Abstract
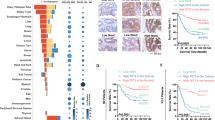
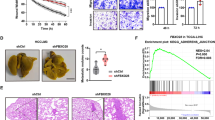
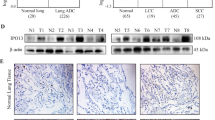
Nucleocytoplasmic transport of proteins is disrupted and dysregulated in cancer cells. Nuclear pore complexes and cargo proteins are two main transportation regulators. However, the mechanism regulating nucleocytoplasmic transport in cancer remains elusive. Here, we identified a S100A2/KPNA2 cotransport complex that transports the tumor-associated transcription factor NFYA in colorectal cancer (CRC). Through the S100A2/KNPA2 complex, depending on its interaction with S100A2, NFYA is transported to the nucleus and inhibits the transcriptional activity of E-cadherin, which in turn promotes CRC metastasis. Targeting the S100A2/KPNA2 binding sites with the specific inhibitor delanzomib is a potential therapeutic approach for CRC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The accession number for RNA sequencing data is PRJNA778318 and other data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
References
Kau TR, Way JC, Silver PA. Nuclear transport and cancer: from mechanism to intervention. Nat Rev Cancer. 2004;4:106–17.
Jamieson C, Sharma M, Henderson BR. Targeting the beta-catenin nuclear transport pathway in cancer. Semin Cancer Biol. 2014;27:20–29.
Chen LF, Greene WC. Regulation of distinct biological activities of the NF-kappaB transcription factor complex by acetylation. J Mol Med (Berl). 2003;81:549–57.
Gravina GL, Senapedis W, McCauley D, Baloglu E, Shacham S, Festuccia C. Nucleo-cytoplasmic transport as a therapeutic target of cancer. J Hematol Oncol. 2014;7:85
Mattaj IW, Englmeier L. Nucleocytoplasmic transport: the soluble phase. Annu Rev Biochem. 1998;67:265–306.
Strom AC, Weis K. Importin-beta-like nuclear transport receptors. Genome Biol. 2001;2:REVIEWS3008.
Stewart M. Molecular mechanism of the nuclear protein import cycle. Nat Rev Mol Cell Biol. 2007;8:195–208.
Kelley JB, Talley AM, Spencer A, Gioeli D, Paschal BM. Karyopherin alpha7 (KPNA7), a divergent member of the importin alpha family of nuclear import receptors. BMC Cell Biol. 2010;11:63.
Chen CF, Li S, Chen Y, Chen PL, Sharp ZD, Lee WH. The nuclear localization sequences of the BRCA1 protein interact with the importin-alpha subunit of the nuclear transport signal receptor. J Biol Chem. 1996;271:32863–8.
Li J, Liu Q, Liu Z, Xia Q, Zhang Z, Zhang R, et al. KPNA2 promotes metabolic reprogramming in glioblastomas by regulation of c-myc. J Exp Clin Cancer Res. 2018;37:194.
Li XL, Jia LL, Shi MM, Li X, Li ZH, Li HF, et al. Downregulation of KPNA2 in non-small-cell lung cancer is associated with Oct4 expression. J Transl Med. 2013;11:232.
Xiang S, Wang Z, Ye Y, Zhang F, Li H, Yang Y, et al. E2F1 and E2F7 differentially regulate KPNA2 to promote the development of gallbladder cancer. Oncogene. 2019;38:1269–81.
Christiansen A, Dyrskjot L. The functional role of the novel biomarker karyopherin alpha 2 (KPNA2) in cancer. Cancer Lett. 2013;331:18–23.
Mueller A, Schafer BW, Ferrari S, Weibel M, Makek M, Hochli M, et al. The calcium-binding protein S100A2 interacts with p53 and modulates its transcriptional activity. J Biol Chem. 2005;280:29186–93.
Masuda T, Ishikawa T, Mogushi K, Okazaki S, Ishiguro M, Iida S, et al. Overexpression of the S100A2 protein as a prognostic marker for patients with stage II and III colorectal cancer. Int J Oncol. 2016;48:975–82.
Bulk E, Sargin B, Krug U, Hascher A, Jun Y, Knop M, et al. S100A2 induces metastasis in non-small cell lung cancer. Clin Cancer Res. 2009;15:22–29.
Sato Y, Harada K, Sasaki M, Nakanuma Y. Clinicopathological significance of S100 protein expression in cholangiocarcinoma. J Gastroenterol Hepatol. 2013;28:1422–9.
Ohuchida K, Mizumoto K, Miyasaka Y, Yu J, Cui L, Yamaguchi H, et al. Over-expression of S100A2 in pancreatic cancer correlates with progression and poor prognosis. J Pathol. 2007;213:275–82.
Wang HJ, Zhang ZD, Li RY, Ang KK, Zhang HZ, Caraway NP, et al. Overexpression of S100A2 protein as a prognostic marker for patients with stage I non small cell lung cancer. Int J Cancer. 2005;116:285–90.
van Bon L, Cossu M, Loof A, Gohar F, Wittkowski H, Vonk M, et al. Proteomic analysis of plasma identifies the Toll-like receptor agonists S100A8/A9 as a novel possible marker for systemic sclerosis phenotype. Ann Rheum Dis. 2014;73:1585–9.
Henze G, Dummer R, Joller-Jemelka HI, Boni R, Burg G. Serum S100−a marker for disease monitoring in metastatic melanoma. Dermatology. 1997;194:208–12.
Takata M, Shimamoto S, Yamaguchi F, Tokuda M, Tokumitsu H, Kobayashi R. Regulation of nuclear localization signal-importin alpha interaction by Ca2+/S100A6. FEBS Lett. 2010;584:4517–23.
Wang CI, Chien KY, Wang CL, Liu HP, Cheng CC, Chang YS, et al. Quantitative proteomics reveals regulation of karyopherin subunit alpha-2 (KPNA2) and its potential novel cargo proteins in nonsmall cell lung cancer. Mol Cell Proteom. 2012;11:1105–22.
Bresnick AR, Weber DJ, Zimmer DB. S100 proteins in cancer. Nat Rev Cancer. 2015;15:96–109.
Li C, Chen Q, Zhou Y, Niu Y, Wang X, Li X. et al. S100A2 promotes glycolysis and proliferation via GLUT1regulation in colorectal cancer. FASEB J. 2020;34:13333–44.
Kirschner RD, Sanger K, Muller GA, Engeland K. Transcriptional activation of the tumor suppressor anddifferentiation gene S100A2 by a novel p63-binding site. Nucleic Acids Res. 2008;36:2969–80.
Kumar M, Srivastava G, Kaur J, Assi J, Alyass A, Leong I, et al. Prognostic significance of cytoplasmic S100A2overexpression in oral cancer patients. J Transl Med. 2015;13:8.
van Dieck J, Brandt T, Teufel DP, Veprintsev DB, Joerger AC, Fersht AR. Molecular basis of S100 proteins interacting with the p53 homologs p63 and p73. Oncogene. 2010;29:2024–35.
Santamaria-Kisiel L, Rintala-Dempsey AC, Shaw GS. Calcium-dependent and -independent interactions of theS100 protein family. Biochem J. 2006;396:201–14.
Naz S, Bashir M, Ranganathan P, Bodapati P, Santosh V, Kondaiah P. Protumorigenic actions of S100A2involve regulation of PI3/Akt signaling and functional interaction with Smad3. Carcinogenesis. 2014;35:14–23.
Narayanan S, Cai CY, Assaraf YG, Guo HQ, Cui Q, Wei L. et al. Targeting the ubiquitin-proteasome pathway toovercome anti-cancer drug resistance. Drug Resist Updat. 2020;48:100663.
Acknowledgements
We thank Qiong Huang and Yangwei Li from the core facility platform of Zhejiang University School of Medicine for their technical support. We thank Professor Jimin Shao lab for providing vectors of pGL3-basic, pRL-TK, pGEX-GST, and pET-28a-His and Run Run Shaw Hospital, Zhejiang University for providing colorectal carcinoma samples and serum from patients. We thank Shenghui Hong and Ping Liu in the Laboratory Animal Center of Zhejiang University for their technical assistance on breeding and management of mice.
Funding
This work is supported by the National Natural Science Foundation of China (81871937, 82001586, 91859204, 82072629, 82072811) and CAMS Innovation Fund for Medical Sciences (CIFMS, 2019-I2M-5-044).
Author information
Authors and Affiliations
Contributions
Conceptualization: HZ and ML; methodology: F Han, LZ, SL, and LQ; investigation: F Han, LZ, SL, F Hou, and JG; resources: F Han, LZ, LQ, and F Hou; writing—original draft: F Han and LZ; writing—review and editing: HZ and ML; supervision: HZ and ML; funding acquisition: HZ and ML.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Han, F., Zhang, L., Liao, S. et al. The interaction between S100A2 and KPNA2 mediates NFYA nuclear import and is a novel therapeutic target for colorectal cancer metastasis. Oncogene 41, 657–670 (2022). https://doi.org/10.1038/s41388-021-02116-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-021-02116-6
This article is cited by
-
S100A2 induces epithelial–mesenchymal transition and metastasis in pancreatic cancer by coordinating transforming growth factor β signaling in SMAD4-dependent manner
Cell Death Discovery (2023)
-
CAV2 promotes the invasion and metastasis of head and neck squamous cell carcinomas by regulating S100 proteins
Cell Death Discovery (2022)