Abstract
Breast Cancer (BC) is the most common form of cancer worldwide, responsible for 25% of cancers in women. Whilst treatment is effective and often curative in early BC, metastatic disease is incurable, highlighting the need for early detection. Currently, early detection relies on invasive procedures, however recent studies have shown extracellular vesicles (EVs) obtained from liquid biopsies may have clinical utility. EVs transport diverse bioactive cargos throughout the body, play major roles in intercellular communication and, importantly, mirror their cell of origin. In cancer cells, EVs alter the behaviour of the tumour microenvironment (TME), forming a bridge of communication between cancerous and non-cancerous cells to alter all aspects of cancer progression, including the formation of a pre-metastatic niche. Through gene regulatory frameworks, non-coding RNAs (ncRNAs) modulate vital molecular and cellular processes and can act as both tumour suppressors and oncogenic drivers in various cancer types. EVs transport and protect ncRNAs, facilitating their use clinically as liquid biopsies for early BC detection. This review summarises current research surrounding ncRNAs and EVs within BC, focusing on their roles in cancer progression through bi-directional communication with the microenvironment and their diagnostic implications.

The role of EV ncRNAs in breast cancer. A representation of the different EV ncRNAs involved in tumourigenic processes in breast cancer. Pro-tumourigenic ncRNAs displayed in green and ncRNAs which inhibit oncogenic processes are shown in red.
Similar content being viewed by others
Introduction
Accounting for 11.7% of cancer diagnoses and 6.9% of cancer deaths, breast cancer (BC) is the most common cancer worldwide, with 1 in 8 women developing BC in their lifetime [1]. Differences in molecular features and expression identifies 4 subtypes of BC: luminal A, luminal B, human epidermal growth factor receptor 2 (HER2)-positive, and triple-negative (TNBC) [2]. Defining BC subtype allows tailoring of treatment to the patient, increasing the chances of tumour eradication, and preventing recurrence or therapy resistance. Unfortunately, metastatic BC remains incurable, and treatment aims to relieve symptoms and prolong survival [3]. Many high-income countries have established screening programmes involving mammograms, ultrasounds, and biopsies to identify early BC, increasing the chances of successful treatment [4]. Unfortunately, these tests can be time consuming, unpleasant, and invasive, discouraging individuals from undergoing screening. It is therefore vital to develop new methods that are less invasive, fast, and accurate to improve the early diagnosis of BC.
Extracellular vesicles
Extracellular vesicles (EVs) are small, non-replicative, lipid bilayer-delimited particles, released by nearly all cell types in every organism [5]. EVs contain bioactive cargo, including lipids, metabolites, nucleic acids, and proteins, and can be categorised based on their biogenesis. Exosomes (typically 30–150 nm) are produced through the endosomal pathway during maturation of early endosomes to late endosomes/multivesicular bodies (MVBs) where the inward budding of the MVB membrane produces intraluminal vesicles (ILVs). ILVs are then released as exosomes when MVBs fuse with the plasma membrane [6]. Microvesicles (~50–1000 nm) are produced when the plasma membrane undergoes outwards budding. Other EV subtypes include apoptotic bodies, an important player in apoptosis [7], and oncosomes which are secreted by cancer cells to aid in tumour growth and the development of the tumour microenvironment (TME) [5].
EVs can alter the behaviour of surrounding cells, which is particularly important in cancer as cancer cells use EVs to change the phenotype of surrounding cells in the TME, promoting growth, metastasis, and therapy resistance. EVs have been proposed as a diagnostic tool as they can shield their cargo from nucleases and proteases in biological fluids and can reflect the phenotype of their cell of origin. Importantly, EVs are found in almost all biological fluids, allowing for easy and non-invasive collection [5].
Non-coding RNAs
Traditionally, RNAs were thought primarily to enable transfer of instructions from DNA to ribosomes to produce proteins. However, recently, many new types of RNA have been discovered and categorised into different classes depending on size and function. MicroRNAs (miRNAs) are approximately 22 nucleotides (nt) in length and are loaded into the RNA-induced silencing complex (RISC) to induce translational repression and/or degradation of target RNAs containing complementary sequences. PIWI-interacting RNAs (piRNAs) are 24–31 nt long and bind to the PIWI family of proteins, allowing epigenetic regulation of chromatin through transposon silencing [8]. Long non-coding RNAs (lncRNAs) are over 200 nt and regulate transcription, nuclear organisation, proteins, and can act as miRNA decoys to regulate gene expression, whereas circular RNAs (circRNAs) are closed loops of RNA with roles in gene expression regulation [9, 10]. Each of these non-coding RNA (ncRNA) classes can work as oncogenes or tumour suppressors, contributing to regulation of cancers, including BC [11].
Regulation of ncRNA sorting into EVs
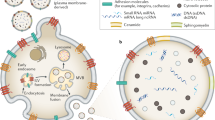
Concise regulation of the ncRNA composition of EVs is critical to maintain homoeostasis, drive angiogenesis, and aid in the response to external stimuli including cellular stress. The ncRNA composition of EVs is not simply a reflection of cellular composition and therefore, there is active regulatory processing governing the loading of ncRNAs into EVs (Fig. 1).
An array of molecular mechanisms are implicated in the regulation of loading a diverse repertoire of ncRNAs, including lncRNAs, miRNAs, circRNAs and tRNA into extracellular vesicles. These appear to involve crucial roles for RNA binding proteins (RBPs) such as hnRNP family members, HuR and YBX1 recognising specific motifs in target RNAs. Loading of ncRNAs into these structures is a tightly regulated process with the RBPs involved controlled by specific cellular conditions, including cellular stress, providing a dynamic system to regulate cell-cell communication under different environmental conditions. ncRNAs themselves have also been suggested to directly influence extracellular vesicle formation, loading of miRNAs containing complementary seed sequences, and docking and therefore appear to lie central to extracellular vesicle biology.
Perhaps the most well-studied within this context is the heterogeneous nuclear ribonucleoprotein (hnRNP) family. For instance, hnRNPA2B1 directly interacts with GAGG motifs, termed EXOmotifs [12], within target RNAs including the miRNA miR-198 [12] and the lncRNAs H19 [13] and LNMAT2 [14], whilst it negatively regulates miR-503 sorting into endothelial cell (EC) EVs [15]. CircRNAs, including circNEIL3, are also loaded into EVs by hnRNPA2B1 [16]. Interestingly, the role of hnRNPA2B1 in EV loading is sensitive to post-translational regulation such as SUMOylation [12] together with O-GlcNAcylation in response to oxidative stress [17]. SUMOylation of hnRNPA1 is important for the regulation of the lncRNA small nucleolar RNA host gene 16 (SNHG16) (otherwise known as ELNAT1) and its packing into EVs [18]. These mechanisms present a beautifully dynamic system for controlling ncRNA secretion through EVs (Fig. 1).
Other members of the hnRNP family have also been implicated in controlling ncRNA sorting into EVs. For example, hnRNPC1 is important for miR-30d loading into EVs [19] whilst Santangelo et al. identified a GGCU motif in miRNAs such as miR-3470a and miR-194-2-3p which was important for hnRNPQ-mediated loading [20, 21]. Additionally, Robinson et al. demonstrated that hnRNPK localises to MVBs in a membrane-raft-dependent mechanism where it recruits specific miRNAs carrying an AsUGnA motif in prostate cancer cells [22]. Interestingly, hnRNPK interacts with the autophagy machinery, in a mechanism defined as LC3-Dependent EV Loading and Secretion (LDELS) [23]. Deficiencies in the LC3-conjugating machinery change the EV ncRNA landscape in a caveolin 1-dependent manner [24]. This mechanism is largely independent of the ESCRT machinery and may reveal a stress-sensitive mechanism for regulating EV content. Therefore, despite their predominantly nuclear localisation, there is overwhelming evidence for nuclear-independent roles for these family members and future work is crucial to understand the molecular mechanisms that govern their shuttling between cellular compartments.
Alongside hnRNP family members, several other RNA binding proteins (RBPs) regulate ncRNA loading into EVs. For example, Lupas La has been demonstrated to drive specific loading of miR-122 into CD63-enriched vesicular high density (vHD) bodies in MDA-MB-231 BC cells [25]. HuR, a potent post-transcriptional regulator, also orchestrates EV-mediated miRNA export, including miR-122, during starvation stress in hepatic cells with this activity sensitive to HuR ubiquitination [26]. HuR has also been implicated in regulating the packaging of miR-1246 into EVs through an interaction with an AUUUU motif [27]. Furthermore, RBP YBX1 has been implicated in the sorting of ncRNAs, including transfer RNAs (tRNAs), YRNAs, viral RNAs (vRNAs) and miR-223 [28, 29], and in regulating miR-133 loading into EVs to promote fibroblast angiogenesis and mesenchymal-endothelial transition [30]. Fragile X messenger ribonucleoprotein 1 (FMR1), another RBP, was also demonstrated to chaperone miRNAs containing an AAUGC motif for internalisation in a process involving the hepatocyte growth factor regulated tyrosine kinase substrate [31]. In addition to those discussed here numerous other RBPs including insulin like growth factor 2 mRNA binding protein 1 (IGF2BP1) [32], major vault protein (MVP) [33], MEX3C1 [34] and annexin A2 (ANXA2) [15, 35] have been implicated in regulating the sorting of ncRNAs into EVs.
The role of protein argonaute-2 (Ago2), a crucial component of the RISC, has also been explored. Whilst there is debate over the presence of Ago2 in EVs which may be driven by technical factors during EV isolation, KRAS mutational status or culture conditions (reviewed in [36]), phosphorylation of Ago2 on Ser 387 has been implicated in the loading of specific miRNAs into EVs in a KRAS and mitogen-activated protein kinase kinase (MEK) activity dependent mechanism [37]. Evidence towards Ago2-miRNA complex loading into EVs was also provided by Lavello et al. where a direct interaction between the adaptor protein Alix, itself involved in EV biogenesis, and Ago2 was presented [38]. They demonstrated that Alix depletion in human liver stem-like cells resulted in a decrease in several miRNAs, including miR-24, miR-16 and miR-125b and inferred that the Alix-Ago2 interaction was important for EV loading [38]. Moreover, an Ago2 knockout mouse model, demonstrated that those miRNAs that are most sensitive to Ago2 depletion are amongst the most highly exported miRNAs [39]. These data provide a foundation for the further work that are required to ascertain the direct role of Ago2-mediated miRNA loading into EVs.
RNA-RNA interaction also influences the RNA content of EVs. For example, Ahadi et al. observed lncRNAs in EVs from prostate cancer cells were enriched in miRNA seed sequences that were similarly enriched within EVs, including members of the let-7 and miR-17 families [40], suggesting these interactions may drive EV presence. The sequence specificity of RNA for localisation to the lipid rafts was also explored using RNA aptamers and identified four motifs that were enriched in both raft-localised aptamers and in pro-tumoral EV-enriched miRNAs, which strikingly included the EXOmotif CCCU previously identified by Villarroya-Belri et al. [12, 41]. In addition to templated RNA motifs, RNA modifications may also participate in ncRNA loading into EVs with untemplated terminal nucleotide additions differentiating between EV enriched miRNAs (3’ uridylated) vs cell enriched (3’ adenylated) miRNAs from B-cells [42]. Interestingly, alongside being actively loaded into EVs, lncRNAs may also regulate the formation of EVs [43, 44]. For instance, PVT1 was shown to promote the docking of MVBs by influencing RAB7 expression and localisation together with promoting palmitoylation of YKT6 and its co-localisation with vesicle-associated membrane protein 3 (VAMP3) [43]. It is conceivable that by influencing the formation of EVs, ncRNAs themselves could control EV content through additional mechanisms other than direct RNA-RNA interactions.
Once the EVs reach the recipient cell membrane they can be internalised either by endocytosis, receptor-ligand interactions, or direct fusion with the cell membrane. The proportion of released ncRNA that is functional is unclear, however, it is likely that the interactions with the RBPs which may drive their initial inclusion into EVs are crucial for their subsequent function within the recipient cell.
The role of ncRNAs in breast cancer
Across the following sections, the roles of specific EV-carried ncRNA’s in different oncogenic processes will be comprehensively discussed. The ncRNA’s discussed are all summarised in Table 1.
Invasion and metastasis
Cancers cause morbidity and mortality through the invasion of local structures and the secondary spread to distant organs (metastasis). Metastasis is a multi-stage process and the leading cause of treatment failure and mortality in cancer. Bi-directional communication between BC and the TME is crucial for both processes. Additionally, the formation of a metastatic niche in distal organs creates a supportive microenvironment for secondary tumours to form. Extensive research has been performed into the role of EV ncRNAs in this process and this section will describe how EV-contained ncRNAs are involved in each step (Fig. 2).
Local invasion
During local invasion, BC cells break though the basement membrane into the surrounding tissue and extracellular matrix. Epithelial-Mesenchymal transition (EMT) is an important part of this process where epithelial cancer cells utilise a complex developmental program to turn off epithelial genes and upregulate EMT transcription factors including those of the SNAIL, TWIST and ZEB families to promote a mesenchymal phenotype [45]. The in vivo relevance of this process is heavily debated due to tissue-specific roles of the different EMT transcription factors and research showing their role in the aggressiveness of non-epithelial tumours [46]. EMT genes may therefore exert effects through altering cell plasticity and de-differentiation in BC rather instead of simply through classical EMT [45]. Therefore, research into EMT in BC is diverse, suggesting many different roles of EMT-related ncRNAs.
Cancer-associated fibroblast (CAF)-derived EVs containing miR-181d-5p enhance BC aggressiveness through targeting CDX2 and downregulating HOXA5 [47]. This process enhances EMT through the regulation of N-cadherin, SLUG, SNAIL1, TWIST1, ZEB1 and ZEB2. A miR-181d-5p inhibitor reversed the effect of CAF EVs on EMT in BC cells and restored HOXA5 expression. miR-18b is upregulated in EVs derived from CAFs compared to normal fibroblasts (NFs) and promotes EMT through transcription elongation factor A like 7 (TCEAL7) inhibition, activating SNAIL through nuclear factor-kappa B (NF-κB) [48]. The authors showed that this pathway also promoted metastasis in a xenograft mouse model, suggesting that CAFs may be a useful target in BC treatment.
Another group identified miR-1910-3p as an important regulator of metastasis and autophagy by targeting myotubularin related protein 3 (MTMR3), activating NF-κB signalling. Overexpression of miR-1910-3p causes upregulation of SLUG and TWIST and a reduction in E-cadherin. Inhibition of miR-1910-3p reduces N-cadherin, Vimentin, SLUG, TWIST, B-cell lymphoma 2 (Bcl-2) and Proliferating cell nuclear antigen (PCNA) expression. Functionally, overexpression of miR-1910-3p promoted proliferation and migration in vivo and EVs from miR-1910-3p overexpressing cells increased invasiveness when taken up by recipient cells. Mechanistically, this was due to inhibition of apoptosis and induction of autophagy, which they suggested was through inhibition of MTMR3 expression and activation of NF-κB and Wnt signalling [49].
Shen et al. identified miR-7641 as a promoter of BC metastasis by using EVs from metastatic MDA-MB-231 or HCC-1937 cells, or non-metastatic MCF-7 cells to treat MCF-7 and primary BC cells in transwell invasion assays and wound-healing assays. EVs from metastatic cell lines promoted invasion and migration in non-metastatic cell lines, with miR-7641 upregulated in the cells and EVs of invasive cells. Additionally, miR-7641 overexpression increased the migration and invasion of both MCF-7 and MDA-MB-231 cells, whereas inhibitors had the opposite effect. This effect was also confirmed in vivo [50]. Separately, in a bioinformatic screen, Liang et al. found miR-7-5p is upregulated in EVs from less invasive BC cells [51]. Comparison of MDA-MB-231 and MCF-7 cell lines showed that EVs from highly invasive BC are more likely to induce migration than EVs from less invasive BC lines, with miR-7-5p mimics promoting E-cadherin expression and inhibiting N-cadherin expression. Overall, they showed that miR-7-5p targets receptor like tyrosine kinase (RYK), reducing c-Jun N-terminal kinase (JNK) phosphorylation and inhibiting EMT [51].
Protease-activated receptor 2 (PAR2) is overexpressed in TNBC [52] and contributes to migration through matrix metalloproteinase (MMP)-2 induction [53]. Upon PAR2 activation, TNBC cells package miR-221 into EVs, promoting EMT through Phosphatase and tensin homologue (PTEN) targeting, causing AKT/NF-κB activation [54]. lncRNAs have also been shown to play a role in EMT. Xia et al. demonstrated that EVs carrying SNHG16 significantly enhance migration, invasion and EMT in BC cells [55], through the regulation of PPAPDC1A by SNHG16, resulting in miR-892b sponging.
Intravasation
The entry of BC cells into the bloodstream or lymphatic system is crucial for dissemination to distant organs. Communication between BC and ECs is critical to enable angiogenesis and intravasation. The cells must then survive in the circulation before they undergo extravasation and form secondary tumours.
Transfer of miR-105 from BC cells to ECs occurs in an EV-mediated fashion [56]. miR-105 is upregulated in metastatic BC cell lines compared to primary breast cell lines and even more so in EVs, indicating selective packaging of miR-105 into EVs. miR-105 targets Zonula occludens-1 (ZO-1), reducing the barrier function of the ECs, increasing vascular permeability. Additionally, overexpression of miR-105 in non-metastatic BC cells is sufficient to induce metastasis in xenograft mouse models. Another group found miR-939 to have an important role in the downregulation of VE-cadherin, destroying the barrier function of ECs, showing upregulation of miR-939 in TNBC and a correlation with poor prognosis and lymph node metastasis [57].
Extravasation
During extravasation, BC cells rely on adherence to the walls of the vasculature, mediated by changes in cell-cell adhesion proteins and bidirectional communication with ECs to exit the lumen of blood vessels and colonise new tissue. miR-214 is a pro-metastatic miRNA in TNBC [58] with well-described roles in increasing cell motility, promoting extravasation and increasing survival to anoikis [59]. Orso et al. demonstrated that BC cells induce the expression of miR-214 in CAFs through interleukin-6 (IL-6)/Signal transducer and activator of transcription 3 (STAT3) signalling. Subsequently miR-214 is packaged into EVs and taken up by BC cells, activating a pro-metastatic program [60].
Another study established a highly metastatic BC cell line through in vivo selection [61]. EVs from these cells were internalised by brain microvascular ECs, reducing trans-endothelial electrical resistance, and increasing blood-brain barrier (BBB) permeability. The lncRNA GS1-600G8.5 was upregulated in the highly metastatic cell line compared to parental cells and GS1-600G8.5 silencing abrogated the BBB permeability phenotype. miR-181c also increases BBB permeability, enabling cancer cell migration through the BBB [62]. Phosphoinositide-dependent protein kinase-1 (PDPK1) downregulation by miR-181c promotes alterations to actin dynamics and localisation due to a reduction in phosphorylated cofilin, enabling trans-BBB migration.
Formation of metastases
Formation of lymph node metastases
Axillary lymph node metastasis is a critical step in the progression of BC and an important prognostic marker in early BC. Due to the modest false negative rate of sentinel lymph node biopsy, a study examined the expression profiles of circulating EVs for biomarkers of lymph node metastasis. The authors showed miR-363-5p was significantly downregulated in EVs from patients with lymph node metastasis where its expression levels correlated with improved survival [63]. Functionally, they determined that miR-363-5p may have a tumour suppressor role, inhibiting colony formation, migration and invasion. Platelet derived growth factor subunit B (PDGFB) was identified as a target of miR-363-5p, suggesting a potential mechanism for the tumour suppressive effect.
miR-222 is an oncogenic miRNA that is highly expressed in BC with lymph node metastasis. Ding et al. found that tumour cell miR-222 overexpression led to increased EV miR-222 that could be transferred to other cells. miR-222 targets the tumour suppressor gene PDLIM2, promoting activation of NF-κB signalling, whilst miR-222 inhibition decreased MDA-MB-231 invasiveness [64]. miR-130a-3p has been shown to inhibit migration and invasion through RAB5B regulation, and is downregulated in circulating EVs in BC patients, whilst overexpression in BC stem cells (BCSCs) inhibits migration and proliferation through G0/G1 arrest. Additionally, low levels of miR-130a-3p correlated with lymph node metastasis, suggesting it may be a useful indicator of lymph node metastases and a potential therapeutic target [65]. RAB22A is an important regulator of intracellular trafficking, and upregulation is associated with lymph node metastasis in BC. RAB22A is a target of miR-193b and RAB22A knockdown or miR-193b overexpression decrease EV release and reduce the ability of EVs to promote proliferation [66].
Tumour-associated macrophages (TAMs) are key regulators of angiogenesis, metastasis and immunosuppression in BC, particularly in the preparation of the pre-metastatic niche and participation in pro-tumourigenic signalling pathways [67]. TAM-derived EVs can also promote metastasis, with one group finding that miR-660 in TAM derived-EVs promotes lymph node metastasis in BC through targeting Kelch like family member 21 (KLHL21) and activating the NF-κB p65 signalling pathway [68]. Another study explored the role of miR-370-3p in BC and found it is highly expressed in EVs, with the level of expression positively correlating with lymph node metastasis. Overexpression of miR-370-3p in BC cells promotes mobility and proliferation whereas knockdown has the opposite effect [69].
Formation of brain metastases
Cancer cells must adapt in order to survive at distal sites, through transcriptome changes and crosstalk with the TME [70]. In a landmark study, Zhang et al. [71] showed that PTEN was downregulated in brain metastases by miRNAs in astrocyte derived-EVs and rescued by the depletion of PTEN-targeting miRNAs in astrocytes, reducing brain metastasis in vivo. They identified increased C-C motif chemokine 2 (CCL2) secretion, recruiting Iba1+ myeloid cells, further promoting proliferation of brain metastatic tumour cells. This study highlights the importance of communication between metastatic tumour cells and the new microenvironment, lending evidence to the seed and soil hypothesis [72], and provides new therapeutic avenues to explore in the inhibition of BC metastasis. Additionally, tGLI1, a transcription factor known to promote brain metastases [73], has been shown to activate astrocytes by EV-mediated transfer of miR-1290 and miR-1246, inhibiting FOXA2 and promoting Ciliary neurotrophic factor (CNTF) cytokine secretion, priming the brain metastatic niche [74].
Morad et al. explored the role of EVs in brain metastasis in TNBC using a brain-seeking variant of MDA-MB-231 cells, generated through sequential passaging in nude mice [75]. Interestingly, they found non-canonical Cdc42-dependent clathrin-independent carrier/GPI-AP-enriched compartments (CLIC/GEEC) endocytosis to be important in astrocyte uptake of BC EVs. Proteomics showed upregulation of surface markers known to be cargo of the CLIC/GEEC endocytic pathway in brain-seeking EVs. The EVs reduced expression of Tissue inhibitor of metalloproteinases 2 (TIMP2) in astrocytes, increasing their migration through miR-301a-3p. Analysis of clinical data also showed that miR-301a-3p levels correlate with decreased survival [76].
In another study, profiling lncRNAs from brain metastatic breast tumours revealed downregulation of X inactive specific transcript (XIST), whilst in xenografts XIST expression inversely correlated with brain metastasis [77]. Mechanistically, XIST downregulation promotes EMT and activates c-Met, promoting stemness. Additionally, EVs from XIST downregulated cells promoted M1-to-M2 conversion in microglia through miR-503 regulation. Finally, the authors showed that fludarbine treatment of XIST low BC cells effectively inhibited brain metastasis in mouse models, demonstrating an interesting new synthetic lethality therapeutic approach.
Formation of bone metastases
Bone metastases occur in most metastatic BC patients [78], leading to complications including bone fracture, severe pain and bone marrow infiltration [79]. To investigate why oestrogen receptor positive (ER+) BC has a preference for bone metastasis, Wu et al. characterised the transcriptomes of bone-tropic and non-bone-tropic BC cells, identifying miR-19a and integrin binding sialoprotein (IBSP) upregulation in bone-tropic ER+ BC cell EVs. These EVs induced osteoclastogenesis in osteoclasts, creating a favourable environment in the bone. The authors also identified miR-19a and IBSP overexpression induced bone metastasis in an ectopic MCF-7 mouse model, whereas neither could promote it alone [80], further demonstrating the importance of ncRNAs in EVs in priming new metastatic sites in BC.
miR-218-5p is significantly upregulated in bone metastatic BC, but not brain metastatic BC [81]. A study showed EVs from miR-218 overexpressing MDA-MB-231 cells significantly downregulated type I collagen expression and deposition by osteoblasts compared to control EVs when injected into mice, contributing to the adaptation of the bone metastatic niche by promoting osteolysis to facilitate bone metastasis [81]. The lncRNA SNHG3 is a key regulator of bone marrow mesenchymal stem cell (MSC) osteogenesis in BC bone metastasis. SNHG3 regulates the miR-1273g-3p/bone morphogenetic protein 3 (BMP3) axis to promote osteogenesis, and SNHG3 levels correlate with increased bone metastasis. BMP3 expression positively correlates with SNHG3 and is regulated by EV-contained miR-1273g-3p [82].
Formation of liver metastases
Liver metastasis is another common occurrence in advanced BC. One group found that EV-contained miR-4443 promotes BC metastasis through TIMP2 downregulation and the upregulation of MMPs, whilst overexpression of miR-4443 in non-invasive BC cells led to liver metastasis [83]. Syndecan-1, which also associates with BC metastasis [84], is suppressed by miR-122-5p. miR-122-5p is enriched in liver cell-derived EVs and increases with liver injury. These EVs increase BC cell motility through Syndecan-1 suppression [85].
Another study found that BCSC-derived EVs increased the proliferation of MDA-MB-231 and SUM149PT cells in vitro and in vivo where they also promote liver metastasis. These EVs were shown to deliver miR-197, targeting PPARG mRNA and promoting EMT and proliferation in the cells [86].
Formation of lung metastases
miR-200c and miR-141 are associated with lung metastasis in BC [87]. In Foxp3 heterozygous Scurfy mutant mice, breast tumours form spontaneously and metastasise to the lung. miR-200c and miR-141 levels in plasma increase throughout tumour progression and this pattern is consistent with human samples. Zhang et al. suggest that EV-contained miR-200c and miR-141 are regulated by the FOXP3-KAT2B axis and may be useful biomarkers for BC metastasis [87].
TAMs also play a role in promoting metastasis. Progranulin knockout in mice was shown to reduce lung metastasis with Progranulin positive BC cells [88]. miR-5100 was upregulated in Progranulin knockout TAMs and the group suggested that, through miR-5100-mediated inhibition of the CXCL12/CXCR4 axis, this reduced the invasiveness and metastatic potential of BC cells due to the pivotal role of CXCL12/CXCR4 axis in cancer cell migration, proliferation and gene regulation. Overall, the authors suggest Progranulin downregulation in TAMs promotes upregulation of miR-5100 in EVs, reducing lung metastasis in BC.
CAF induction by BC cells can occur through several different signalling pathways. Ren et al. described how miR-370-3p from BC cell EVs induces fibroblast activation through CYLD regulation [89]. They found EVs from multiple BC cell lines induced activation of NFs which were then able to enhance migration, invasion and EMT of BC cells. miR-370-3p was responsible for the activation of fibroblasts through downregulation of CYLD, altering NF-κB signalling, ultimately promoting lung metastasis in an in vivo model.
miR-200 is found in EVs derived from metastatic 4T1 cells but not the poorly metastatic 4TO7 cell line [90] and miR-200 family miRNAs suppress EMT through ZEB1 and ZEB2 regulation [91]. A study showed that EVs from MCF10CA1a cells could promote lung colonisation of MDA-MB-231 cells in immunocompromised mice via miR-200. MCF10CA1a cells readily form secondary tumours in nude mice, however MDA-MB-231 cells were reported to be less able to form lung metastases. This study showed the ability of metastatic cells to induce a metastatic phenotype in less metastatic BC cells via EVs, leading to local invasion and the colonisation of the lung [90]. Another group showed that BC-derived EVs contain miR-138-5p which is taken up by macrophages and promotes M2 polarisation through lysine demthylase 6B (KDM6B) downregulation, leading to promotion of lung metastasis [92].
Metastasis promoting roles of TME Cells
In addition to organ specific effects, CAFs have also been shown to generally promote metastasis. miR-3613-3p from CAF EVs promotes metastasis through regulating suppressor of cytokine signalling 2 (SOCS2) expression [93]. miR-3613-3p is upregulated in CAF EVs following education from BT474 and MCF-7 BC cells. The authors proposed that regulation of SOCS2 was essential to this mechanism and clinical data negatively correlated SOCS2 and miR-3613-3p expression in BC samples.
Another group found that miR-185-5p, miR-652-5p, and miR-1246 promoted CAF specialisation [94]. EVs from MDA-MB-231 cells promoted CAF transition, increasing invasiveness in breast epithelial cells. miR-9 also plays a role in CAFs as BC EVs containing miR-9 were shown to promote CAF formation, which was abrogated upon miR-9 inhibition. Interestingly, miR-9 from CAFs could promote invasiveness in BC cells through downregulating E-cadherin [95]. Yang et al. also showed that miR-146a was important in CAF activation. EVs from BC cells containing miR-146a promoted CAF transition as well as BC growth and EMT in nude mice. Thioredoxin interacting protein (TXNIP) was found to be a target of miR-146a, causing WNT activation in fibroblasts [96].
Focal adhesion kinase (FAK) signalling in CAFs may be involved in BC migration and metastasis. Ablation of FAK increases miR-16 and miR-148a in EVs from fibroblasts which are then less able to promote metastasis than wild-type CAFs [97]. In addition to fibroblasts, lymphatic vessel endothelial cells (LECs) have been proposed as contributors to metastasis. ELK3 in LECs is proposed to be essential for the metastasis-promoting properties of LEC-derived EVs, with miR-503-3p, miR-4269 and miR-30e-3p identified as key mediators [98]. Another study found that miR-503 impairs tumour growth and invasiveness and was abundant in EVs released from vascular ECs. They found CCND2 and CCND3 were targets of miR-503 and interestingly, chemotherapy increases release of miR-503 in plasma [99].
MSC-derived EVs also play a role in BC where they induce dormancy and suppress metastasis through miR-205 and miR-31 [100]. Notably, this effect was only seen in parental MDA-MB-231 cells and not organotropic metastatic MDA-MB-231 sublines. In primary tumours, the EVs promoted growth in both parental and organ-specific metastatic lines, whereas they suppress metastasis through promoting dormancy in the parental cell lines. The authors suggested that UBE2N/Ubc13 regulation was involved in the process as it is a target of the miRNAs and silencing of UBE2N/Ubc13 also suppresses migration, invasion, and proliferation of BC cells. Overall, the authors showed that MSCs play a role in promoting dormancy in non-committed metastatic BC cells but do not reduce metastasis in committed BC cells.
Another study also found that bone marrow MSCs play a role in promoting dormancy in metastatic BC cells through suppression of proliferation, protecting cancer cells from chemotherapies [101]. They found that bone metastatic BC cells upregulated miR-23b and downregulated myristoylated alanine rich protein kinase C substrate (MARCKS), reducing cell cycle progression. The authors suggested that transfer of miR-23b from bone marrow MSCs to BC cells was responsible for the promotion of dormancy in the BC cells.
CircRNAs are also implicated in tumour growth. circSKA3 from EVs promotes cell growth and invasiveness, and higher levels of circSKA3 correlate with increased potential to form large colonies [102]. circSKA3 transfer occurs between different BC cells, enabling regulation of less invasive BC cells by more invasive BC cells. An interesting study found that stromal NOTCH-MYC signalling promoted the generation of unshielded RN7SL1-containing EVs. RN7SL1 is normally shielded by the RBP SRP9/14, however when unshielded, it is transferred to immune cells, generating an inflammatory response, acting as a damage-associated molecular pattern (DAMP), activating retinoic acid-inducible gene I (RIG-I), increasing proliferation, metastasis and therapy resistance in BC [103]. These EVs increase myeloid/dendritic cell populations expressing maturation and activation markers in the spleen, however due to the complexity of the immune microenvironment, the mechanism was not elucidated.
Cell growth
Uncontrolled cell growth is a fundamental hallmark of cancers, and is achieved through the activation of proliferative signalling and evasion of growth suppression signals [104]. A growing body of research demonstrates that EV cargoes derived from TME cells and BC themselves can promote proliferation in BC cells, with key studies linking specific ncRNA cargo with proliferation in BC.
Metastasis associated lung adenocarcinoma transcript 1 (MALAT1) is upregulated in BC and BC-derived EVs and associated with progression, where high levels correlate with shorter survival, whilst siRNA against MALAT1 reduces cell proliferation [105]. The lncRNA nuclear paraspeckle assembly transcript 1 (NEAT1) associates with lymph node metastasis and Ki-67 in BC and is overexpressed in serum EVs in BC patients. BC patient EVs promoted proliferation in MCF-7 and MDA-MB-231 cells, whereas healthy volunteer EVs did not, and this was reversed by NEAT1 inhibition. Mechanistically, NEAT1 is a sponge for miR-141 which is frequently downregulated in BC, increasing tumourigenicity and contributes to metastasis and chemoresistance. By sponging miR-141-3p, NEAT1 regulates Krüppel-like factor 12 (KLF12), promoting growth, chemoresistance and metastasis [106].
ADP-ribosylation factor 6 (ARF6) plays a role in CCL18 signalling in BC metastasis. A study found that CCL18 treatment increased ARF6 and p-AMAP1 expression, activating PI3K/Akt signalling. Interestingly, miR-760, which targets ARF6, was found to be highly expressed in EVs secreted from BC cells stimulated by CCL18. MCF-7 cells take up these EVs and become more proliferative and invasive, surprisingly, through miR-760-mediated upregulation of ARF6 and subsequent activation of PI3K/Akt signalling. Overall, this study showed that the M2-derived cytokine, CCL18 promotes upregulation of miR-760 in EVs, resulting in proliferation, chemoresistance and metastasis [107].
miR-106a-5p is upregulated in TNBC compared to healthy tissue, where it associates with poorer prognosis [108]. MSCs release EVs containing miR-106a-5p which are taken up by TNBC cells. HAND2-AS1, an antisense RNA which inhibits miR-106a-5p expression and secretion from MSCs, is negatively correlated with tumour grade and downregulated in TNBC cells. In vivo, HAND2-AS1 injection inhibited tumour growth in nude mice.
One study isolated CAFs and NFs from BCs and adjacent tissue and screened EVs from these cells for miRNAs. They found miR-500a-5p was highly expressed in CAFs and their EVs and upregulated in recipient BC cells after treatment with the EVs. The authors suggested that these EVs promote proliferation and metastasis through the downregulation of ubiquitin-specific peptidase 28 (USP28). In an in vivo model, CAFs overexpressing miR-500a-5p promoted increased tumour size in MDA-MB-231 xenografts [109]. Another study found that miR-1-3p was downregulated in BC tissue and that CAFs from surrounding tissue had reduced miR-1-3p in their EVs compared to NFs. CAF EVs were able to deliver miR-1-3p to BC cells and miR-1-3p overexpression in CAFs promoted suppression of tumour formation and metastasis in BC cells in a coculture system. Krüppel-like zinc-finger protein Gli-similar 1 (GLIS1) was suggested as the target of miR-1-3p and this was confirmed by a dual-luciferase reporter assay and overexpression of GLIS1 abrogated the effects of miR-1-3p on BC development [110].
BC cells also communicate with TAMs to promote growth. miR-222 from adriamycin-resistant BC cells induces M2 polarisation in macrophages. A study showed this led to an increase in proliferation in vivo in miR-222 overexpressing BC cells through targeting of PTEN in macrophages, which, in turn, activated Akt signalling, facilitating M2 polarisation and pro-tumour signalling [111]. Guo et al. showed that BC-derived EVs transferred miR-183-5p to macrophages, downregulating PPP2CA and increasing NF-κB signalling, leading to IL-1β, IL-6, and tumour necrosis factor alpha (TNF-α) expression in macrophages and a pro-inflammatory phenotype [112]. Interestingly, miR-183-5p knockdown in BC suppressed tumour growth and metastasis in a mouse model.
Another study found that endothelial-derived EVs promote tumour growth through induction of an M2-like phenotype in macrophages [113]. miR-142-5p, miR-183-5p and miR-222-3p are released in EVs from ECs which increase M2 signature gene expression. The authors suggest that targeting of PTEN by the miRNAs was responsible for the increase in the M2 markers, arginase-1 (ARG1) and transforming growth factor beta (TGF-β), promoting tumour growth. A comprehensive study of MSC-derived EVs used RNA sequencing, proteomics and lipidomics to analyse MSC EV cargo and found miR-21 and miR-34a to have an important tumour supportive role by promoting proliferation in BC cells [114].
Angiogenesis
Angiogenesis allows tumours to acquire sufficient oxygen and nutrients through the formation of neovasculature whilst facilitating metastases by promoting intravasation. The process is regulated through the balance of pro- and anti-angiogenic factors and the triggering of an “angiogenic switch” depending on the relative abundance of these factors. Tumour cells, particularly in hypoxic conditions, secrete significant quantities of pro-angiogenic factors, which act on ECs to induce angiogenic signalling in the existing vasculature, triggering angiogenic sprouting and new vessel formation. Transfer of biomolecules through EVs plays a role in angiogenic signalling, with EV-associated ncRNAs having both pro- and anti-angiogenic effects.
Overexpression of miR-182-5p in BC tissues correlates with poor patient prognosis, and transfection of miR-182-5p mimic into human umbilical vein endothelial cells (HUVECs) enhanced proliferation, migration and angiogenesis [115]. In this study, BC EVs delivered miR-182-5p to HUVECs, inducing the same angiogenic phenotype. Mechanistically, miR-182-5p reduces expression of CKLF like MARVEL transmembrane domain containing 7 (CMTM7) tumour suppressor, leading to activation of EGFR/AKT signalling and subsequent angiogenic signalling, which was confirmed in vivo [115].
Kong et al. performed a microarray analysis of BC tissues and analysis of The Cancer Genome Atlas (TCGA) data, identifying differentially expressed lncRNA’s in BC. Novel lncRNA AC073352.1 is upregulated in tumour tissues and correlates with poor prognosis. Mechanistically, AC073352.1 binds to YBX1 transcriptional activator to stabilise it and promote metastasis. Notably, YBX1 contributed to the packaging of AC073352.1 into MDA-MB-231 EVs and the uptake of AC073352.1-carrying BC-derived EVs increased angiogenic activity in HUVECs [116].
Hypoxic conditions in a murine BC model increased EV secretion from 4T1 cells, with increased cellular and EV levels of miR-210 reported after desferrioxamine-mediated induction of hypoxia inducible factor 1 alpha (HIF1α) signalling [117]. ECs treated with hypoxic 4T1 cell-derived EVs exhibited increased migration, capillary-like structure formation and proliferation compared cells treated with control EVs. Vascular remodelling proteins and miR-210 targets, ephrin-A3 and PTP1B, were decreased within the TME of tumours treated with hypoxic EVs whereas vascular endothelial growth factor (VEGF) and Ki-67 levels were increased, demonstrating increased angiogenesis [117].
One study found endothelial cell-derived EVs promoted endothelial cell migration in a scratch wound assay, and significantly increased tubule length and sprouting in a Matrigel tubule formation assay. miR-214 was upregulated 3-fold in EVs relative to cells, and EV-mediated induction of endothelial cell migration and tubule formation was dependent on the expression of miR-214, which suppresses cell cycle arrest through ATM downregulation [118].
There are also examples of anti-angiogenic ncRNAs in EVs. Increased calcium levels in MDA-MB-231 cells led to increased EV secretion, and EVs released from BC cells with A23187-treatment elevated intracellular Ca2+ levels significantly increased angiogenic activity in recipient HUVECs [119]. Concurrently, EVs from MDA-MB-231 cells treated with SKF96365 Ca2+ influx inhibitor had anti-angiogenic effects on recipient HUVECs, due to miR-145 and miR-449 upregulation. miR-145 suppresses insulin receptor substrate 1 (IRS1), inhibiting pro-angiogenic PI3K/Akt and MAPK signalling, and IRS1 was downregulated in HUVECs following treatment with anti-angiogenic EVs. STIM1, an essential regulator of Ca2+ signalling, was downregulated in response to calcium depletion, leading to miR-145 upregulation in BC cells, BC-derived EVs and the recipient HUVECs, ultimately decreasing angiogenesis [119].
Similarly, the mitochondrial calcium uniporter (MCU), a key Ca2+ channel implicated in the progression of multiple cancer types, reportedly enhances angiogenesis in the metastatic niche of BC through the downregulation of miR-4488 in BC-derived EVs. MCU suppression in MDA-MB-231 cells led to the secretion of EVs that reduced liver metastasis and angiogenesis in vivo, and modulation of MCU expression in vitro altered the abundance of many miRNAs in EV cargo. A notable negative correlation between cellular MCU expression and EV miR-4488 levels was reported, attributable to MCU-mediated negative selective sorting of miRNA to EV cargo. miR-4488 suppresses angiogenesis by targeting CX3CL1 mRNA, and levels of miR-4488 in serum EVs of TNBC patients were shown to be lower than in non-TNBC patients, highlighting the suppression of miR-4488 sorting into EVs as a mechanism by which MCU expression might promote angiogenesis in the metastatic niche and contributes to a more aggressive disease phenotype [120].
MSCs reportedly have conflicting pro- and anti-tumourigenic roles. Multiple studies have identified that miRNAs carried by MSC EVs have anti-angiogenic effects. One such example identified downregulation of VEGF in 4T1 murine BC cells through the transfer of miR-16 from MSC EVs. Accordingly, EVs from MSCs suppressed angiogenesis in vitro and in vivo [121]. Additionally, Pakravan et al. showed that EV-mediated transfer of miR-100 from MSCs to MDA-MB-231 BC cells significantly reduces VEGF expression in recipient BC cells via miR-100-mediated mTOR downregulation and HIF1α suppression [122].
Omega-3 fatty acid docosahexaenoic acid (DHA), which has anti-cancer efficacy through the suppression of angiogenic factors, significantly decreases the levels of pro-angiogenic miRNAs and increases anti-angiogenic miRNAs in both MDA-MB-231 BC cells and EVs [123]. Additionally, Chang et al. explored the potential of using EVs from Wharton’s Jelly MSCs (WJ-MSCs) therapeutically, showing that WJ-MSC EVs reduce in vitro proliferation, sphere formation, migration and EMT in MDA-MB-231 cells and in vivo metastasis in a murine model. WJ-MSC-derived EVs significantly altered BC cell miRNA expression profiles, upregulating miRNAs associated with inhibited tumour development. The authors highlight miR-125b as significantly elevated in WJ-MSC EV cargo and upregulated in recipient BC cells and show that miR-125b directly regulates HIF1α, hypothesising that this inhibits HIF1 activation and subsequent downstream gene expression changes that drive proliferation, EMT and angiogenesis [124].
Drug resistance
One primary challenge for successful treatment of most cancers remains the development of drug resistance. Many factors contribute to treatment resistance, including upregulation of efflux transporters, mutations, and physical inaccessibility of drugs to poorly vascularised tumour regions. EV-mediated transfer of ncRNAs from resistant cancer cells to sensitive cells is an important mechanism for propagation of tumour drug resistance. This topic has been reviewed deeply, in a 2021 systematic review exploring the role of EV-carried miRNAs in the induction of chemoresistance in multiple cancer types [125], and in recently published BC focused reviews by Weon Yi [126] and Rezaee et al. [127].
The transfer of many different ncRNAs from resistant tumour cells or stromal cells to sensitive BC cells induces resistance to BC chemotherapeutics. miR-222 is transferred in EVs between drug-resistant and sensitive tumour cells, conferring resistance to common BC drugs including tamoxifen, docetaxel and adriamycin by downregulating ERα and PTEN in recipient cells [128,129,130], and EV-mediated transfer of miR-222 and miR-223 from MSCs to BC cells in the bone marrow induces BC cell dormancy, promoting quiescence and resistance to carboplatin in metastatic cells prior to recurrence [131].
One study examined EVs derived from MDA-MB-231 BC cells, finding that MCF-7, BT474 and HCC1937 BC cell survival under doxorubicin, cisplatin and fulvestrant treatment respectively is significantly increased following EV-mediated transfer of miR-887-3p, BTB domain containing 7 (BTBD7) suppression and Notch signalling activation in recipient cells. Notably, inhibition of miR-887-3p in MDA-MB-231 cells abrogated the EV-driven induction of drug resistance in recipient BC cells [132].
Treatment of naïve MDA-MB-231 BC cells with EVs from docetaxel- or doxorubicin-treated MDA-MB-231 cells induces stemness-associated genes like NOTCH1, SOX9 and NANOG. Several miRNAs were shown to be upregulated in “chemo-EVs”, including miR-9-5p, miR-203a-3p and miR-195-5p, which induce this stemness phenotype through inhibition of ONECUT2, a master regulator of cell fate. Importantly, in a xenograft mouse model, the induction of BC stemness by EV-carried miRNAs reduced tumour docetaxel sensitivity [133].
Treatment of 4T1 BC-bearing mice with doxorubicin led to induction of myeloid derived suppressor cells (MDSCs), reportedly promoting tumour growth, metastasis, angiogenesis and anti-inflammatory Th2 responses via EV-carried miR-126a. Inhibition of miR-126a increased the efficacy of chemotherapy against lung metastasis in 4T1 tumour-bearing mice through local angiogenic suppression. This exemplifies how BC tumours adapt to doxorubicin treatment, promoting lung metastasis through induction of angiogenesis and Th2 cell responses in the metastatic niche to facilitate BC cell survival [134].
LncRNAs such as UCA1, APAP2-AS1 and H19 have been separately shown to promote resistance to tamoxifen, trastuzumab and doxorubicin respectively [135,136,137]. Furthermore, the transfer of HIF1A stabilizing long noncoding RNA (HISLA) from TAMs reportedly increases BC resistance to chemotherapeutics through HIF-1α stabilisation and subsequent inhibition of apoptosis [138]. Circ_UBE2D2 and circ-MMP11 also promote tamoxifen and lapatinib resistance through miR-200a-3p and miR-153-3p sponging respectively in BC cells through EV-mediated lncRNA transfer from resistant to sensitive cells [139, 140].
ncRNAs can also enhance treatment sensitivity. One study showed downregulation of miR-134 in EVs from an aggressive clonal variant (Hs578Ts(i)8) of Hs578T BC cells and significantly lower expression in patient breast tumour tissue compared to normal controls. Interestingly, overexpression of miR-134 mimic in Hs578Ts(i)8 reduced expression of anti-apoptotic protein Bcl-2 and increased cisplatin sensitivity. The authors demonstrated that EVs from Hs578Ts(i)8 cells transfected with miR-134 mimic promoted reduced aggressiveness and increased sensitivity to anti-heat shock protein 90 (HSP90) compounds through downregulation of signal transducer and activator of transcription 5B (STAT5B) and HSP90 in recipient TNBC cells [141].
Ectopic overexpression of miR-770 in MDA-MB-468 and MDA-MB-231 cells significantly increased doxorubicin sensitivity in another study. EV packaging of miR-770 and transfer to TAMs, wherein pro-inflammatory M1 polarisation is promoted, suppressed macrophage driven chemo-resistance in TNBC cells. Overexpression of miR-770 significantly decreased stathmin 1 (STMN1) expression, increasing doxorubicin sensitivity and metastasis in both BC lines and, in a xenograft mouse model, miR-770 promoted TNBC treatment sensitivity through the EV-mediated transfer of miR-770 to TAMs, suggesting that TNBC loss of miR-770 represents a key mechanism of chemo-resistance acquisition [142].
Finally, miR-342-3p is enriched MSC-derived EVs, which suppressed invasion and increased doxorubicin, fluorouracil and cisplatin sensitivity in MCF-7 cells in a study from Yu et al. Conversely, inhibition of miR-342-3p in SKBR-3 cells significantly increased invasion and migration and chemotherapeutic resistance. ID4 is an miR-342-3p target, and inhibitor Of DNA binding 4 (ID4) inhibition in BC cells increased MCF-7 chemo-sensitivity and suppressed tumour growth and EMT in vivo [143].
Metabolism
Cellular metabolism in most healthy cells involves glucose uptake and its conversion to pyruvate, then acetyl coenzyme A, fuelling the tricarboxylic acid (TCA) cycle. The TCA cycle produces a range of molecules, including nicotinamide adenine dinucleotide (NADH) which is used in the electron transport chain (ETC) and oxidative phosphorylation (OXPHOS), driving adenosine triphosphate (ATP) synthesis. This process is highly efficient but requires oxygen for the ETC to function properly. In hypoxic conditions, cells instead convert glucose-derived pyruvate to lactate using lactate dehydrogenase (LDH), sacrificing the efficiency of TCA/OXPHOS metabolism for rapid LDH-driven ATP production.
Rapidly proliferating cells typically exhibit aerobic glycolysis metabolism, preferentially converting glucose-derived pyruvate to lactate irrespective of oxygen availability, conferring proliferative advantages. Many factors contribute to the establishment of this altered metabolic state in tumour cells, including the expression of the M2 isoform of pyruvate kinase (PKM2) and aberrant activation of HIF1 signalling. Although such studies are limited, there is growing appreciation of the role of ncRNA EV cargo in reprogramming cellular metabolism.
A circRNA array in BCSC-derived EVs, identified circCARM1 elevation in EVs from breast tumour spheroids compared to spheroids formed from adjacent normal tissue. These EVs promoted glycolysis in recipient MDA-MB-231 cells, whilst short hairpin RNA (shRNA)-mediated suppression of circCARM1 abrogated the pro-glycolytic phenotype. CircCARM1 interacts with miR-1252-5p in vitro and negatively correlates with miR-1252-5p in BC tissues, and expression of circCARM1 promotes the upregulation of glycolytic enzyme 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 2 (PFKFB2) through miR-1252-5p sequestration [144].
Co-culturing TAMs and MDA-MB-231 BC cells in a transwell system increased glucose consumption and lactate production in MDA-MB-231 cells and elevated expression of glycolytic enzymes like PKM2 and LDHA, stabilising HIF1α. In this study, strong upregulation of 5 lncRNAs (HISLA, LINC01234, LINC02432, LINC01678 and LINC01493) in an EV-secretion-dependent manner was validated by qRT-PCR and suppression of HISLA in TAMs abrogated the co-culture driven induction of aerobic glycolysis in BC cells. HISLA contributes to HIF1α activation through disrupting its interaction with prolyl hydroxylase domain-containing protein 2 (PHD2), leading to HIF1α stabilisation [138].
Similarly, miR-503-3p is upregulated in BC cell lines and tissues, correlating with more advanced disease [145]. miR-503-3p directly targets and suppresses dishevelled binding antagonist of beta catenin 2 (DACT2), activating WNT/β-catenin signalling and a glycolytic shift in metabolic activity. Importantly, M2 macrophage-derived EVs carry miR-503-3p, and recipient BC cells also exhibit metabolic changes, further highlighting how macrophage-derived EVs within the TME regulate tumour cell metabolism to promote growth [145].
One study found that uptake of CAF-derived EVs by BC cells impairs mitochondrial function and elevates extracellular acidification rate, suggesting increased aerobic glycolysis. Secretion of the lncRNA SNHG3 was significantly higher from CAFs than normal breast MCF-10A cells, and treatment of MDA-MB-453 cells with EVs from SNHG3-suppressed CAFs reduced BC proliferation and restored mitochondrial metabolism. SNHG3 suppresses miR-330-5p in BC cells leading to pyruvate kinase upregulation and increased glycolysis, which was validated in vivo using MDA-MB-453 tumours in mice [146].
Conversely, uptake of BC-derived EVs by CAFs leads to a MYC-dependent glycolytic increase, mediated by miR-105. Yan et al. showed that miR-105 is carried by MDA-MB-231 EVs and directly targets MYC-antagonist MXI1. miR-105 reprogrammes CAF metabolism in response to nutrient availability, promoting glycolysis and glutaminolysis in nutrient rich contexts to produce fuels for nearby cancer cells, or promoting detoxification of waste by-products lactic acid and ammonium in nutrient-starved conditions to enhance tumour cell survival. Orthotopic implantation of patient-derived BC xenografts with anti-miR-105 expressing CAFs in a female Non-Obese Diabetic (NOD)/Severe Combined Immunodeficiency (SCID)/IL2Rγ-null (NSG) mouse, demonstrated that mice with miR-105-resistant CAFs exhibit significantly reduced tumour growth [147].
miR-122 was shown to be highly secreted in EVs from MDA-MB-231 BC cells compared to MCF-10A, and BC-EV-carried miR-122 suppressed glucose metabolism through downregulation of PKM2 expression in recipient cells. miR-122 suppressed glucose uptake in lung fibroblasts and astrocytes, and EVs containing high levels of miR-122 were shown to be taken up by lung and brain tissues in vivo. Notably, though overexpression of miR-122 in xenograft tumours reduced primary tumour growth, these animals exhibited significantly increased metastasis to brain and lung tissues, where glucose availability was higher due to reduced local tissue utilisation [148].
Treatment of SCID mice with MDA-MB-231 BC EVs or EVs from miR-122 overexpressing MCF-10A cells suppressed insulin signalling, inducing endogenous glucose production. Suppressed insulin signalling was attributed to reduced insulin secretion, due to PKM2 suppression in pancreatic islet β-cells following uptake of miR-122-carrying EVs. Knockout of miR-122 in BC cells improved insulin signalling, lowered blood glucose levels and reduced tumour growth and proliferation, and corresponding results were seen in a patient-derived xenograft model. This demonstrates the regulation of β-cell insulin signalling by BC EVs, tying the effect to miR-122-mediated suppression of PKM2 by demonstrating that exogenous expression of PKM2 rescues the phenotype in the mouse model [149].
EV ncRNA as a diagnostic biomarker in breast cancer
Current BC screening and diagnosis relies on mammography, ultrasound, MRI, and biopsy. Although these techniques have led to a reduction in BC mortality, they are invasive, costly, time-consuming, associated with false-negative results, and can lead to patient anxiety [150]. Additionally, the current investigations have limited spatial resolution which limits their ability to be used to determine the minimal residual disease status of patients following treatment. Therefore, the implementation of a novel diagnostic biomarker would be invaluable in BC diagnosis and monitoring.
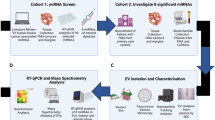
The most well-studied EV ncRNA is miRNA. Key miRNAs with differing expression profiles include miR-9, miR-16, miR-21, miR-429, miR-320a, miR-1246 and miR-4433b-5p which have been collected from serum, plasma, or urine EVs [151,152,153]. Furthermore, to improve their role as a biomarker, panels of miRNAs have been developed and shown to lead to a more accurate diagnosis, with a combination of miR-21, miR-16, miR-9, and miR-429 enabling discrimination between different BC subtypes and healthy controls (sensitivity 96.8% and specificity 80.0%) [151] and a predictive model of 16 EV miRNAs outperforming routine diagnostic methods [154] (Fig. 3).
Other studies have also investigated EVs from other biofluids including urine and tears. Schirmer tear test strips have been used to show miR-21 and miR-200c were significantly higher in BC patient EVs compared to healthy controls [155]. Another study tested a miRNA panel of miR-424, miR-423, miR-660, and let7-i on EVs isolated from urine of recently diagnosed BC patients and healthy controls which showed 98.6% sensitivity and 100% specificity [156].
Screening miRNAs in EV serum samples of BC patients and healthy controls identified miR-142-5p, miR-320a, and miR-4433b-5p as differentially expressed [157]. Collectively, these miRNAs differentiate between various tumour characteristics, including subtype, size and grade. Significantly higher expression of miR-150-5p (AUC = 0.705), miR-576-3p (AUC = 0.691), and miR-4665-5p (AUC = 0.681) in plasma EVs from TNBC patients was shown to be a potential biomarker for recurrence [158]. Furthermore, EVs can be separated from tumour tissues removed during surgery to inspect miR-342-3p levels which correlate with chemo-resistance [143]. Another study found miR-1246 (AUC = 0.750, 78.1% sensitivity and 75% specificity) and miR-155 (AUC = 0.877, 68.8% sensitivity and 97.2% specificity) were significantly upregulated in EVs isolated from blood samples from trastuzumab-resistant compared to trastuzumab-sensitive BC patients [159].
Some EV lncRNAs also demonstrate potential as diagnostic markers for BC such as H19 [137, 160], XIST [161], and HOX transcript antisense RNA (HOTAIR) [162]. BC cells upregulate HOTAIR compared to adjacent tissues and healthy controls, whilst serum samples of BC patients showed increased levels of HOTAIR in EVs compared to healthy controls. Increased HOTAIR levels correlate with shorter survival, poor response to therapies [163] and HER2 positivity [164].
BC patients, especially those with TNBC, have higher levels of lncRNA SUMO1P3 in tissue and serum-derived EVs compared to healthy controls [165]. In this study, increased serum EV SUMO1P3 positively correlated with increased invasion, metastasis, and worse overall survival. After chemotherapy, SUMO1P3 levels decreased significantly in chemo-sensitive patients, but remained unchanged in chemo-resistant patients, highlighting SUMO1P3 as a potential prognostic EV biomarker for BC. Interestingly, SUMO1P3 negatively regulates miR-320a [166] which has tumour suppressive qualities and is found in the EVs of patients with smaller, early-stage BC tumours [157]. Therefore, a higher level of SUMO1P3 combined with a lower level of miR-320a could be used in combination as a prognostic marker for more aggressive BC tumours (Table 2).
To provide clinical utility, EV based biomarkers will need to demonstrate improvements compared to the internationally accepted standard of care investigations. The current studies investigating the use of EV ncRNA as biomarkers in BC have been focussed on discovery and initial technical validation. To progress further, researchers will need to perform much larger studies focussing on identifying the optimal role for these novel biomarkers in BC diagnostics and monitoring. These studies would need to be designed carefully and with appropriate power to demonstrate superior diagnostic ability compared to current investigations, either through improved patient acceptability or increased sensitivity. Alternatively, they could be studied as an additional test to the standard of care investigations to further reduce the false positive and false negative rates. Given the non-invasive nature of such proposed tests, it is likely these methods would demonstrate significant improvements in patient acceptability, though the relative sensitivity remains to be seen. With around 30 biofluids in humans, including saliva, ascites and lymph all potentially acting as reservoirs for cancer-derived EVs, the field of EV ncRNA biomarker discovery for BC is still in its early stages and the possibility of finding and implementing a highly specific and sensitive diagnostic, prognostic or predictive panel of ncRNA biomarkers for BC still remains [5].
Despite the identification of numerous promising biomarker ncRNAs in EVs for BC, significant challenges remain, particularly those associated with the separation of EVs from biofluids and their subsequent analysis. EV isolation is a technically challenging procedure that can be costly, time-consuming and highly variable with different protocols enriching different EV subpopulations [5]. As such, the detection of rare EV subpopulations in biofluids will need to be suitably robust to meet the requirements for large-scale, reliable diagnostics.
Conclusions
A vast number of studies have demonstrated the importance of ncRNAs in EV-mediated communication between cancer cells and the TME. Various forms of ncRNA have been linked to diverse processes in BC progression, including invasion and metastasis, growth, angiogenesis, metabolic changes, and drug resistance. Targeting pathways relevant to the regulatory role of ncRNAs in the TME may therefore represent a useful strategy for BC treatment. As a biomarker, EV-contained ncRNA could be useful for diagnosis, monitoring and predicting therapy responses due to the high specificity and sensitivity of ncRNAs, particularly combined as panels. Less invasive diagnostic techniques are preferable due to increased cooperation from patients, and so the further development and implementation of ncRNA-based diagnostic techniques could therefore increase early detection of BC, leading to improved survival.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–52.
Waks AG, Winer EP. Breast cancer treatment: a review. JAMA. 2019;321:288–300.
Niell BL, Freer PE, Weinfurtner RJ, Arleo EK, Drukteinis JS. Screening for breast cancer. Radio Clin North Am. 2017;55:1145–62.
Thery C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750.
Zhang Y, Liu Y, Liu H, Tang WH. Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci. 2019;9:19.
Santavanond JP, Rutter SF, Atkin-Smith GK, Poon IKH. Apoptotic bodies: mechanism of formation, isolation and functional relevance. Subcell Biochem. 2021;97:61–88.
Iwasaki YW, Siomi MC, Siomi H. PIWI-interacting RNA: its biogenesis and functions. Annu Rev Biochem. 2015;84:405–33.
Kopp F, Mendell JT. Functional classification and experimental dissection of long noncoding RNAs. Cell. 2018;172:393–407.
Chan JJ, Tay Y. Noncoding RNA:RNA regulatory networks in cancer. Int J Mol Sci. 2018;19:1310.
Yan H, Bu P. Non-coding RNA in cancer. Essays Biochem. 2021;65:625–39.
Villarroya-Beltri C, Gutiérrez-Vázquez C, Sánchez-Cabo F, Pérez-Hernández D, Vázquez J, Martin-Cofreces N, et al. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat Commun. 2013;4:2980.
Lei Y, Guo W, Chen B, Chen L, Gong J, Li W. Tumor‑released lncRNA H19 promotes gefitinib resistance via packaging into exosomes in non‑small cell lung cancer. Oncol Rep. 2018;40:3438–46.
Chen C, Luo Y, He W, Zhao Y, Kong Y, Liu H, et al. Exosomal long noncoding RNA LNMAT2 promotes lymphatic metastasis in bladder cancer. J Clin Invest. 2020;130:404–21.
Pérez-Boza J, Boeckx A, Lion M, Dequiedt F, Struman I. hnRNPA2B1 inhibits the exosomal export of miR-503 in endothelial cells. Cell Mol Life Sci. 2020;77:4413–28.
Pan Z, Zhao R, Li B, Qi Y, Qiu W, Guo Q, et al. EWSR1-induced circNEIL3 promotes glioma progression and exosome-mediated macrophage immunosuppressive polarization via stabilizing IGF2BP3. Mol Cancer. 2022;21:16.
Lee H, Li C, Zhang Y, Zhang D, Otterbein LE, Jin Y. Caveolin-1 selectively regulates microRNA sorting into microvesicles after noxious stimuli. J Exp Med. 2019;216:2202–20.
Chen C, Zheng H, Luo Y, Kong Y, An M, Li Y, et al. SUMOylation promotes extracellular vesicle-mediated transmission of lncRNA ELNAT1 and lymph node metastasis in bladder cancer. J Clin Invest. 2021;131:e146431.
Balaguer N, Moreno I, Herrero M, González M, Simón C, Vilella F. Heterogeneous nuclear ribonucleoprotein C1 may control miR-30d levels in endometrial exosomes affecting early embryo implantation. Mol Hum Reprod. 2018;24:411–25.
Santangelo L, Giurato G, Cicchini C, Montaldo C, Mancone C, Tarallo R, et al. The RNA-binding protein SYNCRIP is a component of the hepatocyte exosomal machinery controlling microRNA sorting. Cell Rep. 2016;17:799–808.
Hobor F, Dallmann A, Ball NJ, Cicchini C, Battistelli C, Ogrodowicz RW, et al. A cryptic RNA-binding domain mediates Syncrip recognition and exosomal partitioning of miRNA targets. Nat Commun. 2018;9:831.
Robinson H, Ruelcke JE, Lewis A, Bond CS, Fox AH, Bharti V, et al. Caveolin-1-driven membrane remodelling regulates hnRNPK-mediated exosomal microRNA sorting in cancer. Clin Transl Med. 2021;11:e381.
Leidal AM, Debnath J. LC3-dependent extracellular vesicle loading and secretion (LDELS). Autophagy. 2020;16:1162–3.
Leidal AM, Huang HH, Marsh T, Solvik T, Zhang D, Ye J, et al. The LC3-conjugation machinery specifies the loading of RNA-binding proteins into extracellular vesicles. Nat Cell Biol. 2020;22:187–99.
Temoche-Diaz MM, Shurtleff MJ, Nottingham RM, Yao J, Fadadu RP, Lambowitz AM, et al. Distinct mechanisms of microRNA sorting into cancer cell-derived extracellular vesicle subtypes. Elife. 2019;8:e47544.
Mukherjee K, Ghoshal B, Ghosh S, Chakrabarty Y, Shwetha S, Das S, et al. Reversible HuR-microRNA binding controls extracellular export of miR-122 and augments stress response. EMBO Rep. 2016;17:1184–203.
Shi Y, Wang Z, Zhu X, Chen L, Ma Y, Wang J, et al. Exosomal miR-1246 in serum as a potential biomarker for early diagnosis of gastric cancer. Int J Clin Oncol. 2020;25:89–99.
Shurtleff MJ, Temoche-Diaz MM, Karfilis KV, Ri S, Schekman R. Y-box protein 1 is required to sort microRNAs into exosomes in cells and in a cell-free reaction. Elife. 2016;5:e19276.
Shurtleff MJ, Yao J, Qin Y, Nottingham RM, Temoche-Diaz MM, Schekman R, et al. Broad role for YBX1 in defining the small noncoding RNA composition of exosomes. Proc Natl Acad Sci USA. 2017;114:E8987–e95.
Lin F, Zeng Z, Song Y, Li L, Wu Z, Zhang X, et al. YBX-1 mediated sorting of miR-133 into hypoxia/reoxygenation-induced EPC-derived exosomes to increase fibroblast angiogenesis and MEndoT. Stem Cell Res Ther. 2019;10:263.
Wozniak AL, Adams A, King KE, Dunn W, Christenson LK, Hung WT, et al. The RNA binding protein FMR1 controls selective exosomal miRNA cargo loading during inflammation. J Cell Biol. 2020;219:e201912074.
Ghoshal A, Rodrigues LC, Gowda CP, Elcheva IA, Liu Z, Abraham T, et al. Extracellular vesicle-dependent effect of RNA-binding protein IGF2BP1 on melanoma metastasis. Oncogene. 2019;38:4182–96.
Teng Y, Ren Y, Hu X, Mu J, Samykutty A, Zhuang X, et al. MVP-mediated exosomal sorting of miR-193a promotes colon cancer progression. Nat Commun. 2017;8:14448.
Lu P, Li H, Li N, Singh RN, Bishop CE, Chen X, et al. MEX3C interacts with adaptor-related protein complex 2 and involves in miR-451a exosomal sorting. PLoS One. 2017;12:e0185992.
Hagiwara K, Katsuda T, Gailhouste L, Kosaka N, Ochiya T. Commitment of annexin A2 in recruitment of microRNAs into extracellular vesicles. FEBS Lett. 2015;589:4071–8.
Weaver AM, Patton JG. Argonautes in extracellular vesicles: artifact or selected cargo? Cancer Res. 2020;80:379–81.
McKenzie AJ, Hoshino D, Hong NH, Cha DJ, Franklin JL, Coffey RJ, et al. KRAS-MEK signaling controls Ago2 sorting into exosomes. Cell Rep. 2016;15:978–87.
Iavello A, Frech VS, Gai C, Deregibus MC, Quesenberry PJ, Camussi G. Role of alix in miRNA packaging during extracellular vesicle biogenesis. Int J Mol Med. 2016;37:958–66.
Guduric-Fuchs J, O’Connor A, Camp B, O’Neill CL, Medina RJ, Simpson DA. Selective extracellular vesicle-mediated export of an overlapping set of microRNAs from multiple cell types. BMC Genomics. 2012;13:357.
Ahadi A, Brennan S, Kennedy PJ, Hutvagner G, Tran N. Long non-coding RNAs harboring miRNA seed regions are enriched in prostate cancer exosomes. Sci Rep. 2016;6:24922.
Janas T, Janas P, Sapoń K, Janas T. Binding of RNA aptamers to membrane lipid rafts: implications for exosomal miRNAs transfer from cancer to immune cells. Int J Mol Sci. 2020;21:8503.
Koppers-Lalic D, Hackenberg M. Bijnsdorp IV, van Eijndhoven MAJ, Sadek P, Sie D, et al. Nontemplated nucleotide additions distinguish the small RNA composition in cells from exosomes. Cell Rep. 2014;8:1649–58.
Sun C, Wang P, Dong W, Liu H, Sun J, Zhao L. LncRNA PVT1 promotes exosome secretion through YKT6, RAB7, and VAMP3 in pancreatic cancer. Aging. 2020;12:10427–40.
Yang L, Peng X, Li Y, Zhang X, Ma Y, Wu C, et al. Long non-coding RNA HOTAIR promotes exosome secretion by regulating RAB35 and SNAP23 in hepatocellular carcinoma. Mol Cancer. 2019;18:78.
Brabletz T, Kalluri R, Nieto MA, Weinberg RA. EMT in cancer. Nat Rev Cancer. 2018;18:128–34.
Kahlert UD, Joseph JV, Kruyt FAE. EMT- and MET-related processes in nonepithelial tumors: importance for disease progression, prognosis, and therapeutic opportunities. Mol Oncol. 2017;11:860–77.
Wang H, Wei H, Wang J, Li L. Chen A, Li Z. MicroRNA-181d-5p-containing exosomes derived from CAFs promote EMT by regulating CDX2/HOXA5 in breast cancer. Mol Ther Nucleic Acids. 2020;19:654–67.
Yan Z, Sheng Z, Zheng Y, Feng R, Xiao Q, Shi L, et al. Cancer-associated fibroblast-derived exosomal miR-18b promotes breast cancer invasion and metastasis by regulating TCEAL7. Cell Death Dis. 2021;12:1120.
Wang B, Mao JH, Wang BY, Wang LX, Wen HY, Xu LJ, et al. Exosomal miR-1910-3p promotes proliferation, metastasis, and autophagy of breast cancer cells by targeting MTMR3 and activating the NF-kappaB signaling pathway. Cancer Lett. 2020;489:87–99.
Shen S, Song Y, Zhao B, Xu Y, Ren X, Zhou Y, et al. Cancer-derived exosomal miR-7641 promotes breast cancer progression and metastasis. Cell Commun Signal. 2021;19:20.
Liang Z, Liu L, Gao R, Che C, Yang G. Downregulation of exosomal miR-7-5p promotes breast cancer migration and invasion by targeting RYK and participating in the atypical WNT signalling pathway. Cell Mol Biol Lett. 2022;27:88.
Su S, Li Y, Luo Y, Sheng Y, Su Y, Padia RN, et al. Proteinase-activated receptor 2 expression in breast cancer and its role in breast cancer cell migration. Oncogene. 2009;28:3047–57.
Das K, Prasad R, Ansari SA, Roy A, Mukherjee A, Sen P. Matrix metalloproteinase-2: a key regulator in coagulation proteases mediated human breast cancer progression through autocrine signaling. Biomed Pharmacother. 2018;105:395–406.
Das K, Paul S, Singh A, Ghosh A, Roy A, Ansari SA, et al. Triple-negative breast cancer-derived microvesicles transfer microRNA221 to the recipient cells and thereby promote epithelial-to-mesenchymal transition. J Biol Chem. 2019;294:13681–96.
Xia W, Liu Y, Cheng T, Xu T, Dong M, Hu X. Extracellular vesicles carry lncRNA SNHG16 to promote metastasis of breast cancer cells via the miR-892b/PPAPDC1A axis. Front Cell Dev Biol. 2021;9:628573.
Zhou W, Fong MY, Min Y, Somlo G, Liu L, Palomares MR, et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell. 2014;25:501–15.
Di Modica M, Regondi V, Sandri M, Iorio MV, Zanetti A, Tagliabue E, et al. Breast cancer-secreted miR-939 downregulates VE-cadherin and destroys the barrier function of endothelial monolayers. Cancer Lett. 2017;384:94–100.
Kalniete D, Nakazawa-Miklasevica M, Strumfa I, Abolins A, Irmejs A, Gardovskis J, et al. High expression of miR-214 is associated with a worse disease-specific survival of the triple-negative breast cancer patients. Hered Cancer Clin Pr. 2015;13:7.
Penna E, Orso F, Cimino D, Tenaglia E, Lembo A, Quaglino E, et al. microRNA-214 contributes to melanoma tumour progression through suppression of TFAP2C. EMBO J. 2011;30:1990–2007.
Orso F, Virga F, Dettori D, Dalmasso A, Paradzik M, Savino A, et al. Stroma-derived miR-214 coordinates tumor dissemination. J Exp Clin Cancer Res. 2023;42:20.
Lu Y, Chen L, Li L, Cao Y. Exosomes derived from brain metastatic breast cancer cells destroy the blood-brain barrier by carrying lncRNA GS1-600G8.5. Biomed Res Int. 2020;2020:7461727.
Tominaga N, Kosaka N, Ono M, Katsuda T, Yoshioka Y, Tamura K, et al. Brain metastatic cancer cells release microRNA-181c-containing extracellular vesicles capable of destructing blood-brain barrier. Nat Commun. 2015;6:6716.
Wang X, Qian T, Bao S, Zhao H, Chen H, Xing Z, et al. Circulating exosomal miR-363-5p inhibits lymph node metastasis by downregulating PDGFB and serves as a potential noninvasive biomarker for breast cancer. Mol Oncol. 2021;15:2466–79.
Ding J, Xu Z, Zhang Y, Tan C, Hu W, Wang M, et al. Exosome-mediated miR-222 transferring: an insight into NF-kappaB-mediated breast cancer metastasis. Exp Cell Res. 2018;369:129–38.
Kong X, Zhang J, Li J, Shao J, Fang L. MiR-130a-3p inhibits migration and invasion by regulating RAB5B in human breast cancer stem cell-like cells. Biochem Biophys Res Commun. 2018;501:486–93.
Sun L, He M, Xu N, Xu DH, Ben-David Y, Yang ZY, et al. Regulation of RAB22A by mir-193b inhibits breast cancer growth and metastasis mediated by exosomes. Int J Oncol. 2018;53:2705–14.
Qiu SQ, Waaijer SJH, Zwager MC, de Vries EGE, van der Vegt B, Schroder CP. Tumor-associated macrophages in breast cancer: Innocent bystander or important player? Cancer Treat Rev. 2018;70:178–89.
Li C, Li R, Hu X, Zhou G, Jiang G. Tumor-promoting mechanisms of macrophage-derived extracellular vesicles-enclosed microRNA-660 in breast cancer progression. Breast Cancer Res Treat. 2022;192:353–68.
Mao J, Wang L, Wu J, Wang Y, Wen H, Zhu X, et al. miR-370-3p as a novel biomarker promotes breast cancer progression by targeting FBLN5. Stem Cells Int. 2021;2021:4649890.
Park ES, Kim SJ, Kim SW, Yoon SL, Leem SH, Kim SB, et al. Cross-species hybridization of microarrays for studying tumor transcriptome of brain metastasis. Proc Natl Acad Sci USA. 2011;108:17456–61.
Zhang L, Zhang S, Yao J, Lowery FJ, Zhang Q, Huang WC, et al. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature. 2015;527:100–4.
Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989;8:98–101.
Sirkisoon SR, Carpenter RL, Rimkus T, Doheny D, Zhu D, Aguayo NR, et al. TGLI1 transcription factor mediates breast cancer brain metastasis via activating metastasis-initiating cancer stem cells and astrocytes in the tumor microenvironment. Oncogene. 2020;39:64–78.
Sirkisoon SR, Wong GL, Aguayo NR, Doheny DL, Zhu D, Regua AT, et al. Breast cancer extracellular vesicles-derived miR-1290 activates astrocytes in the brain metastatic microenvironment via the FOXA2–>CNTF axis to promote progression of brain metastases. Cancer Lett. 2022;540:215726.
Yoneda T, Williams PJ, Hiraga T, Niewolna M, Nishimura R. A bone-seeking clone exhibits different biological properties from the MDA-MB-231 parental human breast cancer cells and a brain-seeking clone in vivo and in vitro. J Bone Min Res. 2001;16:1486–95.
Morad G, Daisy CC, Otu HH, Libermann TA, Dillon ST, Moses MA. Cdc42-dependent transfer of mir301 from breast cancer-derived extracellular vesicles regulates the matrix modulating ability of astrocytes at the blood-brain barrier. Int J Mol Sci. 2020;21:3851.
Xing F, Liu Y, Wu SY, Wu K, Sharma S, Mo YY, et al. Loss of XIST in breast cancer activates MSN-c-Met and reprograms microglia via exosomal miRNA to promote brain metastasis. Cancer Res. 2018;78:4316–30.
Patanaphan V, Salazar OM, Risco R. Breast cancer: metastatic patterns and their prognosis. South Med J. 1988;81:1109–12.
Coleman R, Body JJ, Aapro M, Hadji P, Herrstedt J, Group EGW. Bone health in cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol. 2014;25:iii124–37.
Wu K, Feng J, Lyu F, Xing F, Sharma S, Liu Y, et al. Exosomal miR-19a and IBSP cooperate to induce osteolytic bone metastasis of estrogen receptor-positive breast cancer. Nat Commun. 2021;12:5196.
Liu X, Cao M, Palomares M, Wu X, Li A, Yan W, et al. Metastatic breast cancer cells overexpress and secrete miR-218 to regulate type I collagen deposition by osteoblasts. Breast Cancer Res. 2018;20:127.
Sun Z, Hu J, Ren W, Fang Y, Hu K, Yu H, et al. LncRNA SNHG3 regulates the BMSC osteogenic differentiation in bone metastasis of breast cancer by modulating the miR-1273g-3p/BMP3 axis. Biochem Biophys Res Commun. 2022;594:117–23.
Wang J, Zhang Q, Wang D, Yang S, Zhou S, Xu H, et al. Microenvironment-induced TIMP2 loss by cancer-secreted exosomal miR-4443 promotes liver metastasis of breast cancer. J Cell Physiol. 2020;235:5722–35.
Sayyad MR, Puchalapalli M, Vergara NG, Wangensteen SM, Moore M, Mu L, et al. Syndecan-1 facilitates breast cancer metastasis to the brain. Breast Cancer Res Treat. 2019;178:35–49.
Uen Y, Wang JW, Wang C, Jhang Y, Chung JY, Tseng T, et al. Mining of potential microRNAs with clinical correlation - regulation of syndecan-1 expression by miR-122-5p altered mobility of breast cancer cells and possible correlation with liver injury. Oncotarget. 2018;9:28165–75.
Li L, Xiong Y, Wang N, Zhu M, Gu Y. Breast cancer stem cells-derived extracellular vesicles affect PPARG expression by delivering microRNA-197 in breast cancer cells. Clin Breast Cancer. 2022;22:478–90.
Zhang G, Zhang W, Li B, Stringer-Reasor E, Chu C, Sun L, et al. MicroRNA-200c and microRNA- 141 are regulated by a FOXP3-KAT2B axis and associated with tumor metastasis in breast cancer. Breast Cancer Res. 2017;19:73.
Yue S, Ye X, Zhou T, Gan D, Qian H, Fang W, et al. PGRN(-/-) TAMs-derived exosomes inhibit breast cancer cell invasion and migration and its mechanism exploration. Life Sci. 2021;264:118687.
Ren Z, Lv M, Yu Q, Bao J, Lou K, Li X. MicroRNA-370-3p shuttled by breast cancer cell-derived extracellular vesicles induces fibroblast activation through the CYLD/Nf-kappaB axis to promote breast cancer progression. FASEB J. 2021;35:e21383.
Le MT, Hamar P, Guo C, Basar E, Perdigao-Henriques R, Balaj L, et al. miR-200-containing extracellular vesicles promote breast cancer cell metastasis. J Clin Invest. 2014;124:5109–28.
Park SM, Gaur AB, Lengyel E, Peter ME. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 2008;22:894–907.
Xun J, Du L, Gao R, Shen L, Wang D, Kang L, et al. Cancer-derived exosomal miR-138-5p modulates polarization of tumor-associated macrophages through inhibition of KDM6B. Theranostics. 2021;11:6847–59.
Liu Y, Yang Y, Du J, Lin D, Li F. MiR-3613-3p from carcinoma-associated fibroblasts exosomes promoted breast cancer cell proliferation and metastasis by regulating SOCS2 expression. IUBMB Life. 2020;72:1705–14.
Scognamiglio I, Cocca L, Puoti I, Palma F, Ingenito F, Quintavalle C, et al. Exosomal microRNAs synergistically trigger stromal fibroblasts in breast cancer. Mol Ther Nucleic Acids. 2022;28:17–31.
Baroni S, Romero-Cordoba S, Plantamura I, Dugo M, D’Ippolito E, Cataldo A, et al. Exosome-mediated delivery of miR-9 induces cancer-associated fibroblast-like properties in human breast fibroblasts. Cell Death Dis. 2016;7:e2312.
Yang SS, Ma S, Dou H, Liu F, Zhang SY, Jiang C, et al. Breast cancer-derived exosomes regulate cell invasion and metastasis in breast cancer via miR-146a to activate cancer associated fibroblasts in tumor microenvironment. Exp Cell Res. 2020;391:111983.
Wu HJ, Hao M, Yeo SK, Guan JL. FAK signaling in cancer-associated fibroblasts promotes breast cancer cell migration and metastasis by exosomal miRNAs-mediated intercellular communication. Oncogene. 2020;39:2539–49.
Kim KS, Park JI, Oh N, Cho HJ, Park JH, Park KS. ELK3 expressed in lymphatic endothelial cells promotes breast cancer progression and metastasis through exosomal miRNAs. Sci Rep. 2019;9:8418.
Bovy N, Blomme B, Freres P, Dederen S, Nivelles O, Lion M, et al. Endothelial exosomes contribute to the antitumor response during breast cancer neoadjuvant chemotherapy via microRNA transfer. Oncotarget. 2015;6:10253–66.
Vallabhaneni KC, Penfornis P, Xing F, Hassler Y, Adams KV, Mo YY, et al. Stromal cell extracellular vesicular cargo mediated regulation of breast cancer cell metastasis via ubiquitin conjugating enzyme E2 N pathway. Oncotarget. 2017;8:109861–76.
Ono M, Kosaka N, Tominaga N, Yoshioka Y, Takeshita F, Takahashi RU, et al. Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Sci Signal. 2014;7:ra63.
Du WW, Li X, Ma J, Fang L, Wu N, Li F, et al. Promotion of tumor progression by exosome transmission of circular RNA circSKA3. Mol Ther Nucleic Acids. 2022;27:276–92.
Nabet BY, Qiu Y, Shabason JE, Wu TJ, Yoon T, Kim BC, et al. Exosome RNA unshielding couples stromal activation to pattern recognition receptor signaling in cancer. Cell 2017;170:352–66.e13.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74.
Zhang P, Zhou H, Lu K, Lu Y, Wang Y, Feng T. Exosome-mediated delivery of MALAT1 induces cell proliferation in breast cancer. Onco Targets Ther. 2018;11:291–9.
Zhou D, Gu J, Wang Y, Wu H, Cheng W, Wang Q, et al. Long non-coding RNA NEAT1 transported by extracellular vesicles contributes to breast cancer development by sponging microRNA-141-3p and regulating KLF12. Cell Biosci. 2021;11:68.
Huang X, Lai S, Qu F, Li Z, Fu X, Li Q, et al. CCL18 promotes breast cancer progression by exosomal miR-760 activation of ARF6/Src/PI3K/Akt pathway. Mol Ther Oncolytics. 2022;25:1–15.
Xing L, Tang X, Wu K, Huang X, Yi Y, Huan J. LncRNA HAND2-AS1 suppressed the growth of triple negative breast cancer via reducing secretion of MSCs derived exosomal miR-106a-5p. Aging. 2020;13:424–36.
Chen B, Sang Y, Song X, Zhang D, Wang L, Zhao W, et al. Exosomal miR-500a-5p derived from cancer-associated fibroblasts promotes breast cancer cell proliferation and metastasis through targeting USP28. Theranostics. 2021;11:3932–47.
Tao S, Li H, Ma X, Ma Y, He J, Gao Y, et al. Elevating microRNA-1-3p shuttled by cancer-associated fibroblasts-derived extracellular vesicles suppresses breast cancer progression and metastasis by inhibiting GLIS1. Cancer Gene Ther. 2021;28:634–48.
Chen WX, Wang DD, Zhu B, Zhu YZ, Zheng L, Feng ZQ, et al. Exosomal miR-222 from adriamycin-resistant MCF-7 breast cancer cells promote macrophages M2 polarization via PTEN/Akt to induce tumor progression. Aging. 2021;13:10415–30.
Guo J, Duan Z, Zhang C, Wang W, He H, Liu Y, et al. Mouse 4T1 breast cancer cell-derived exosomes induce proinflammatory cytokine production in macrophages via miR-183. J Immunol. 2020;205:2916–25.
Njock MS, O’Grady T, Nivelles O, Lion M, Jacques S, Cambier M, et al. Endothelial extracellular vesicles promote tumour growth by tumour-associated macrophage reprogramming. J Extracell Vesicles. 2022;11:e12228.
Vallabhaneni KC, Penfornis P, Dhule S, Guillonneau F, Adams KV, Mo YY, et al. Extracellular vesicles from bone marrow mesenchymal stem/stromal cells transport tumor regulatory microRNA, proteins, and metabolites. Oncotarget. 2015;6:4953–67.
Lu C, Zhao Y, Wang J, Shi W, Dong F, Xin Y, et al. Breast cancer cell-derived extracellular vesicles transfer miR-182-5p and promote breast carcinogenesis via the CMTM7/EGFR/AKT axis. Mol Med. 2021;27:78.
Kong X, Li J, Li Y, Duan W, Qi Q, Wang T, et al. A novel long non-coding RNA AC073352.1 promotes metastasis and angiogenesis via interacting with YBX1 in breast cancer. Cell Death Dis. 2021;12:670.
Jung KO, Youn H, Lee CH, Kang KW, Chung JK. Visualization of exosome-mediated miR-210 transfer from hypoxic tumor cells. Oncotarget. 2017;8:9899–910.
van Balkom BW, de Jong OG, Smits M, Brummelman J, den Ouden K, de Bree PM, et al. Endothelial cells require miR-214 to secrete exosomes that suppress senescence and induce angiogenesis in human and mouse endothelial cells. Blood. 2013;121:3997–4006. S1-15
Pan S, Zhao X, Shao C, Fu B, Huang Y, Zhang N, et al. STIM1 promotes angiogenesis by reducing exosomal miR-145 in breast cancer MDA-MB-231 cells. Cell Death Dis. 2021;12:38.
Zheng X, Lu S, He Z, Huang H, Yao Z, Miao Y, et al. MCU-dependent negative sorting of miR-4488 to extracellular vesicles enhances angiogenesis and promotes breast cancer metastatic colonization. Oncogene. 2020;39:6975–89.
Lee JK, Park SR, Jung BK, Jeon YK, Lee YS, Kim MK, et al. Exosomes derived from mesenchymal stem cells suppress angiogenesis by down-regulating VEGF expression in breast cancer cells. PLoS One. 2013;8:e84256.
Pakravan K, Babashah S, Sadeghizadeh M, Mowla SJ, Mossahebi-Mohammadi M, Ataei F, et al. MicroRNA-100 shuttled by mesenchymal stem cell-derived exosomes suppresses in vitro angiogenesis through modulating the mTOR/HIF-1alpha/VEGF signaling axis in breast cancer cells. Cell Oncol. 2017;40:457–70.
Ghaffari-Makhmalbaf P, Sayyad M, Pakravan K, Razmara E, Bitaraf A, Bakhshinejad B, et al. Docosahexaenoic acid reverses the promoting effects of breast tumor cell-derived exosomes on endothelial cell migration and angiogenesis. Life Sci. 2021;264:118719.
Chang YH, Vuong CK, Ngo NH, Yamashita T, Ye X, Futamura Y, et al. Extracellular vesicles derived from Wharton’s Jelly mesenchymal stem cells inhibit the tumor environment via the miR-125b/HIF1alpha signaling pathway. Sci Rep. 2022;12:13550.
Campos A, Sharma S, Obermair A, Salomon C. Extracellular vesicle-associated miRNAs and chemoresistance: a systematic review. Cancers. 2021;13:4608.
Yi YW. Therapeutic implications of the drug resistance conferred by extracellular vesicles derived from triple-negative breast cancer cells. Int J Mol Sci. 2023;24:3704.
Rezaee M, Mohammadi F, Keshavarzmotamed A, Yahyazadeh S, Vakili O, Milasi YE, et al. The landscape of exosomal non-coding RNAs in breast cancer drug resistance, focusing on underlying molecular mechanisms. Front Pharm. 2023;14:1152672.
Chen WX, Liu XM, Lv MM, Chen L, Zhao JH, Zhong SL, et al. Exosomes from drug-resistant breast cancer cells transmit chemoresistance by a horizontal transfer of microRNAs. PLoS One. 2014;9:e95240.
Wei Y, Lai X, Yu S, Chen S, Ma Y, Zhang Y, et al. Exosomal miR-221/222 enhances tamoxifen resistance in recipient ER-positive breast cancer cells. Breast Cancer Res Treat. 2014;147:423–31.
Yu DD, Wu Y, Zhang XH, Lv MM, Chen WX, Chen X, et al. Exosomes from adriamycin-resistant breast cancer cells transmit drug resistance partly by delivering miR-222. Tumour Biol. 2016;37:3227–35.
Bliss SA, Sinha G, Sandiford OA, Williams LM, Engelberth DJ, Guiro K, et al. Mesenchymal stem cell-derived exosomes stimulate cycling quiescence and early breast cancer dormancy in bone marrow. Cancer Res. 2016;76:5832–44.
Wang B, Wang Y, Wang X, Gu J, Wu W, Wu H, et al. Extracellular vesicles carrying miR-887-3p promote breast cancer cell drug resistance by targeting BTBD7 and activating the Notch1/Hes1 signaling pathway. Dis Markers. 2022;2022:5762686.
Shen M, Dong C, Ruan X, Yan W, Cao M, Pizzo D, et al. Chemotherapy-induced extracellular vesicle miRNAs promote breast cancer stemness by targeting ONECUT2. Cancer Res. 2019;79:3608–21.
Deng Z, Rong Y, Teng Y, Zhuang X, Samykutty A, Mu J, et al. Exosomes miR-126a released from MDSC induced by DOX treatment promotes lung metastasis. Oncogene. 2017;36:639–51.
Xu CG, Yang MF, Ren YQ, Wu CH, Wang LQ. Exosomes mediated transfer of lncRNA UCA1 results in increased tamoxifen resistance in breast cancer cells. Eur Rev Med Pharm Sci. 2016;20:4362–8.
Zheng Z, Chen M, Xing P, Yan X, Xie B. Increased expression of exosomal AGAP2-AS1 (AGAP2 Antisense RNA 1) in breast cancer cells inhibits trastuzumab-induced cell cytotoxicity. Med Sci Monit. 2019;25:2211–20.
Wang X, Pei X, Guo G, Qian X, Dou D, Zhang Z, et al. Exosome-mediated transfer of long noncoding RNA H19 induces doxorubicin resistance in breast cancer. J Cell Physiol. 2020;235:6896–904.
Chen F, Chen J, Yang L, Liu J, Zhang X, Zhang Y, et al. Extracellular vesicle-packaged HIF-1alpha-stabilizing lncRNA from tumour-associated macrophages regulates aerobic glycolysis of breast cancer cells. Nat Cell Biol. 2019;21:498–510.
Hu K, Liu X, Li Y, Li Q, Xu Y, Zeng W, et al. Exosomes mediated transfer of Circ_UBE2D2 enhances the resistance of breast cancer to tamoxifen by binding to MiR-200a-3p. Med Sci Monit. 2020;26:e922253.
Wu X, Ren Y, Yao R, Zhou L, Fan R. Circular RNA circ-MMP11 contributes to lapatinib resistance of breast cancer cells by regulating the miR-153-3p/ANLN axis. Front Oncol. 2021;11:639961.
O’Brien K, Lowry MC, Corcoran C, Martinez VG, Daly M, Rani S, et al. miR-134 in extracellular vesicles reduces triple-negative breast cancer aggression and increases drug sensitivity. Oncotarget. 2015;6:32774–89.
Li Y, Liang Y, Sang Y, Song X, Zhang H, Liu Y, et al. MiR-770 suppresses the chemo-resistance and metastasis of triple negative breast cancer via direct targeting of STMN1. Cell Death Dis. 2018;9:14.
Yu S, Zhou Y, Niu L, Qiao Y, Yan Y. Mesenchymal stem cell-derived exosome mir-342-3p inhibits metastasis and chemo-resistance of breast cancer through regulating ID4. Genes Genomics. 2022;44:539–50.
Liu Y, Ma L, Hua F, Min Z, Zhan Y, Zhang W, et al. Exosomal circCARM1 from spheroids reprograms cell metabolism by regulating PFKFB2 in breast cancer. Oncogene. 2022;41:2012–25.
Huang S, Fan P, Zhang C, Xie J, Gu X, Lei S, et al. Exosomal microRNA-503-3p derived from macrophages represses glycolysis and promotes mitochondrial oxidative phosphorylation in breast cancer cells by elevating DACT2. Cell Death Discov. 2021;7:119.
Li Y, Zhao Z, Liu W, Li X. SNHG3 functions as miRNA sponge to promote breast cancer cells growth through the metabolic reprogramming. Appl Biochem Biotechnol. 2020;191:1084–99.
Yan W, Wu X, Zhou W, Fong MY, Cao M, Liu J, et al. Cancer-cell-secreted exosomal miR-105 promotes tumour growth through the MYC-dependent metabolic reprogramming of stromal cells. Nat Cell Biol. 2018;20:597–609.
Fong MY, Zhou W, Liu L, Alontaga AY, Chandra M, Ashby J, et al. Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat Cell Biol. 2015;17:183–94.
Cao M, Isaac R, Yan W, Ruan X, Jiang L, Wan Y, et al. Cancer-cell-secreted extracellular vesicles suppress insulin secretion through miR-122 to impair systemic glucose homeostasis and contribute to tumour growth. Nat Cell Biol. 2022;24:954–67.
McDonald ES, Clark AS, Tchou J, Zhang P, Freedman GM. Clinical diagnosis and management of breast cancer. J Nucl Med. 2016;57:9S–16S. Suppl 1
Kim MW, Park S, Lee H, Gwak H, Hyun KA, Kim JY, et al. Multi-miRNA panel of tumor-derived extracellular vesicles as promising diagnostic biomarkers of early-stage breast cancer. Cancer Sci. 2021;112:5078–87.
Carvalho TM, Brasil GO, Jucoski TS, Adamoski D, de Lima RS, Spautz CC, et al. MicroRNAs miR-142-5p, miR-150-5p, miR-320a-3p, and miR-4433b-5p in serum and tissue: potential biomarkers in sporadic breast cancer. Front Genet. 2022;13:865472.
Hannafon BN, Trigoso YD, Calloway CL, Zhao YD, Lum DH, Welm AL, et al. Plasma exosome microRNAs are indicative of breast cancer. Breast Cancer Res. 2016;18:90.
Pan Y, Xu X, Luo T, Yang S, Zhou D, Zeng Y. A breast cancer prediction model based on a panel from circulating exosomal miRNAs. Biomed Res Int. 2022;2022:5170261.
Inubushi S, Kawaguchi H, Mizumoto S, Kunihisa T, Baba M, Kitayama Y, et al. Oncogenic miRNAs identified in tear exosomes from metastatic breast cancer patients. Anticancer Res. 2020;40:3091–6.
Hirschfeld M, Rucker G, Weiss D, Berner K, Ritter A, Jager M, et al. Urinary exosomal microRNAs as potential non-invasive biomarkers in breast cancer detection. Mol Diagn Ther. 2020;24:215–32.
Ozawa PMM, Vieira E, Lemos DS, Souza ILM, Zanata SM, Pankievicz VC, et al. Identification of miRNAs enriched in extracellular vesicles derived from serum samples of breast cancer patients. Biomolecules. 2020;10:150.
Wu H, Wang Q, Zhong H, Li L, Zhang Q, Huang Q, et al. Differentially expressed microRNAs in exosomes of patients with breast cancer revealed by next‑generation sequencing. Oncol Rep. 2020;43:240–50.
Zhang Z, Zhang L, Yu G, Sun Z, Wang T, Tian X, et al. Exosomal miR-1246 and miR-155 as predictive and prognostic biomarkers for trastuzumab-based therapy resistance in HER2-positive breast cancer. Cancer Chemother Pharm. 2020;86:761–72.
Zhong G, Wang K, Li J, Xiao S, Wei W, Liu J. Determination of serum exosomal H19 as a noninvasive biomarker for breast cancer diagnosis. Onco Targets Ther. 2020;13:2563–71.
Lan F, Zhang X, Li H, Yue X, Sun Q. Serum exosomal lncRNA XIST is a potential non-invasive biomarker to diagnose recurrence of triple-negative breast cancer. J Cell Mol Med. 2021;25:7602–7.
Abba MC, Fabre ML, Lee J, Tatineni P, Kil H, Aldaz CM. HOTAIR modulated pathways in early-stage breast cancer progression. Front Oncol. 2021;11:783211.
Tang S, Zheng K, Tang Y, Li Z, Zou T, Liu D. Overexpression of serum exosomal HOTAIR is correlated with poor survival and poor response to chemotherapy in breast cancer patients. J Biosci. 2019;44:37.
Centers for Disease C. Multistate outbreak of Shigella sonnei gastroenteritis–United States. MMWR Morb Mortal Wkly Rep. 1987;36:440–2. 8-9
Na-Er A, Xu YY, Liu YH, Gan YJ. Upregulation of serum exosomal SUMO1P3 predicts unfavorable prognosis in triple negative breast cancer. Eur Rev Med Pharm Sci. 2021;25:154–60.
Liu J, Song Z, Feng C, Lu Y, Zhou Y, Lin Y, et al. The long non-coding RNA SUMO1P3 facilitates breast cancer progression by negatively regulating miR-320a. Am J Transl Res. 2017;9:5594–602.
Ando W, Kikuchi K, Uematsu T, Yokomori H, Takaki T, Sogabe M, et al. Novel breast cancer screening: combined expression of miR-21 and MMP-1 in urinary exosomes detects 95% of breast cancer without metastasis. Sci Rep. 2019;9:13595.
Li S, Zhang M, Xu F, Wang Y, Leng D. Detection significance of miR-3662, miR-146a, and miR-1290 in serum exosomes of breast cancer patients. J Cancer Res Ther. 2021;17:749–55.
Funding
This work was supported by Action Against Cancer.
Author information
Authors and Affiliations
Contributions
MS, WJ, BT, CT, SR and GG contributed to the writing and editing of this manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
GG is editor of Cancer Gene Therapy and Oncogene and founder/chief scientific officer of Stingray Bio. The other authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Samuels, M., Jones, W., Towler, B. et al. The role of non-coding RNAs in extracellular vesicles in breast cancer and their diagnostic implications. Oncogene 42, 3017–3034 (2023). https://doi.org/10.1038/s41388-023-02827-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-023-02827-y