significant differences in proportion of participants with mental and motor impairment (development index score of ≤85) and cerebral palsy were noted (Table 1).
Publication bias and findings of GRADE assessment
There was no evidence of publication bias for all the outcomes considered in the meta-analysis (Supplemental Figure S1; A-H (online)). For all the outcomes, degree of heterogeneity was not substantial (I2 < 50%), and, therefore, meta-regression was not performed. On risk of bias assessment, all seven studies reportedly adopted random sequence generation (Supplemental Table S2 (online)). All studies, except the one by Romeo et al.,39 reported having followed allocation concealment, ensured appropriate blinding of participants and the intervention delivery team, as well as those that assessed outcome. Attrition bias was assessed to be absent in all the included studies. Additional major bias that could have potentially affected the findings was judged to be absent in the included studies (Supplemental Table S2 (online)). The quality of evidence using GRADE was judged as “low” for the cognitive and motor outcomes and for risk of neurodevelopmental impairment (Table 2). For outcomes related to the risk of cerebral palsy, hearing, and visual impairment, the quality of evidence was judged as “very low.” The explanations for downgrading the quality of evidence are mentioned as footnote of Table 2.
Discussion
The current meta-analysis shows that probiotics were neither harmful nor advantageous with respect to neurodevelopmental outcomes. The available evidence is of low to very low quality, mostly because the included studies had low sample size that was not powered to capture the small differences in neurodevelopmental outcomes. There were no differences in risk of cognitive, motor, and visual impairments, as well as cerebral palsy among the intervention and control groups. However, we found that the risk of hearing impairment was reduced but the quality of evidence is very low. The subgroup analyses suggest that early supplementation, that is, within 7 days of birth and using formulation with more than one strain might have some advantage in reducing the risk of hearing impairment. Similarly, supplementation within 7 days of birth may lead to an improvement in mean motor scores. It is possible that these are chance findings and until they are replicated in future studies; interpretation must be done with caution.
Previous studies have documented that the risk of poor cognitive and psychomotor development is higher in preterm infants with NEC and late-onset sepsis, compared to those who did not have these conditions.46,47,48 It is surprising to note that even when the role of probiotics in reducing the incidence of NEC and late-onset sepsis is well established,21,22 the findings of the meta-analysis did not suggest any significant benefit of probiotic supplementation on neurodevelopmental outcomes. Therefore, certainly more methodologically robust randomized controlled trials are needed to explore long-lasting effects of probiotics on brain functions. In the current meta-analysis, almost all the studies measured neurodevelopment outcomes as a secondary objective and were accordingly not optimally designed and powered to detect differences in neurodevelopmental outcomes. The duration of the intervention could also have a bearing on the observed effect. In all the included trials, probiotics were given only for first 1 to 1.5 months of life. It could be possible that introducing an effect on neurodevelopmental outcomes may have required longer periods of intervention. Another possible hypothesis for the observed lack of effect could be the probiotic strains used. Effects of probiotics are known to be strain and dose specific.49 It could be possible that lack of influence on neurodevelopmental outcomes is because the most appropriate probiotic strain(s) or optimal dose required for modulation of the brain–gut–microbiome axis was not used in the included studies. In the included studies, probiotics were given few days after birth, depending upon the time when the infant started to take enteral feeds. Most serious complications such as germinal matrix intraventricular hemorrhage and periventricular leukomalacia, and even the inflammatory processes that affect the neonatal brain would have occurred before this critical period of first few days after birth.50,51 This in part might explain the lack of observed benefits.
There are various unaddressed issues that limit our understanding of the effects of probiotics on neurodevelopment and future trials should aim to fill these gaps in knowledge. First, there is a need to identify potential probiotic strains for the effective gut–brain axis modulation. Second, there is an issue of optimal dosage for producing ascertainable benefits. Studies looking at the effect of probiotic preparations on gastrointestinal diseases such as acute onset infectious diarrhea, antibiotic associated diarrhea, inflammatory bowel diseases, and irritable bowel syndrome suggest that a dose in the range of 107–109 colony-forming units (CFUs) per day seems effective.52,53 No data exist for the optimal dosage required for prevention and/or management of neurobehavioural disorders, particularly in infants and children. However, it is important to acknowledge that before an optimal dosage is discovered, it will be essential to prove that probiotics are effective for improving neurodevelopment. Third, there is not enough understanding about the appropriate duration of administration to discern a significant effect. Majority of the probiotic strains do not colonize the gut and are no longer recoverable from stools 1–4 weeks after stopping the consumption.54 They exert their effect during the time they are present in the gut. The effect of longer duration of probiotic consumption on neurodevelopment in preterm infants is something that is worth testing. However, we need to be cognizant of the potential side effects that may occur due to longer administration, considering recent emerging evidences on probable increased risk of inflammatory and allergic conditions after probiotic supplementation in children.23,24,25
There are certain limitations of this analysis which should be considered while interpreting the findings. First, the method of assessment of neurodevelopmental outcomes and age of assessments varied across the included studies which could influence the validity of the pooled estimates. Second, we could not generate pooled estimates for effect of prebiotic supplementation as there were only two studies which were related. Third, the trials included in the meta-analysis tested the effect of intervention on neurodevelopmental outcomes as a secondary objective and therefore not powered to detect statistically significant differences. Fourth, the number of trials and of participants in the meta-analytic sample may be too small for subgroup analysis to have adequate statistical power, whether to demonstrate significance of intervention effect or heterogeneity. Fifth, the risk of neurodevelopmental impairment is documented to be more in preterms <32 weeks of gestation and in those with birth weight <1500 g, more specifically in those <1000 g. While six out of seven included studies were done in preterms <33 weeks of gestation and/or birth weight <1500 g, there were no studies with participants specifically having birth weight of <1000 g. We were therefore unable to document the synthesized effect of the intervention on this vulnerable subset of infants. Lastly, we did not have access to EMBASE and CINAHL, and therefore limited our search to PubMed and CENTRAL.
Conclusion
Prebiotics and probiotics, because of their suggested role in modulating gut–brain axis, are promising candidates to be tested for their impact on long-term neurodevelopmental outcomes in preterm infants. The findings of this meta-analysis refer to pooled results of preterm very low birth weight children receiving “any” probiotic strain against those that never received any strain. The findings were equivocal and did not show either an increased or a reduced risk for cognitive, motor and other neurodevelopmental impairments in preterm infants for probiotic supplementation. The available trials are more empirical in nature and the quality of synthesized evidence generated is of “low to very low quality”, as per GRADE criteria. We could not generate pooled estimates for neurodevelopmental outcomes in relation to prebiotic supplementation because of the limited availability of studies. In nutshell, there is need for methodologically robust trials that should aim to test the effect of prebiotic and probiotic supplementation in preterm very low birth weights on their long-term neurodevelopment.
References
Carabotti, M. et al. The gut–brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 28, 203–209 (2015).
Mayer, E. A. et al. Gut microbes and the brain: paradigm shift in neuroscience. J. Neurosci. 34, 15490–15496 (2014).
Tognini, P. Gut microbiota: a potential regulator of neurodevelopment. Front. Cell Neurosci. 11, 25 (2017).
Cryan, J. F. & Dinan, T. G. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 13, 701–712 (2012).
Foster, J. A., Rinaman, L. & Cryan, J. F. Stress & the gut–brain axis: regulation by the microbiome. Neurobiol. Stress 7, 124–136 (2017).
Huang, R., Wang, K. & Hu, J. Effect of probiotics on depression: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 8 (2016). pii: E483. https://doi.org/10.3390/nu8080483
Lima-Ojeda, J. M., Rupprecht, R. & Baghai, T. C. “I Am I And My Bacterial Circumstances”: linking gut microbiome, neurodevelopment, and depression. Front. Psychiatry 8, 153 (2017).
Round, J. L. & Mazmanian, S. K. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 9, 313–323 (2009).
Joint FAO/WHO expert consultation on evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Córdoba, Argentina Oct. 2001. Available at: http://www.fao.org/3/a-a0512e.pdf
Bermudez-Brito, M. et al. Probiotic mechanisms of action. Ann. Nutr. Metab. 61, 160–174 (2012).
Gibson, G. R. et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 14, 491–502 (2017).
Holscher, H. D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 8, 172–184 (2017).
Allen, A. P. et al. Bifidobacterium longum 1714 as a translational psychobiotic: modulation of stress, electrophysiology and neurocognition in healthy volunteers. Transl. Psychiatry 6, e939 (2016).
Messaoudi, M. et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br. J. Nutr. 105, 755–764 (2011).
Savignac, H. M. et al. Prebiotic feeding elevates central brain derived neurotrophic factor, N-methyl-d-aspartate receptor subunits and d-serine. Neurochem. Int. 63, 756–764 (2013).
Schmidt, K. et al. Prebiotic intake reduces the waking cortisol response and alters emotional bias in healthy volunteers. Psychopharmacology (Berl.) 232, 1793–1801 (2015).
Allotey, J. et al. Cognitive, motor, behavioural and academic performances of children born preterm: a meta-analysis and systematic review involving 64 061 children. BJOG 125, 16–25 (2018).
Pascal, A. et al. Neurodevelopmental outcome in very preterm and very-low-birthweight infants born over the past decade: a meta-analytic review. Dev. Med. Child Neurol. https://doi.org/10.1111/dmcn.13675 (2018).
Gritz, E. C. & Bhandari, V. The human neonatal gut microbiome: a brief review. Front. Pediatr. 3, 17 (2015).
Groer, M. W. et al. Development of the preterm infant gut microbiome: a research priority. Microbiome 2, 38 (2014).
Rao, S. C. et al. Probiotic supplementation and late-onset sepsis in preterm infants: a meta-analysis. Pediatrics 137, e20153684 (2016).
Alfaleh, K., Anabrees, J. & Bassler, D. Probiotics reduce the risk of necrotizing enterocolitis in preterm infants: a meta-analysis. Neonatology 97, 93–99 (2010).
Kopp, M. V. et al. Randomized, double-blind, placebo-controlled trial of probiotics for primary prevention: no clinical effects of Lactobacillus GG supplementation. Pediatrics 121, e850–e856 (2008).
Taylor, A. L., Dunstan, J. A. & Prescott, S. L. Probiotic supplementation for the first 6 months of life fails to reduce the risk of atopic dermatitis and increases the risk of allergen sensitization in high-risk children: a randomized controlled trial. J. Allergy Clin. Immunol. 119, 184–191 (2007).
Kalliomäki, M. et al. Probiotics during the first 7 years of life: a cumulative risk reduction of eczema in a randomized, placebo-controlled trial. J. Allergy Clin. Immunol. 119, 1019–1021 (2007).
Lee, S. E. et al. General intelligence is associated with subclinical inflammation in Nepalese children: a population-based plasma proteomics study. Brain Behav. Immun. 56, 253–263 (2016).
Mackinnon, N. et al. Association between childhood infection, serum inflammatory markers and intelligence: findings from a population-based prospective birth cohort study. Epidemiol. Infect. 146, 256–264 (2018).
British Library. EthOS: E-theses Online Service. http://ethos.bl.uk/Home.do. Accessed 15 Jul 2018.
Open Access Theses and Dissertations. https://oatd.org/. Accessed 15 Jul 2018.
Networked Digital Library of Theses and Dissertations (NDLTD). http://www.ndltd.org/resources. Accessed 18 Jul 2018.
ProQuest Dissertation and Theses Global. http://www.proquest.com/products-services/pqdtglobal.html. Accessed 20 Jul 2018.
Open Thesis. http://www.openthesis.org/. Accessed 21 Jul 2018.
PRISMA: Transparent reporting of systematic reviews and meta-analysis. http://www.prisma-statement.org/
Higgins, J. P. T. & Green, S. (eds). Cochrane Handbook for Systematic Review of Interventions. Version 5.1.0, updated March 2011. http://handbook-5-1.cochrane.org/
Harris, R. J. et al. Metan: fixed and random effects meta-analysis. Stata J. 8, 3–28 (2008).
Atkins, D. et al. Grading quality of evidence and strength of recommendations. BMJ 328, 1490 (2004).
Guyatt, G. H. et al. GRADE guidelines 6. Rating the quality of evidence—imprecision. J. Clin. Epidemiol. 64, 1283–1293 (2011).
Chou, I. C. et al. Lack of effects of oral probiotics on growth and neurodevelopmental outcomes in preterm very low birth weight infants. J. Pediatr. 156, 393–396 (2010).
Romeo, M. G. et al. Role of probiotics in the prevention of the enteric colonization by Candida in preterm newborns: incidence of late-onset sepsis and neurological outcome. J. Perinatol. 31, 63–69 (2011).
Sari, F. N. et al. Do oral probiotics affect growth and neurodevelopmental outcomes in very low-birth-weight preterm infants? Am. J. Perinatol. 29, 579–586 (2012).
LeCouffe, N. E. et al. Neurodevelopmental outcome during the first year of life in preterm infants after supplementation of a prebiotic mixture in the neonatal period: a follow-up study. Neuropediatrics 45, 22–29 (2014).
van den Berg, J. P. et al. Neurodevelopment of preterm infants at 24 months after neonatal supplementation of a prebiotic mix: a Randomized Trial. J. Pediatr. Gastroenterol. Nutr. 63, 270–276 (2016).
Akar, M. et al. Impact of oral probiotics on neurodevelopmental outcomes in preterm infants. J. Matern. Fetal Neonatal Med. 30, 411–415 (2017).
Jacobs, S. E. et al. Probiotics, prematurity and neurodevelopment: follow-up of a randomised trial. BMJ Paediatr. Open 1, e000176 (2017).
World Bank Country and Lending Groups. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups
Martin, C. R. et al. Neurodevelopment of extremely preterm infants who had necrotizing enterocolitis with or without late bacteremia. J. Pediatr. 157, 751–6.e1 (2010).
Hentges, C. R. et al. Association of late-onset neonatal sepsis with late neurodevelopment in the first two years of life of preterm infants with very low birth weight. J. Pediatr. (Rio J.) 90, 50–57 (2014).
Mitha, A. et al. Neonatal infection and 5-year neurodevelopmental outcome of very preterm infants. Pediatrics 132, e372–e380 (2013).
Shanahan, F. Molecular mechanisms of probiotic action: it’s all in the strains! Gut 60, 1026–1027 (2011).
Itakura, A. et al. Timing of periventricular leukomalacia using neonatal electroencephalography. Int. J. Gynaecol. Obstet. 55, 111–115 (1996).
Al-Abdi, S. Y. & Al-Aamri, M. A. A systematic review and meta-analysis of the timing of early intraventricular hemorrhage in preterm neonates: clinical and research implications. J. Clin. Neonatol. 3, 76–88 (2014).
Minelli, E. B. & Benini, A. Relationship between number of bacteria and their probiotic effects. Microb. Ecol. Health Dis. 20, 180–183 (2008).
Ritchie, M. L. & Romanuk, T. N. A meta-analysis of probiotic efficacy for gastrointestinal diseases. PLoS ONE 7, e34938 (2012).
Sanders, M. E. Impact of probiotics on colonizing microbiota of the gut. J. Clin. Gastroenterol. 45(Suppl.), S115–S119 (2011).
Acknowledgements
The study was funded by Knowledge Integration and Technology Platform (KnIT), a Grand Challenges Initiative of the Department of Biotechnology and Biotechnology Industry Research Assistance Council (BIRAC) of Government of India and Bill & Melinda Gates Foundation.
Author contributions
R.P.U. conceptualized the idea, designed the search strategy, identified eligible studies, performed data extraction and quality assessment of included studies, conducted the statistical analyses, and GRADE assessments, as well as prepared the first draft. S.T. conceptualized the idea, reviewed the search strategy, supervised statistical analysis and provided inputs in the preparation of the manuscript. R.C. was involved in identifying eligible studies, assisted in data abstraction, and in quality assessment of the included studies. T.A.S. and N.B. were involved in providing technical inputs in the preparation of the manuscript and also provided overall supervision in the conduct of the meta-analysis. All authors (R.P.U., S.T., R.C., T.A.S., N.B.) approved the final version of the manuscript for submission.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Data sharing statement:
All the data used in the analysis have been provided in the main manuscript and in the supplementary files. For additional queries, communication can be directed to the corresponding author.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Upadhyay, R.P., Taneja, S., Chowdhury, R. et al. Effect of prebiotic and probiotic supplementation on neurodevelopment in preterm very low birth weight infants: findings from a meta-analysis. Pediatr Res 87, 811–822 (2020). https://doi.org/10.1038/s41390-018-0211-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-018-0211-9
This article is cited by
-
Growth and neuro-developmental outcomes of probiotic supplemented preterm infants—a systematic review and meta-analysis
European Journal of Clinical Nutrition (2023)
-
Probiotic sepsis in preterm neonates—a systematic review
European Journal of Pediatrics (2022)