Abstract
Background
High sodium intake has been linked to the prevalence of non-alcoholic fatty liver disease (NAFLD), but underlying mechanism remains unclear. This study aims to explore the role of chronic inflammation in the association between sodium and NAFLD. We also observed whether β-carotene, which had a strong anti-inflammatory effect, lowers the odds of NAFLD.
Methods
We performed mediation analyses to assess the mediating effects of C-reactive protein (CRP) and red cell distribution width (RDW) on the relationship between dietary sodium and NAFLD defined by the hepatic steatosis index (HSI) and the fatty liver index (FLI), respectively.
Results
A total of 6725 participants were included in this study. Compared with the high sodium-low carotene group, participants in the high sodium-high carotene group had 16% and 26% lower odds for HSI and FLI-defined NAFLD, respectively. There were positive indirect effects of dietary sodium intake on the HSI-defined NAFLD (indirect effect: 0.0057, 95% CI: 0.0021–0.0091, P < 0.0001), as well as the FLI defined NAFLD (indirect effect: 0.0081, 95% CI: 0.0024–0.0162, P < 0.0001) when C-reactive protein (CRP) was considered as a mediator. The mediating effects were somewhat attenuated after further adjusting for dietary β-carotene intake. Similar results were found when RDW was considered as a mediator in the HSI-defined NAFLD analysis.
Conclusions
Higher sodium intake increases the odds of NAFLD by upregulating inflammation. Dietary β-carotene may attenuate this association by down regulating inflammation.
Similar content being viewed by others
Introduction
High sodium intake has been confirmed as one of the most important risk factors for high blood pressure [1,2,3]. Excessive sodium intake also has been linked to higher risk of overweight/obesity as well as cardiovascular diseases [4,5,6,7]. Our previous study reported that dietary sodium intake was positively associated with non-alcoholic fatty liver disease (NAFLD) in a representative US general population [8]. NAFLD is a liver condition defined as the accumulation of intracellular fat in hepatocytes in the absence of excessive alcohol intake, and it is considered the hepatic expression of the metabolic syndrome. To date, the mechanisms underlying sodium intake in relation to NAFLD remain unclear. As components of metabolic syndrome, NAFLD, high blood pressure, and overweight/obesity share a common pathophysiology, that is, chronic inflammation [9,10,11]. As a systemic inflammation biomarker, C-reactive protein (CRP) has been linked to disease and mortality risk [9]. Recently, red cell distribution width (RDW) has also been suggested as a marker that reflect an underlying chronic inflammatory state [12,13,14]. We assume that high sodium intake increases the risk of NAFLD by upregulating inflammation. If this hypothesis is true, down regulating inflammation will improve hepatic steatosis caused by high sodium intake. β-carotene, a retinol precursor and antioxidant that is found in certain fruits and vegetables, has been found to have a strong anti-inflammatory effect [15,16,17]. Our second hypothesis, accordingly, is that high dietary β-carotene intake attenuates the effects of high sodium intake on NAFLD by down regulating inflammation. The present study aims to use mediation analyses to test the above two hypotheses by utilizing data from two cycles (cycle 2007–2008 and cycle 2009–2010) of the National Health and Nutrition Examination Survey (NHANES).
Subjects and methods
Study population
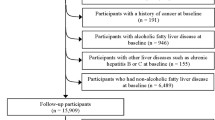
Data were derived from two cycles of NHANES (2007–2008 and 2009–2010). A detailed description of the NHANES study design and methods is available elsewhere [18]. In total, 20,686 individuals completed the NHANES surveys between 2007 and 2010 within two cycles (10,149 in NHANES 2007–2008 and 10,537 in NHANES 2009–2010). In this study, we included individuals aged ≥20 years who completed two 24-h recalls with valid energy intakes (n = 9477). A valid level was defined as total energy intake from both 24-h recalls within 500–5000 kcal/day for women and 500–8000 kcal/day for men [19]. We further excluded 2719 subjects for the following reasons: (1) pregnant women (n = 106); (2) heavy alcohol drinking defined as >21 drinks per week for men and >14 drinks per week for women (n = 1715) [8]; (3) serum hepatitis B surface antigen (HBsAg) or serum hepatitis C antibody was positive (n = 156); (4) important covariates were not available (n = 775). This resulted in 6725 participants included in the hepatic steatosis index (HSI) defined NAFLD analysis. A subsample of 3237 participants was included in the fatty liver index (FLI) defined NAFLD analysis because FLI requires fasting blood tests. A detailed flowchart depicting participant selection is shown in Fig. 1. The National Center for Health Statistics institutional review board approved the study protocol (#2005–06), and all participants provided written informed consent. All data used in this manuscript are de-identified and freely available to the public through https://wwwn.cdc.gov/nchs/nhanes/.
Data collection
Data were collected by trained interviewers during interviews in the participants’ homes and through medical examination and subsequent laboratory assessments in the Mobile Examination Center (MEC). The questionnaire contains a number of questions on demographics, health conditions, and lifestyles such as smoking, drinking, house income, and passive sedentary behavior.
The ethnicity was divided into Hispanic, non-Hispanic White, non-Hispanic Black, and other races. As the house income variable in our analysis, family monthly poverty level index was calculated by dividing family income by the poverty guidelines, specific to family size, as well as the appropriate year and state. The index was grouped into three categories (≤1.30, 1.31–1.85, and >1.85).
According to participants’ answers to the questionnaire, smoking status was categorized into current, former, and never smoker. Drinkers were defined as participants drinking alcohol at least 12 times in the previous year. Sedentary time, defined as total time spent sitting in a typical day not including time spent sleeping, was used as a passive sedentary behavior indicator in our analysis.
Dietary intake was documented and validated utilizing the 24-h dietary history interview. Participants received two 24-h dietary recall interviews. The first dietary recall interview was collected face-to-face in the MEC, and the second was collected by telephone 3 to 10 days later. Dietary intake was estimated by the mean of the two dietary recalls. Dietary sodium (in mg/d) and β-carotene (in µg/day) intakes were used as the main exposure variables. We calculated the dietary inflammatory index (DII) score according to the calculating methods provided by a previous study [20]. DII is a literature-derived dietary tool for measuring individual dietary inflammatory potential. The DII score was used as a covariates in the analysis. Body weight (in kg) was measured using a digital weight scale and standing height (cm) was measured using a stadiometer. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Waist circumference (WC) was measured to the nearest 0.1 cm at the end of the participant’s normal expiration using a tape by the standard method.
Laboratory tests
Blood specimens were processed, stored, and shipped to Fairview Medical Center Laboratory at the University of Minnesota, Minneapolis, Minnesota for analysis. Serum alanine aminotransferase (ALT) was measured by a kinetic rate method in Beckman Coulter UniCel® DxC800 Synchron Clinical System. Serum aspartate aminotransferase (AST) and gamma-glutamyl transferase (GGT) were measured by enzymatic rate methods in Beckman Coulter UniCel® DxC800 Synchron Clinical System. Hemoglobin A1c (HbA1c) was measured by high-performance liquid chromatography (HPLC). The analyzer dilutes the whole blood specimen with Hemolysis & Wash Solution, and then injects a small volume of the treated specimen onto the HPLC analytical column. Separation is achieved by utilizing differences in ionic interactions between the cation exchange group on the column resin surface and the hemoglobin components. The separated hemoglobin components pass through the photometer flow cell where the analyzer measures changes in absorbance at 415 nm. The analyzer integrates and reduces the raw data, and then calculates the relative percentages of each hemoglobin fraction. We defined diabetes as HbA1c ≥ 6.5% and/or current treatment with a hypoglycemic agent or insulin [21]. The laboratory method used for triglycerides (TG) measurement was enzymatic assay using Roche/Hitachi Modular P Chemistry Analyzer. CRP was measured by latex-enhanced nephelometry on a Behring Nephelometer. Particle-enhanced assays are based on the reaction between a soluble analyte and the corresponding antigen or antibody bound to polystyrene particles. For the quantification of CRP, particles consisting of a polystyrene core and a hydrophilic shell are used in order to link anti-CRP antibodies covalently. A dilute solution of test sample is mixed with latex particles coated with mouse monoclonal anti-CRP antibodies. CRP concentrations are calculated by using a calibration curve. Data reduction of the signals is performed by using a storable logit-log function for the calibration curve. These assays are performed on a Siemens/Behring Nephelometer for quantitative CRP determination. Red cell distribution width represents the size distribution spread of the erythrocyte population derived from the red blood cell (RBC) histogram. It is the coefficient of variation, expressed in percent, of the RBC size distribution. RBC was directly measured on the Coulter® HMX. A detailed description of laboratory methods and materials used in the tests is available at https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/labmethods.aspx?BeginYear=2007.
Definition of NAFLD
NAFLD was defined using HSI and FLI, both of which have been validated externally and used in epidemiologic studies, in the absence of other causes of chronic liver disease [22,23,24]. HSI and FLI were calculated using the following two formulas:
(1) HSI = 8 × (ALT/AST ratio) + BMI (+2, if female; +2, if diabetes). In this case, NAFLD was defined as HSI > 36.
(2) FLI = [e0.953✕loge (TG) + 0.139✕BMI + 0.718✕loge (GGT) + 0.053✕WC − 15.745]/[1 + e0.953✕loge (TG) + 0.139✕BMI + 0.718✕loge (GGT) + 0.053✕WC – 15.745] ✕ 100. In this case, NAFLD was defined as FLI ≥ 60.
Statistical analysis
Data were presented as mean ± standard deviation (SD) for continuous variables and as frequency (percentage) for categorical variables. Dietary sodium and β-carotene intake were dichotomized using their median values and were cross combined into four groups (Low sodium-High carotene, Low sodium-Low carotene, High sodium-High carotene, and High sodium-Low carotene). We tested differences in baseline characteristics among the above four groups with one-way analysis of variance for continuous variables and Chi-square tests for categorical variables. The odds ratios (ORs) and 95% confidence intervals (CIs) for NAFLD of each group were estimated using unconditional binary logistic regression models. Potential covariates such as age, sex, ethnicity, education level, family monthly poverty level, passive sedentary hours, smoking status, drinking status, total energy intake, DII, and serum creatinine were included in multivariable analyses. The selection of covariates was based on clinical knowledge but not specific statistical way (e.g., forward or backward). The restricted cubic spline model was used for the dose–response analysis. When we analyzing the dose–response relationship between sodium or β-carotene and NAFLD separately, we mutually adjusted for β-carotene and sodium in the model.
When performing mediation analysis, we followed the AGReMA statement, a guideline for reporting mediation analyses [25]. The variables of sodium intake, CRP, and RDW were standardized prior to analyses in order to compare effects across different variables by solving shifting or scaling issues. The mediation analyses include the following steps. First, linear regression was applied to examine the associations between sodium and CRP (or RDW), adjusting for age, sex, ethnicity, education level, family monthly poverty level, passive sedentary hours, smoking status, drinking status, total energy intake, DII, and serum creatinine (path α). Second, CRP (or RDW) was additionally controlled in the logistic regression model to estimate the direct effect of sodium intake on NAFLD, adjusting for the same covariates (path γ’). Third, the mediate function from the R mediation package with the causal analytical approach was applied to quantify the indirect effect (path α & path β) and the total effect (path γ) [26, 27]. To observe the effect of β-carotene on the mediating effects, we repeated the above analyses with adjustment for β-carotene. The mediation package is freely available for download via the Comprehensive R Archive Network (CRAN) at http://CRAN.R-project.org/package=mediation. A detailed description of the construction and statistic approaches for the mediation package is available at https://cran.r-project.org/web/packages/mediation/vignettes/mediation.pdf. The mediation analyses diagram for the association of dietary sodium and β-carotene intakes with NAFLD is shown in Fig. 2.
Note: Path α represents the regression coefficient for the association of dietary sodium intake with CRP or RDW. Path β represents the regression coefficient for the association of CRP or RDW with NAFLD. The product of regression coefficients α and β represents the mediated effect (indirect effect) of CRP or RDW (α*β). Path γ’ represents the direct effect of dietary sodium intake with NAFLD, after adjustment for CRP or RDW. Path γ represents the simple total effect of dietary sodium intake on NAFLD, without adjustment for CRP and RDW.
A two-tailed P < 0.05 was considered significant. Analyses were performed using SAS version 9.4 (SAS Institute, Cary, North Carolina) and R version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
A total of 6725 participants (3119 men and 3606 women) with a mean age of 53.2 ± 17.4 years were included in this study. The characteristics of the study population by the combination groups of dietary sodium and β-carotene intakes are presented in Table 1. Compared with participants with high sodium and low β-carotene intakes, those with high sodium and high β-carotene intakes were older and more likely to be white race, to have higher education level, to have higher family income, to have longer passive sedentary time, and to have lower DII and log-transformed CRP level. In addition, those with high sodium and high β-carotene intakes were more likely to be drinker and less likely to be current smoker.
Table 2 presents ORs and 95% CIs for the HSI and FLI defined NAFLD, respectively, by different combinations of dietary sodium and β-carotene intakes. Compared with the high sodium-low carotene group, participants in the high sodium-high carotene group had a lower odds for both the HSI and FLI defined NAFLD. The corresponding ORs and 95% CIs were 0.84 (0.73–0.98) for the HSI-defined NAFLD and 0.74 (0.60–0.92) for the FLI defined NAFLD after adjusting for age, sex, ethnicity, education level, family monthly poverty level, smoking, drinking, sedentary time, total energy intake, DII, and serum creatinine. Besides, participants in the low sodium-high carotene group had the lowest odds for NAFLD compared with the high sodium-low carotene group.
The dose–response relations of dietary sodium and β-carotene intakes with NAFLD defined by HSI and FLI were shown in Fig. 3. There was a significant positive dose–response relationship between dietary sodium intake and NAFLD after adjusting for covariates described above plus β-carotene (P < 0.001). In contrast, there was a significant inverse association between dietary β-carotene intake and NAFLD after adjustment for covariates including dietary sodium intake (P < 0.05).
The restricted cubic spline model was basically adjusted for age, sex, ethnicity, education level, family monthly poverty level, smoking, drinking, sedentary time, total energy intake, dietary inflammatory index, and serum creatinine. A and C additionally adjusted for dietary β-carotene intake; B and D additionally adjusted for dietary sodium intake.
Results from the mediation analysis for the association between dietary sodium intake and NAFLD with or without adjustment for dietary β-carotene intake were shown in Table 3. There were positive indirect effects of dietary sodium intake on the HSI-defined NAFLD (indirect effect: 0.0057, 95% CI: 0.0021–0.0091, P < 0.0001) as well as the FLI defined NAFLD (indirect effect: 0.0081, 95% CI: 0.0024–0.0162, P < 0.0001) when CRP was considered as a mediator. As expected, the mediating effects were somewhat attenuated when we further adjusted for dietary β-carotene intake. Similar results were found when RDW was considered as a mediator in the HSI analysis. However, the mediating effect of RDW on the relationship between dietary sodium intake and FLI defined NAFLD did not reach the statistical significant level.
Discussion
In this study on a representative sample of US adults, we found that dietary sodium intake was positively associated with NAFLD, while dietary β-carotene intake was inversely associated with NAFLD. Participants who had high sodium and high β-carotene intakes had a lower odds of NAFLD compared with those had high sodium and low β-carotene intakes. The mediation analysis supports the association of dietary sodium with the NAFLD, being, at least partially, mediated by the effect of inflammation.
In recent years, several previous studies including our own study reported an independent relationship between dietary sodium intake and NAFLD. A study using data from the Korea National Health and Nutrition Examination Surveys (2008–2010) found significant associations between higher estimated 24-h urinary sodium excretion and NAFLD as assessed by both HSI (OR: 1.39, 95% CI: 1.26–1.55) and FLI (OR: 1.75, 95% CI: 1.39–2.20) [28]. Another study conducted in the Korea population also suggested that higher dietary sodium intake was associated with a greater prevalence of NAFLD in young and middle-aged general adults [29]. Results from the Prevention of Renal and Vascular End-Stage Disease (PREVEND) cohort study showed that higher dietary sodium intake was positively associated with NAFLD as defined by the FLI, with an OR per SD increment of 1.30 (95% CI: 1.21–1.41). Similar results were also shown for the HSI-defined NAFLD, where the corresponding OR and 95% CI were 1.40 (1.31–1.51) [30]. Our previous study based on NHANES found that comparing with the lowest quartile of dietary sodium intake, the highest quartile had a multivariable-adjusted OR and 95 % CI of 1.46 (1.29–1.65) for NAFLD as defined by HSI, and 1.41 (1.18–1.69) for NAFLD as defined by FLI [8]. The dose–response relationship between dietary sodium intake and NAFLD deprived from the restricted cubic regression model in the present study is in line with previous observational studies. However, the mechanism underlying this relationship remains unclear.
Carotenoids form an important part of the human diet, consumption of which has been associated with many health benefits. β-carotene is one of the main constituents of carotenoids [31]. A community-based cross-sectional study conducted in a Chinese population observed a dose-dependent inverse association between β-carotene and NAFLD. The OR and 95% CI of NAFLD for the highest quartile was 0.32 (0.25–0.41) compared with the lowest quartile [32]. A case–control study enrolled 24 control subjects and 62 NAFLD patients, whose liver biopsies were collected and histological characteristics were assessed. Compared with individuals without hepatic steatosis, those with moderate steatosis had significantly decreased serum β-carotene (0.34 ± 0.03 vs. 0.21 ± 0.02 μmol/L). Moreover, β-carotene gradually decreased along disease progression from normal liver, simple steatosis to borderline steatohepatitis (P for trend ≤0.001) [33]. A prospective cohort study enrolled 2687 subjects who completed both NAFLD tests were classified into stable, improved and progressed groups according to changes in the degree of NAFLD between two visits. Analysis of covariance showed that ln-transformed serum β-carotene was positively associated with NAFLD improvement (P for trend < 0.05). After multivariable adjustment, mean difference in serum β-carotene was higher by 29.6% in the improved vs. progressed subjects [34].
The pro-inflammatory effect of sodium has been reported in several previous studies. In animal experiments, high sodium diet triggers TH17 (interleukin-17 (IL-17)-producing helper T cells) development and promotes tissue inflammation. TH17 cells are highly pro-inflammatory cells that are critical for clearing extracellular pathogens and for inducing multiple autoimmune diseases [35,36,37]. Results from a recent study showed that mice fed with a high-salt diet displayed obvious hepatic steatosis and inflammation. All these pathological changes persisted after salt withdrawal, displaying a memory phenomenon [38]. In human studies, high sodium intake was reported to be associated with changes in inflammatory markers including tumor necrosis factor-α (TNF-α) and adiponectin [39,40,41]. Contrary to the pro-inflammatory effect of sodium, β-carotene has a strong anti-inflammatory effect [42]. A previous study suggested that β-carotene decreases the severity of C-C motif chemokine ligand 4 (CCL4) induced hepatic inflammation and fibrosis in rats [43]. Another study reported that tomato powder (rich in β-carotene) inhibits hepatic steatosis and inflammation potentially through restoring sirturin 1 (SIRT1) activity and adiponectin function [44].
To the best of our knowledge, this is the first study to explore the pro-inflammatory mechanism of higher sodium intake in relation to an increased odds of NAFLD in humans through a mediation analysis. Moreover, we also found that dietary β-carotene attenuates the mediating effects of inflammation on the association between dietary sodium intake and NAFLD. Our study has limitations: First, dietary sodium intake was measured from two 24-h dietary recalls instead of multiple 24-h urine collections. The latter is considered as the gold-standard for estimating sodium intake in humans. However, the lack of urinary sodium measurements in this study limits our efforts to use more objective sodium measurement indicators to verify the reliability of the results. Second, NAFLD was defined using HSI- and FLI- based predictive equations rather than hepatic biopsy. Although hepatic biopsy method has a better accuracy for the diagnosis of NAFLD, it is difficult to apply in large-scale population studies. Third, given the enormous complexity of the inflammatory response, CRP and RDW provide only limited information. Future research should thus focus on additional biomarkers, such as IL-1β, IL-6, TNF-α, and CD4 + T cell subsets. Lastly, the cross-sectional study design is subject to recall bias and this design cannot prove causality in itself. Findings from our study should be further confirmed in well-designed prospective cohort studies using more reliable sodium measurements in future.
Conclusions
This study suggests that higher sodium intake increases the odds of NAFLD by upregulating inflammation. Dietary β-carotene may attenuate this association by down regulating inflammation.
Data availability
All data used in this manuscript are de-identified and freely available to the public through https://www.cdc.gov/nchs/nhanes/
References
He FJ, Li J, Macgregor GA. Effect of longer term modest salt reduction on blood pressure: Cochrane systematic review and meta-analysis of randomised trials. BMJ. 2013;346:f1325.
Stamler J, Chan Q, Daviglus ML, Dyer AR, Van Horn L, Garside DB, et al. Relation of dietary sodium (salt) to blood pressure and its possible modulation by other dietary factors: The INTERMAP Study. Hypertension. 2018;71:631–7.
Huang L, Trieu K, Yoshimura S, Neal B, Woodward M, Campbell N, et al. Effect of dose and duration of reduction in dietary sodium on blood pressure levels: systematic review and meta-analysis of randomised trials. BMJ. 2020;368:m315.
Zhou L, Stamler J, Chan Q, Van Horn L, Daviglus ML, Dyer AR, et al. Salt intake and prevalence of overweight/obesity in Japan, China, the United Kingdom, and the United States: the INTERMAP Study. Am J Clin Nutr. 2019;110:34–40.
Zhou L, Wen X, Peng Y, Zhao L, Yu Y. Salt added to food and body mass index: a bidirectional Mendelian randomisation study. Nutr Diet. 2021;78:315–23.
Strazzullo P, D’Elia L, Kandala NB, Cappuccio FP. Salt intake, stroke, and cardiovascular disease: meta-analysis of prospective studies. BMJ. 2009;339:b4567.
Aburto NJ, Ziolkovska A, Hooper L, Elliott P, Cappuccio FP, Meerpohl JJ. Effect of lower sodium intake on health: systematic review and meta-analyses. BMJ. 2013;346:f1326.
Zhou L, Yang Y, Feng Y, Zhao X, Fan Y, Rong J, et al. Association between dietary sodium intake and non-alcoholic fatty liver disease in the US population. Public Health Nutr. 2021;24:993–1000.
Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019;25:1822–32.
Polimeni L, Del BM, Baratta F, Perri L, Albanese F, Pastori D, et al. Oxidative stress: New insights on the association of non-alcoholic fatty liver disease and atherosclerosis. World J Hepatol. 2015;7:1325–36.
Stefan N, Häring HU, Cusi K. Non-alcoholic fatty liver disease: causes, diagnosis, cardiometabolic consequences, and treatment strategies. Lancet Diabetes Endocrinol. 2019;7:313–24.
Dogan S, Celikbilek M, Zararsiz G, Deniz K, Sivgin S, Guven K, et al. Red blood cell distribution width as a non-invasive marker for the assessment of inflammation in non-alcoholic steatohepatitis. Hepatogastroenterology. 2015;62:393–8.
Kim J, Im JS, Choi CH, Park CH, Lee JI, Son KH, et al. The Association between Red Blood Cell Distribution Width and Sarcopenia in U.S. Adults. Sci Rep. 2018;8:11484.
Ferreira JP, Lamiral Z, Bakris G, Mehta C, White WB, Zannad F. Red cell distribution width in patients with diabetes and myocardial infarction: an analysis from the EXAMINE trial. Diabetes Obes Metab. 2021;23:1580–7.
Rodríguez-Rodríguez E, López-Sobaler AM, Navia B, Andrés P, Jiménez-Ortega AI, Ortega RM. β-carotene concentration and its association with inflammatory biomarkers in Spanish school children. Ann Nutr Metab. 2017;71:80–7.
Schultz H, Ying G, Dunaief JL, Dunaief DM. Rising plasma beta-carotene is associated with diminishing C-reactive protein in patients consuming a dark green leafy vegetable-rich, low inflammatory foods everyday (LIFE) diet. Am J Lifestyle Med. 2019;15:634–43.
Cheng J, Balbuena E, Miller B, Eroglu A. The role of β-carotene in colonic inflammation and intestinal barrier integrity. Front Nutr. 2021;8:723480.
Zipf G, Chiappa M, Porter KS, Ostchega Y, Lewis BG, Dostal J. National health and nutrition examination survey: plan and operations, 1999-2010. Vital Health Stat. 2013;1:1–37.
Zhou L, Feng Y, Yang Y, Zhao X, Fan Y, Rong J, et al. Diet behaviours and hypertension in US adults: the National Health and Nutrition Examination Survey 2013–2014. J Hypertens. 2019;37:1230–8.
Liu N, Ma F, Feng Y, Ma X. The association between the Dietary Inflammatory Index and Thyroid Function in U.S. adult males. Nutrients. 2021;13:3330.
Zhou L, Li X, Li S, Wen X, Peng Y, Zhao L. Relationship between dietary choline intake and diabetes mellitus in the National Health and Nutrition Examination Survey 2007–2010. J Diabetes. 2021;13:554–61.
Lee JH, Kim D, Kim HJ, Lee CH, Yang JI, Kim W, et al. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis. 2010;42:503–8.
Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, et al. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33.
Meffert PJ, Baumeister SE, Lerch MM, Mayerle J, Kratzer W, Völzke H. Development, external validation, and comparative assessment of a new diagnostic score for hepatic steatosis. Am J Gastroenterol. 2014;109:1404–14.
Lee H, Cashin AG, Lamb SE, Hopewell S, Vansteelandt S, VanderWeele TJ, et al. A guideline for reporting mediation analyses of randomized trials and observational studies: the AGReMA Statement. JAMA. 2021;326:1045–56.
Tingley D, Yamamoto T, Hirose K, Keele L, Imai K. Mediation: R package for causal mediation analysis. J Stat Softw. 2014;59:1–38.
Lee H, Herbert RD, McAuley JH. Mediation analysis. JAMA. 2019;321:697–8.
Huh JH, Lee KJ, Lim JS, Lee MY, Park HJ, Kim MY, et al. High dietary sodium intake assessed by estimated 24-h urinary sodium excretion is associated with NAFLD and hepatic fibrosis. PLoS ONE. 2015;10:e143222.
Choi Y, Lee JE, Chang Y, Kim MK, Sung E, Shin H, et al. Dietary sodium and potassium intake in relation to non-alcoholic fatty liver disease. Br J Nutr. 2016;116:1447–56.
van den Berg EH, Gruppen EG, Blokzijl H, Bakker S, Dullaart R. Higher sodium intake assessed by 24 h urinary sodium excretion is associated with non-alcoholic fatty liver disease: The PREVEND Cohort Study. J Clin Med. 2019;8:2157.
Clugston RD. Carotenoids and fatty liver disease: current knowledge and research gaps. Biochim Biophys Acta Mol Cell Biol Lipids. 2020;1865:158597.
Cao Y, Wang C, Liu J, Liu ZM, Ling WH, Chen YM. Greater serum carotenoid levels associated with lower prevalence of nonalcoholic fatty liver disease in Chinese adults. Sci Rep. 2015;5:12951.
Wang L, Ding C, Zeng F, Zhu H. Low levels of serum β-carotene and β-carotene/retinol ratio are associated with histological severity in nonalcoholic fatty liver disease patients. Ann Nutr Metab. 2019;74:156–64.
Xiao ML, Chen GD, Zeng FF, Qiu R, Shi WQ, Lin JS, et al. Higher serum carotenoids associated with improvement of non-alcoholic fatty liver disease in adults: a prospective study. Eur J Nutr. 2019;58:721–30.
Kleinewietfeld M, Manzel A, Titze J, Kvakan H, Yosef N, Linker RA, et al. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature. 2013;496:518–22.
Wu C, Yosef N, Thalhamer T, Zhu C, Xiao S, Kishi Y, et al. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature. 2013;496:513–7.
Edwards JK. Inflammation: differential effects of salt on immune cell activity. Nat Rev Nephrol. 2016;12:2.
Gao P, You M, Li L, Zhang Q, Fang X, Wei X, et al. Salt-induced hepatic inflammatory memory contributes to cardiovascular damage through epigenetic modulation of SIRT3. Circulation. 2022;145:375–91.
Costa AP, de Paula RC, Carvalho GF, Araújo JP, Andrade JM, de Almeida OL, et al. High sodium intake adversely affects oxidative-inflammatory response, cardiac remodelling and mortality after myocardial infarction. Atherosclerosis. 2012;222:284–91.
Yilmaz R, Akoglu H, Altun B, Yildirim T, Arici M, Erdem Y. Dietary salt intake is related to inflammation and albuminuria in primary hypertensive patients. Eur J Clin Nutr. 2012;66:1214–8.
Rodrigues TL, de Carvalho BG, Caramori JC, Castro JH, Martin LC, Barretti P. Effect of dietary sodium restriction on body water, blood pressure, and inflammation in hemodialysis patients: a prospective randomized controlled study. Int Urol Nephrol. 2014;46:91–7.
Lee Y, Hu S, Park YK, Lee JY. Health benefits of carotenoids: a role of carotenoids in the prevention of non-alcoholic fatty liver disease. Prev Nutr Food Sci. 2019;24:103–13.
Seifert WF, Bosma A, Hendriks HF, van Leeuwen RE, van Thiel-de RG, Seifert-Bock I, et al. Beta-carotene (provitamin A) decreases the severity of CCl4-induced hepatic inflammation and fibrosis in rats. Liver. 1995;15:1–8.
Li CC, Liu C, Fu M, Hu KQ, Aizawa K, Takahashi S, et al. Tomato powder inhibits hepatic steatosis and inflammation potentially through restoring SIRT1 activity and adiponectin function independent of carotenoid cleavage enzymes in mice. Mol Nutr Food Res. 2018;62:e1700738.
Author information
Authors and Affiliations
Contributions
L Zhou and GL contributed to the study design and implementation, and is the guarantor of this work. L Zhou, YC, MW, and FC contributed to study conception and data interpretation. L Zhou contributed to data acquisition. L Zhou, YC, MW, and FC contributed to data analysis and drafted the manuscript. XW, L Zhao, and GL contributed to revision of this manuscript. All authors have approved the final version to be published.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, Y., Wu, M., Chen, F. et al. Potential role of inflammation in relation to dietary sodium and β-carotene with non-alcoholic fatty liver disease: a mediation analysis. Nutr. Diabetes 12, 40 (2022). https://doi.org/10.1038/s41387-022-00218-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41387-022-00218-y
This article is cited by
-
Genetically determined circulating micronutrients and the risk of nonalcoholic fatty liver disease
Scientific Reports (2024)