Abstract
Background
Obesity is reported to be a risk factor for severe disease in patients with coronavirus disease 2019 (COVID-19). However, there are no specific reports on the risk of severe disease according to body mass index (BMI) in Japan. Thus, this study aimed to investigate the effect of obesity stratified by BMI on the severity of COVID-19 in the general Japanese population.
Methods
From February 2020 to May 2021, 1 837 patients aged ≥18 years were enrolled in the Japan COVID-19 Task Force. Patients with known BMI and disease severity were analyzed. Severity was defined as critical if the patient was treated in the intensive care unit, required invasive mechanical ventilation, or died.
Results
Class 1 obesity (25.0 ≤ BMI < 30.0 kg/m2), class 2 obesity (30.0 ≤ BMI < 35.0 kg/m2), and class 3 or 4 obesity (BMI ≥ 35 kg/m2) were present in 29%, 8%, and 3% of the cases, respectively. Multiple logistic regression analysis with known risk factors for critical illness indicated that class 2 obesity was an independent risk factor for oxygenation (adjusted odds ratio, 4.75) and critical cases (adjusted odds ratio, 1.81). Class 1 obesity and class 3 or 4 obesity were independent risk factors for oxygen administration (adjusted odds ratios 2.01 and 3.12, respectively), but not for critical cases. However, no differences in the mortality rates were observed between the BMI classes (P = 0.5104).
Conclusion
Obesity is a risk factor for respiratory failure in Japanese patients with COVID-19, regardless of the degree of obesity. However, it may not cause severe COVID-19 in a dose–response relationship with BMI. COVID-19 patients with mild obesity may benefit from aggressive intensive care.
Similar content being viewed by others
Introduction
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by the severe acute respiratory syndrome coronavirus 2 [1]. It is widespread in many countries and progresses from mild viral illness to hypoxia, multiple organ failure, acute respiratory distress syndrome, and death [2]. Although several factors that contribute to the development of severe COVID-19 have been identified, such as increasing age, male sex, geographic region, and multiple chronic comorbidities, obesity is emerging as a significant risk factor, especially in industrialized countries [3,4,5]. In the United States of America, severe obesity with a body mass index (BMI) ≥ 35 kg/m2 has been reported to be a risk factor for invasive mechanical ventilation (IMV), intensive care unit (ICU) admission, and hospital death [4, 5].
The prevalence of obesity in the Japanese population is lower than that in Westerners [6]. In the United States of America, 40% of the population have obesity (BMI ≥ 30 kg/m2), and 9% of the population has a BMI ≥ 40 kg/m2 [7]. Meanwhile, the percentage of people with obesity in Japan is approximately 4.5%, and the percentage of people with a BMI > 35 kg/m2 is approximately 0.9% [8]. Recently, a genome-wide association study has identified host genetic factors that contribute to the risk of developing severe COVID-19 with respiratory failure [9, 10]. We have conducted a nationwide multicenter consortium to overcome the COVID-19 pandemic in Japan (https://www.covid19-taskforce.jp/en/home/). Previously, we reported the association between obesity-related genes and COVID-19 severity using a Mendelian randomization analysis [11]. Thus, obesity may be a significant comorbidity in Japanese patients with COVID-19. However, the relationship between obesity and COVID-19 severity in the Japanese population, which differs greatly from that of Westerners (white and black) in terms of the number of infections and deaths and the percentage of obesity, has not yet been clarified. Therefore, we hypothesized that the frequency and impact of obesity on disease severity might be different from those reported in Westerners. This study aimed to investigate the effect of obesity stratified by BMI on the severity of COVID-19 in the general Japanese population.
Subjects and methods
Study design and settings
All cases affected by COVID-19 were recruited through the Japan COVID-19 Task Force [11]. From February 2020 to May 2021, data from consecutive patients aged ≥18 years who were diagnosed with COVID-19 using severe acute respiratory syndrome coronavirus 2 polymerase chain reaction test results at one of more than 100 affiliated hospitals and who agreed to cooperate in the study were registered in an electronic case record form by the study subspecialist at the affiliated research institute and were analyzed in this retrospective cohort study. The inclusion criteria were: (i) non-Japanese patients and (ii) patients with incomplete medical records, such as inability to obtain BMI and critical outcome information. Of the 2 079 patients who met the exclusion criteria, we excluded 54 non-Japanese patients and 188 patients without BMI and outcome information. Thus, 1837 patients were included in the analysis (Fig. 1).
All consecutive patients with COVID-19 aged ≥18 years who were hospitalized during the study period and recruited through the Japan COVID-19 Task Force between February 2020 and May 2021 were included. After excluding 242 patients, 1837 patients were enrolled in this study. *A critical case was defined as receiving treatment in the intensive care unit, using invasive mechanical ventilation, or hospital death.
This study was approved by the Ethics Committee of Keio University School of Medicine (ID: 20200061), and written or oral informed consent was obtained. The study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Data collection
Actual measurement values of height and weight at admission were obtained from physicians, and BMI was calculated. The following data were extracted from the electronic case record form: age, sex, clinical symptoms and signs, laboratory and radiographic findings on admission, comorbidities, and disease severity (ICU entry, using IMV, and survival status). All laboratory tests were performed according to the clinical care needs of the patients. Symptoms and signs were included at the time of referral and admission and at the time of hospitalization. Laboratory and radiographic results were collected within 48 h of the initial visit or admission. The collected data were reviewed by a team of respiratory clinicians. If core data were missing, the clinician was contacted to collect the data. Missing data in the patient background were noted as unknown.
Outcomes and statistics
The primary exposure in all analyses was BMI. BMI was calculated using the height and weight recorded during hospitalization. BMI categories were defined using the following Ministry of Health, Labour and Welfare, Japan criteria: underweight (BMI < 18.5 kg/m2), normal weight (18.5 ≤ BMI < 25.0 kg/m2), class 1 obesity (25.0 ≤BMI < 30.0 kg/m2), class 2 obesity (30.0 ≤BMI < 35.0 kg/m2), and class 3 or 4 obesity (BMI ≥ 35 kg/m2). The primary outcome was critical illness, defined as treatment in the ICU, using IMV, or death [5, 12]. Continuous and categorical variables are presented as mean ± standard deviation (SD) or number (proportion), respectively. Data were compared among the five groups using an analysis of variance and the χ2 test as appropriate. Additionally, among patients with BMI ≥ 25 kg/m2, we compared clinical information between the critical and non-critical groups. Student’s t test and the χ2 test were used to compare the two groups.
We performed univariate and multivariate logistic regression analyses to evaluate the relationship between BMI and COVID-19 severity: oxygen administration, ICU treatment, IMV use, and critical illness. Multivariate logistic regression analyses were performed on factors reported as risk factors for severe disease in previous studies and factors selected in previous studies (BMI groups, age, sex, and presence of comorbidities: hypertension, diabetes, prior cardiovascular disease, and chronic kidney disease) [13,14,15,16,17]. Odds ratios (ORs) and adjusted odds ratios (aORs) with 95% CIs were used in the comparison. In all outcome analyses, we predefined the group without obesity (underweight or normal: BMI < 25 kg/m2) as the reference group. All P-values were two-tailed, and statistical significance was set at P < 0.05. All data were analyzed using the JMP 16 program (SAS Institute Japan Ltd., Tokyo, Japan).
Results
Comparison of baseline characteristics by obesity classes
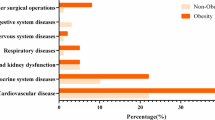
The baseline characteristics of the patients are shown in Table 1. The proportions of each BMI category were underweight (6%), normal weight (54%), class 1 obesity (29%), class 2 obesity (8%), and class 3 or 4 obesity (3%). High BMI classes were associated with younger age (P < 0.0001). Additionally, patients of high BMI classes were more likely to be male (P < 0.0001) and had more comorbidities such as hypertension (P < 0.0001), diabetes (P < 0.0001), hyperuricemia (P < 0.0001), and chronic liver disease (P = 0.023). On admission, patients with a higher BMI had a higher prevalence of fever (P = 0.0068), cough (P = 0.0001), shortness of breath (P < 0.0001), and sense of fatigue (P = 0.0008).
Comparison of laboratory and imaging findings by obesity classes
The laboratory parameters and imaging findings of the patients are shown in Table 2. Patients with higher BMI had higher levels of hemoglobin (P < 0.0001), aspartate aminotransferase (P < 0.0001), alanine aminotransferase (P < 0.0001), γ-glutamyl transpeptidase (P < 0.0001), lactate dehydrogenase (P < 0.0001), uric acid (P < 0.0001), complement C3 (P < 0.0001), serum ferritin (P < 0.0001), triglyceride (P < 0.0001), Krebs von den Lungen-6 (P = 0.0001), and hemoglobin A1c (P < 0.0001). Such patients also had a higher frequency of bilateral ground glass opacity on chest radiography (P < 0.0001), computed tomography (P = 0.018), and consolidation on chest radiography (P < 0.0001) and computed tomography (P = 0.0004).
Association between clinical outcomes and obesity classes
The outcomes of patients stratified by BMI are shown in Fig. 2. Patients with higher BMI had higher rates of oxygen therapy (P < 0.0001), ICU treatment (P = 0.0108), and IMV (P = 0.0315). In contrast, in-hospital deaths were few and not significantly different (P = 0.5104). The results of the univariate and multivariate logistic regression analyses are shown in the supplementary file and Fig. 3. The univariate logistic regression analysis indicated that compared to underweight or normal, patients with class 1 obesity (OR = 1.86 [1.50–2.30]), class 2 obesity (OR = 3.30 [2.28–4.77]), and class 3 or 4 obesity (OR = 1.91 [1.12–3.25]) were at higher risk of requiring oxygen therapy (supplementary file). However, class 2 obesity had the highest OR. Treatment in the ICU was associated with a higher risk of having class 1 (OR = 1.28 [1.00–1.63]) and class 2 obesity (OR = 1.82 [1.25–2.64]) than being without obesity. Using IMV (OR = 1.70 [1.06–2.72]) and critical illness (OR = 1.63 [1.13–2.36]) were at a higher risk than non-obesity only for class 2 obesity. Multivariate logistic regression analysis also showed that patients with class 1 obesity (aOR = 2.01 [1.56–2.57]), class 2 obesity (aOR = 4.75 [3.08–7.32]), and class 3 or 4 obesity (OR = 3.12 [1.68–5.77]) were at higher risk of oxygen therapy than those with no obesity (Fig. 3). Moreover, ICU treatment (aOR = 1.99 [1.32–2.98]), using IMV (aOR = 1.68 [1.00–2.83]), and critical illness (aOR = 1.81 [1.21–2.70]) were at higher risk than non-obesity only for class 2 obesity.
Forest plot of adjusted odds ratio and 95% confidence intervals according to BMI category by multivariate logistic regression analyses. Outcomes were adjusted for BMI groups, age, sex, and presence of comorbidities such as hypertension, diabetes, prior cardiovascular disease, and chronic kidney disease.
Comparison of baseline characteristics in the obesity category according to critical illness
The characteristics of patients with higher BMI classes classified as critical and non-critical are shown in Table 3. No significant differences in BMI were observed between critical or non-critical illness group (P = 0.174). Age was significantly higher in the critical illness group than in the non-critical illness group (P < 0.0001). Additionally, the critical illness group had significantly more comorbidities such as hypertension (P = 0.004), diabetes (P = 0.0184), chronic obstructive pulmonary disease (P = 0.0352), and chronic kidney disease (P = 0.0009) than the non-critical illness group. These results indicated that among patients in the high BMI category, older age and comorbidities play a more significant role in COVID-19 severity than among those with higher BMI.
Discussion
To the best of our knowledge, this is the first large-scale study to investigate the prevalence of obesity stratified by BMI and the clinical characteristics of Japanese COVID-19 patients with obesity. In comparison with the population of the Japanese National Nutrition Survey [8], Japanese patients hospitalized for COVID-19 were more likely to have obesity. Moreover, the number of patients with obesity was lower than that reported in Western studies [17,18,19]. One of the major strengths of this study is the comprehensive assessment of clinical data, including BMI. Only one previous study has reported the impact of obesity in Japanese patients with COVID-19 [20]. However, missing BMI data were supplemented by physicians. Given the large number of cases with detailed clinical data and accurate BMI measured by physicians, we were able to reveal that there is no dose–response relationship between obesity and COVID-19 severity and that mild obesity is important in Japanese people.
In many Western reports, severe obesity has been associated with poor outcomes [21, 22]. However, our study is clinically significant to note that even patients with class 2 obesity had poor outcomes other than death. These patients had high rates of respiratory failure, ICU admission, and IMV use. Patients with class 1 obesity also had poor outcomes on oxygen administration. However, no differences in the mortality rates were observed between the BMI classes, suggesting that patients with class 1 obesity or class 2 obesity might benefit from aggressive intensive care. In the United States and Europe, there are reports of increased severity of illness and mortality, especially in patients with severe obesity [4, 18, 19]. Here, severe obesity had no significant effect on the outcome, although this may be an underestimation due to the small number of patients with severe obesity. Our study revealed that patients with obesity and critical illness had significantly more comorbidities than those with obesity without critical illness. However, no significant difference in BMI was observed between the two groups. These results were consistent with the absence of a dose–response relationship between BMI and clinical outcomes in this study, indicating that obesity is an essential factor contributing to poor COVID-19 outcomes, while critical illness in patients with obesity is a multifaceted condition involving other factors such as comorbidities.
Obesity-related adverse events in COVID-19 may involve both mechanical and inflammatory mechanisms. First, obesity suppresses diaphragmatic movement and limits chest wall mobility, which may adversely affect lung function and cause hypoxemia due to atelectasis and shunting, thereby contributing to worsened breathing during infection [18, 23, 24]. Second, severe acute respiratory syndrome coronavirus 2 cell invasion is mediated by angiotensin-converting enzyme 2 receptor [25]. Individuals with obesity have enhanced expression of angiotensin-converting enzyme 2 receptor in the adipocytes. Thus, the presence of excess adipose tissue may increase the severity of the infection, and obesity and COVID-19 severity may be associated [26, 27]. Third, obesity is associated with chronic inflammation due to increased pro-inflammatory cytokines and leptin by adipocytes and immune cells, including elevated C-reactive protein, interleukin 6, and ferritin [28, 29]. In COVID-19, higher inflammatory biomarkers indicate greater disease severity [12, 30]. However, in the present study, ferritin levels suggested a significant difference in comparison between groups according to BMI, while C-reactive protein levels did not indicate a significant difference. A previous study has also reported no correlation between high BMI and interleukin 6, C-reactive protein, and ferritin levels in patients with COVID-19 [19, 30, 31]. Ultimately, several factors may contribute to the pathophysiology of obesity and COVID-19 severity, and further studies are needed.
This study had several limitations. The first limitation was the selection of patients; only hospitalized patients were included. Since only inpatients were included in the study, the number of patients who became seriously ill increased, and the risk of obesity causing serious illness may have been overestimated. Second, the analysis was based only on the BMI and host factors. In the early stages of the disease, such as the first wave in Japan, steroids were avoided, although corticosteroids were later found to be effective [32]. The number of effective treatments such as remdesivir, baricitinib, and tocilizumab increased [33, 34]. As described above, significant differences were observed between the first wave of treatment and current treatment, which may have affected the outcomes since the present study did not analyze the timing of treatment. Third, the virus strains have mutated in the data collection, and each mutated strain may have different characteristics [35]. Since the mutant strains were not included in the analysis in this study, the results may be different if they were included in the analysis. Fourth, East Asian ethnic groups are characterized by more visceral fat for lower BMI, and visceral fat has been reported to be a major prognostic factor for COVID-19 [36,37,38]. This study may have underestimated visceral fat in the Japanese population. It would be good if waist circumference or, even better, chest computed tomography-derived visceral fat itself could be used as a parameter, but this was not possible in the present study. Fifth, the number of patients with class 3 or 4 obesity was small in this study, and it is possible that the impact of severe obesity on COVID-19 severity was not accurately assessed. Therefore, further large-scale studies are desirable in the future. Owing to these limitations, further studies are required.
In conclusion, the number of patients with obesity was lower than that reported in Westerners. Patients with higher BMI classes had more comorbidities and a higher prevalence of respiratory and systemic symptoms. All higher BMI classes were associated with oxygen administration. However, obesity may not increase severity in a dose-dependent manner; only class 2 obesity may be associated with critical illness. COVID-19 patients with mild obesity may benefit from aggressive intensive care.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl J Med. 2020;382:727–33. https://doi.org/10.1056/NEJMoa2001017.
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. https://doi.org/10.1016/S0140-6736(20)30183-5.
Chu Y, Yang J, Shi J, Zhang P, Wang X. Obesity is associated with increased severity of disease in COVID-19 pneumonia: A systematic review and meta-analysis. Eur J Med Res. 2020;25:64. https://doi.org/10.1186/s40001-020-00464-9.
Kompaniyets L, Goodman AB, Belay B, Freedman DS, Sucosky MS, Lange SJ, et al. Body mass index and risk for COVID-19-Related hospitalization, Intensive Care Unit admission, invasive mechanical ventilation, and death - United States, March-December 2020. MMWR Morb Mortal Wkly Rep. 2021;70:355–61. https://doi.org/10.15585/mmwr.mm7010e4.
Petrilli CM, Jones SA, Yang J, Rajagopalan H, O’Donnell L, Chernyak Y, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: Prospective cohort study. BMJ. 2020;369:m1966. https://doi.org/10.1136/bmj.m1966.
Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–81. https://doi.org/10.1016/S0140-6736(14)60460-8.
Craig M, Hales MDC, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity Among adults: United States, 2017–2018. NCHS Data Brief. 2020;360:1–8.
Report of the national health and nutrition survey, 2019. https://www.mhlw.go.jp/bunya/kenkou/kenkou_eiyou_chousa.html.
Beckman MF, Mougeot FB, Mougeot JC. Comorbidities and susceptibility to COVID-19: a generalized gene set data mining approach. J Clin Med. 2021; 10. https://doi.org/10.3390/jcm10081666.
Covid S-19 GWAS Group, Ellinghaus D, Degenhardt F, Bujanda L, Buti M, Albillos A, et al. Genomewide association study of severe Covid-19 with respiratory failure. N Engl J Med. 2020;383:1522–34. https://doi.org/10.1056/NEJMoa2020283.
Namkoong H, Edahiro R, Fukunaga K, Shirai Y, Sonehara K, Tanaka H, et al. Japan COVID-19 Task Force: A Nation-Wide Consortium to Elucidate Host Genetics of COVID-19 Pandemic in Japan. 2021; https://doi.org/10.1101/2021.05.17.21256513.
Chow DS, Glavis-Bloom J, Soun JE, Weinberg B, Loveless TB, Xie X, et al. Development and external validation of a prognostic tool for COVID-19 critical disease. PLoS ONE. 2020;15:e0242953. https://doi.org/10.1371/journal.pone.0242953.
Geng L, He C, Kan H, Zhang K, Mao A, Zhang C, et al. The association between blood pressure levels and mortality in critically ill patients with COVID-19 in Wuhan, China: a case-series report. Hypertens Res. 2021;44:368–70. https://doi.org/10.1038/s41440-020-00594-x.
Huang I, Lim MA, Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia—a systematic review, meta-analysis, and meta-regression. Diabetes Metab Syndr. 2020;14:395–403. https://doi.org/10.1016/j.dsx.2020.04.018.
Santoso A, Pranata R, Wibowo A, Al-Farabi MJ, Huang I, Antariksa B. Cardiac injury is associated with mortality and critically ill pneumonia in COVID-19: a meta-analysis. Am J Emerg Med. 2021;44:352–7. https://doi.org/10.1016/j.ajem.2020.04.052.
Singh J, Malik P, Patel N, Pothuru S, Israni A, Chakinala RC, et al. Kidney disease and COVID-19 disease severity-systematic review and meta-analysis. Clin Exp Med. 2022;22:125–35. https://doi.org/10.1007/s10238-021-00715-x.
Hendren NS, de Lemos JA, Ayers C, Das SR, Rao A, Carter S, et al. Association of body mass index and age With morbidity and mortality in patients hospitalized With COVID-19: Results From the American Heart Association COVID-19 cardiovascular disease registry. Circulation. 2021;143:135–44. https://doi.org/10.1161/CIRCULATIONAHA.120.051936.
Anderson MR, Geleris J, Anderson DR, Zucker J, Nobel YR, Freedberg D, et al. Body mass index and risk for intubation or death in SARS-CoV-2 infection: a retrospective cohort study. Ann Intern Med. 2020;173:782–90. https://doi.org/10.7326/M20-3214.
Guerson-Gil A, Palaiodimos L, Assa A, Karamanis D, Kokkinidis D, Chamorro-Pareja N, et al. Sex-specific impact of severe obesity in the outcomes of hospitalized patients with COVID-19: a large retrospective study from the Bronx, New York. Eur J Clin Microbiol Infect Dis. 2021;40:1963–74. https://doi.org/10.1007/s10096-021-04260-z.
Terada M, Ohtsu H, Saito S, Hayakawa K, Tsuzuki S, Asai Y, et al. Risk factors for severity on admission and the disease progression during hospitalisation in a large cohort of patients with COVID-19 in Japan. BMJ Open. 2021;11:e047007. https://doi.org/10.1136/bmjopen-2020-047007.
Palaiodimos L, Kokkinidis DG, Li W, Karamanis D, Ognibene J, Arora S, et al. Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx, New York. Metabolism. 2020;108:154262. https://doi.org/10.1016/j.metabol.2020.154262.
Frank RC, Mendez SR, Stevenson EK, Guseh JS, Chung M, Silverman MG. Obesity and the risk of intubation or death in patients With coronavirus Disease 2019. Crit Care Med. 2020;48:e1097–e1101. https://doi.org/10.1097/CCM.0000000000004553.
Jones RL, Nzekwu MM. The effects of body mass index on lung volumes. Chest. 2006;130:827–33. https://doi.org/10.1378/chest.130.3.827.
Watson RA, Pride NB, Thomas EL, Fitzpatrick J, Durighel G, McCarthy J, et al. Reduction of total lung capacity in obese men: Comparison of total intrathoracic and gas volumes. J Appl Physiol (1985). 2010;108:1605–12. https://doi.org/10.1152/japplphysiol.01267.2009.
Hoffmann M, Kleine-Weber H, Pöhlmann S. A multibasic cleavage site in the spike protein of SARS-CoV-2 is essential for infection of human lung cells. Mol Cell. 2020;78:779–84. https://doi.org/10.1016/j.molcel.2020.04.022.
Kassir R. Risk of COVID-19 for patients with obesity. Obes Rev. 2020;21:e13034. https://doi.org/10.1111/obr.13034.
Al-Benna S. Association of high level gene expression of ACE2 in adipose tissue with mortality of COVID-19 infection in obese patients. Obes Med. 2020;19:100283. https://doi.org/10.1016/j.obmed.2020.100283.
Sattar N, McInnes IB, McMurray JJV. Obesity is a risk factor for severe COVID-19 infection: multiple potential mechanisms. Circulation. 2020;142:4–6. https://doi.org/10.1161/CIRCULATIONAHA.120.047659.
Demeulemeester F, de Punder K, van Heijningen M, van Doesburg F. Obesity as a risk factor for severe COVID-19 and complications: a review. Cells. 2021; 10. https://doi.org/10.3390/cells10040933.
Coomes EA, Haghbayan H. Interleukin-6 in Covid-19: a systematic review and meta-analysis. Rev Med Virol. 2020;30:1–9. https://doi.org/10.1002/rmv.2141.
Ullah W, Roomi S, Nadeem N, Saeed R, Tariq S, Ellithi M, et al. Impact of body mass index on COVID-19-Related in-hospital outcomes and mortality. J Clin Med Res. 2021;13:230–6. https://doi.org/10.14740/jocmr4239.
RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in hospitalized patients with Covid-19. N. Engl J Med. 2021;384:693–704. https://doi.org/10.1056/NEJMoa2021436.
WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group, Shankar-Hari M, Vale CL, Godolphin PJ, Fisher D, Higgins JPT, et al. Association Between Administration of IL-6 Antagonists and Mortality Among Patients Hospitalized for COVID-19: A Meta-analysis. JAMA. 2021;326:499–518. https://doi.org/10.1001/jama.2021.11330.
Kalil AC, Patterson TF, Mehta AK, Tomashek KM, Wolfe CR, Ghazaryan V, et al. Baricitinib plus remdesivir for hospitalized adults with Covid-19. N. Engl J Med. 2021;384:795–807. https://doi.org/10.1056/NEJMoa2031994.
Lauring AS, Hodcroft EB. Genetic variants of SARS-CoV-2-What do they mean?. JAMA. 2021;325:529–31. https://doi.org/10.1001/jama.2020.27124.
Yang Y, Ding L, Zou X, Shen Y, Hu D, Hu X, et al. Visceral adiposity and high intramuscular fat deposition independently predict critical illness in patients with SARS-CoV-2. Obes (Silver Spring). 2020;28:2040–8. https://doi.org/10.1002/oby.22971.
Watanabe M, Caruso D, Tuccinardi D, Risi R, Zerunian M, Polici M, et al. Visceral fat shows the strongest association with the need of intensive care in patients with COVID-19. Metabolism. 2020;111:154319. https://doi.org/10.1016/j.metabol.2020.154319.
Chandarana H, Dane B, Mikheev A, Taffel MT, Feng Y, Rusinek H. Visceral adipose tissue in patients with COVID-19: Risk stratification for severity. Abdom Radio (NY). 2021;46:818–25.
Acknowledgements
The authors would like to thank all the participants involved in this study, and all members of the Japan COVID-19 Task Force engaged in clinical and research work on COVID-19 every day. All the members contributed to this study.
Author information
Authors and Affiliations
Consortia
Contributions
Conceptualization: HL, SC, HN, KM, HK, MI, NH, and KF. Data curation: HL, KN, HT, SO, AM, TF, MW, and TK. Formal analysis: HL, SC. Methodology: HL, SC, and HN. Supervision: HL, NH, KM, HK, MI, NH, NH, TU, SU, TI, KA, FS, TY, YN, YM, YS, KM, YO, RK, YK, AK, SI, SM, SO, TK, and KF. Visualization: HL and HN. Writing—original draft: HL, SC. Writing—review and editing: HL, SC, NH, KM, HK, MI, NH, NH, TU, SU, TI, KA, FS, TY, YN, YM, YS, KM, YO, RK, YK, AK, SI, SM, SO, TK, and KF.
Corresponding authors
Ethics declarations
Competing interests
This study was supported by AMED (JP20nk0101612, JP20fk0108415, JP21jk0210034, JP21km0405211, JP21km0405217), JST CREST (JPMJCR20H2), JST PRESTO (JPMJPR21R7), MHLW (20CA2054), Takeda Science Foundation, Mitsubishi Foundation, and Bioinformatics Initiative of Osaka University Graduate School of Medicine, Osaka University.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A full list of members and their affiliations appears in the Supplementary Information.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, H., Chubachi, S., Namkoong, H. et al. Effects of mild obesity on outcomes in Japanese patients with COVID-19: a nationwide consortium to investigate COVID-19 host genetics. Nutr. Diabetes 12, 38 (2022). https://doi.org/10.1038/s41387-022-00217-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41387-022-00217-z
This article is cited by
-
Prognostic significance of hypertension history and blood pressure on admission in Japanese patients with coronavirus disease 2019: integrative analysis from the Japan COVID-19 Task Force
Hypertension Research (2024)
-
Clinical utilization of artificial intelligence-based COVID-19 pneumonia quantification using chest computed tomography – a multicenter retrospective cohort study in Japan
Respiratory Research (2023)
-
Insulin-like growth factor-1 levels are associated with high comorbidity of metabolic disorders in obese subjects; a Japanese single-center, retrospective-study
Scientific Reports (2022)
-
Characteristics of hospitalized patients with COVID-19 during the first to fifth waves of infection: a report from the Japan COVID-19 Task Force
BMC Infectious Diseases (2022)