Abstract
In most social species, the attainment of social dominance is strongly affected by personality traits. Dominant individuals show better cognitive abilities, however, whether an individual’s cognition can determine its social status has remained inconclusive. We found that mice show better cognitive abilities tend to possess a higher social rank after cohousing. The dynamic release of acetylcholine (ACh) in the prelimbic cortex (PL) is correlated with mouse dominance behavior. ACh enhanced the excitability of the PL neurons via acetylcholine muscarinic M1 receptors (M1). Inhibition of M1 impaired mice cognitive performance and induced losing in social competition. Mice with M1 deficiency in the PL performed worse on cognitive behavioral tests, and exhibited lower status when re-grouped with others. Elevating ACh level in the PL of subordinate mice induced winning. These results provide direct evidence for the involvement of M1 in social hierarchy and suggest that social rank can be tuned by altering cognition through cholinergic system.
Similar content being viewed by others
Introduction
Dominance hierarchy is widely observed among social species, and the social hierarchy plays a crucial role in determining resource access and significantly impacts survival, health, reproductive success, as well as various behavioral patterns [1, 2]. The social hierarchy can be influenced by variations in individual characteristics that impact social success, such as bravery, persistence, and motivational drive [3,4,5]. Studies found that across multiple species, the cognitive task performance of individual is different by social rank, with dominant individuals performing more efficiently on operant learning [6], spatial learning [7, 8] and spatial memory tasks [9]. However, the causality between social hierarchy and cognitive abilities is extremely complicated and remains to be further elucidated.
Cognitive abilities are considered to be one of the characteristics that is crucial for individuals to achieve social dominance [10,11,12]. Learning capacity allows individuals to adapt to changing environments. For example, social learning can inform individuals about conspecifics motivations and consequently guide future social interactions [13]. Thus, individuals with more proficient social learning abilities have been found to be higher ranking [14]. Performances on operant foraging and spatial learning tasks are also reported as higher in dominant individuals [6, 8, 9]. Individuals which are inherently good at cognitive performing are more efficient at beneficial behaviors such as foraging, mate choice, and navigation which are crucial for the propagation of the population. Nevertheless, the impact of cognitive abilities on social hierarchy has been essentially unknown.
The medial prefrontal cortex (mPFC) has been implicated in the regulation of social dominance. Human imaging studies have shown that PFC activity is engaged with social hierarchies [15]. A rodent study indicated that the dominant rats exhibit significantly higher levels of c-fos immunoreactivity in the prelimbic and infralimbic subcortical regions of the mPFC that project to the amygdala [16]. The strength of excitatory transmission in layer V pyramidal neurons of the mPFC plays a crucial role in determining social hierarchy, as bidirectional relationship between males manipulation of synaptic strength switched the dominance [17]. Furthermore, the activation of the mPFC is both necessary and sufficient to rapidly induce winning in social competitions [18]. On the other hand, the mPFC is widely recognized as a crucial component in cognitive regulation. The mPFC has been linked to the function of working memory, as evidenced by disruptions in working memory tasks following mPFC lesions [19, 20]. Evidence from in vivo electrophysiology studies shows that single mPFC neurons exhibit a variety of behavioral correlates during navigation and working memory tasks [21, 22]. Modulating mPFC neural activities influences mice spatial working memory [23, 24]. Meanwhile, The mPFC is a key regulator in social cognition [25, 26]. A more recent study found that NAc-projecting pyramidal neurons in the mPFC is preferentially involved in social memory [27]. These findings imply that the mPFC plays a crucial role in social hierarchy as well as cognitive function.
Innervation of the mPFC through acetylcholine (ACh) releasing from the basal forebrain cholinergic system has a critical modulatory role in cognition [28]. The influence of ACh is mediated by ionotropic nicotinic and metabotropic muscarinic receptors, modulating synaptic transmission, inducing synaptic plasticity, and coordinating the firing patterns of neuronal groups [29]. Several pharmacological studies have reported that administration of acetylcholine muscarinic M1 receptors (M1) agonist in the mPFC of mice could effectively improve their performance in cognitive relative tests [30,31,32]. Physiological studies have demonstrated that layer V pyramidal neurons in the mPFC are activated by ACh via M1 [33, 34]. These studies indicated that the cholinergic system in the mPFC may be the key components for the mPFC to regulate cognitive functions. However, the precise role of the cholinergic system in the prefrontal cortex in mediating social hierarchy remains elusive.
In this study, using the dominance tube test [35], we found that mice show better cognitive abilities tend to possess a higher social status after cohousing. Subsequently, by using fiber photometry recording, electrophysiological recording, viral and pharmacological approaches, we provide evidence demonstrates that the modulation of mice cognition through M1 in the prefrontal cortex plays a pivotal role for social dominance.
Materials and methods
Subjects
Male C57 BL/6 J mice were obtained from the Southern Medical University Animal Center (Guangzhou, China), Chat-Cre mice were a gift from Tianming Gao at Southern Medical University (Guangzhou, China). The behavioral experiment adhered to the guidelines set by the Chinese Council on Animal Care. For additional details, see Supplementary Information.
Stereotaxic injections and optic fiber/cannula implantations
The mice were positioned in a stereotactic apparatus to undergo stereotaxic injections and optic fiber/cannula implantations. For the PL microinfusion, the dummy cannula was replaced with an infusion cannula, The infusion cannula was connected to a 10-μL microsyringe, which was mounted on a microinfusion pump. AAV-U6-M1shRNA-CMV-RFP was injected into the PL of mice to genetically interfere with M1 expression. Immunoblotting was used to confirm the efficiency of M1 knockdown. For additional details, see Supplementary Information.
Fiber photometry recording
Photometry was performed as described before [36]. A tube with a width-adjustable slit is used for mice implanted with optic connectors.The fluorescence signals were acquired using a fiber photometry system. The data was initially segmented based on the behavioral events. The average fluorescence and frequency of signal were then computed for both the baseline and event phases. For additional details, see Supplementary Information.
Behavior analysis
Tube test for social hierarchy
The tube test assay was applied as described [17]. In brief, mice were first underwent a two-day habituation period (day 1 and day 2) in the test chamber. During the subsequent two days (day 3 and day 4), mice underwent behavioral training. After training, testing was performed from day 5. Each mouse explored the tube once from each side before the confrontation trials. A pair of cage mates was allowed to enter the tube from opposite ends and meet in the middle. The trial ended when 1 of the mice retreated with all 4 paws out of the tube, becoming the subordinate. The mouse that forced its cage mate to retreat was termed dominant. For additional details, see Supplementary Information.
Y-maze test
The Y-maze test was applied as described [37]. In brief, the Y-maze apparatus comprised three identical arms made of black plastic. The start arm, novel arm, and other arm were randomly assigned in the test. The novel arm was obstructed during the training trial. In the training trial, mice were given a 10-min period to explore the alternative arm. After a 1-h interval, the mice were reintroduced to the maze for a 5-min period of unrestricted exploration, during which all three arms were made accessible (test trial). For additional details, see Supplementary Information.
Three-chamber test
The Y-maze test was applied as described [38]. In brief, The apparatus consisted of a box divided into three equal compartments. During the sociability test, a stimulus mouse was placed inside a wire containment cup in a compartment. The other was equipped with an empty cup. The test mouse was allowed to freely explore the chambers for 10 min. The time that the test mice spent investigating each containment cup was measured. During the social novelty test, we introduced a second stimulus mouse into an identical cup placed in the chamber opposite to that of the test mouse, allowing for a 10-min exploration. The time spent by the test mice in exploring the cup containing either the familiar or the unfamiliar was measured. For additional details, see Supplementary Information.
Electrophysiological recordings and procedures
All electrophysiological recording protocols were performed in accordance with our previous studies [39, 40]. For additional details, see Supplementary Information.
Statistical analysis
Statistical analysis was performed using Prism 8 and SPSS (version 17.0). No specific method was used to predetermine the ideal sample size or to randomly assign the animals to the experimental groups. The normality of the data distribution was confirmed by the Shapiro-Wilk normality test. Statistical differences of normally distributed data were then determined using two-way repeated-measures ANOVA followed by Bonferroni’s multiple comparisons test or one-way ANOVA followed by Dunnett’s multiple comparisons test. Two experimental groups were compared using a two-tailed paired Student’s t test test or unpaired Student’s t test test. *p < 0.05, **p < 0.01, ***p < 0.001, n.s. not significant.
Results
Higher cognitive abilities predict mice social dominance after cohousing
We employed the tube test to assess the dominant–subordinate relationship in rodents (Fig. 1A). After identifying their social hierarchy through the tube test, mice were subsequently subjected to cognitive tests (Fig. 1B). In Y-maze tests, the dominant mice exhibited prolonged duration (Fig. 1C, D; D, one-way ANOVA, F3,24 = 19.11, p < 0.001) and heightened frequency (Fig. 1E; One-way ANOVA, F3,24 = 9.610, p < 0.001) of exploration in the novel arm compared to the other three subordinate mice. This observation suggests that the dominant mice exhibit higher short-term spatial working memory capabilities.
A Tube-test ranking for social hierarchy. B The timeline of experiments. C Illustration of the Y-maze and the timeline of sample and test trial phases. D Dominant mice spent more time in the novel arm during the test trial phase compared with subordinate mice (One-way ANOVA with Dunnett’s multiple comparisons test, n = 7 mice per group; Rank1 vs Rank2, p = 0.007; Rank1 vs Rank3, p < 0.001; Rank1 vs Rank4, p < 0.001). E Dominant mice showed significant increase in the percentage of entries into the novel arm during the test trial phase compared subordinate mice (One-way ANOVA with Dunnett’s multiple comparisons test, n = 7 mice per group; Rank1 vs Rank2, p = 0.0273; n = 7 mice per group, Rank1 vs Rank3, p < 0.001; Rank1 vs Rank4, p < 0.001). F Illustration of the three-chamber sociability and social novelty tests. G Sociability test: time spent in the empty chamber and mouse chamber for dominant and subordinate mice (Bonferroni’s multiple comparisons test; (S–E) Rank1, p < 0.001, Rank2, p < 0.001, Rank3, p < 0.001, Rank1, p < 0.001). H Dominant and subordinate mice show no difference in preference for the social stimulus (One-way ANOVA with Dunnett’s multiple comparisons test, n = 7 mice per group; Rank1 vs Rank2, p = 0.9954; Rank1 vs Rank3, p = 0.8844; Rank1 vs Rank4, p = 0.9616). I Examples of individual dominant (Rank1) and subordinate mice (Rank4) performing the three-chamber social novelty test. Heat map indicates time in location. J Social novelty test: time spent in the familiar mouse chamber and stranger mouse chamber for dominant and subordinate mice (Bonferroni’s multiple comparisons test; S-F Rank1, p < 0.001, Rank2, p = 0.0028, Rank3, p = 0.0576, Rank1, p = 0.280). K Dominant mice show greater preference for the familiar mouse compared with subordinate mice (One-way ANOVA with Dunnett’s multiple comparisons test, n = 7 mice per group; Rank1 vs Rank2, p = 0.0370; Rank1 vs Rank3, p = 0.01; Rank1 vs Rank4, p = 0.0032). L The timeline of experiments. M Time in the novel arm during the test trial phase in Y-maze test and time in the stranger mouse chamber during the social novelty test can predict the rank of individual mice after cohousing (Ordinal logistic regression; Y-maze: odds ratio [OR], 0.921 [95% CI 0.862–0.983], p = 0.014; social novelty test: OR, 0.941 [95% CI 0.904–0.979], p = 0.003). The data are presented as the mean ± s.e.m. *p < 0.05, **p < 0.01, ***p < 0.001, n.s. not significant.
In the three-chamber tests, which test the sociability and social novelty (Fig. 1F), the social ability of mice in different social ranks showed no discernible variation, as there was no difference observed in the duration spent in the social zone during the three-chamber sociability test (Fig. 1G; Two-way ANOVA, chamber main effect: F3,48 = 4.970, p = 0.0044 ; rank main effect: F1,48 = 127.5, p < 0.001; no interaction; Fig. 1H; One-way ANOVA, F3,24 = 0.2503, p = 0.8603). Nevertheless, the dominant mice show more preference for strange individuals during social novelty test (Fig. 1I–K; J, Two-way ANOVA, chamber main effect: F3,48 = 6.830, p < 0.001 ; rank main effect: F1,48 = 60.24, p < 0.001; interaction: F3,48 = 6.369, p = 0.001; K; One-way ANOVA, F3,24 = 5.438, p = 0.0054). Moreover, mice show no difference in the anxiety level (Fig. S1A; One-way ANOVA, F3,24 = 0.4891, p = 0.6931), body weight (Fig. S1B; One-way ANOVA, F3,24 = 0.1930, p = 0.9001) and locomotion (Fig. S1C, D; One-way ANOVA; S1C, F3,24 = 0.4860, p = 0.6952; S1D, F3,24 = 0.2442, p = 0.8646). The findings, consistent with previous studies [8, 41,42,43], indicated that the dominant mice exhibit higher cognitive abilities.
Next, mice were firstly single housed for two weeks to exclude the possible influence of social rank on cognition [43]. Then, mice were subjected to Y-maze and three-chamber tests. Subsequently, the mice were group-housed in cages based on their performance in behavioral tests, ensuring that each cage accommodated four mice with distinct cognitive abilities (Fig. 1L). Remarkably, mice exhibiting better cognitive performance maintain higher social rank (Fig. 1M; Ordinal logistic regression; Y-maze: odds ratio [OR], 0.921 [95% CI 0.862–0.983], p = 0.014; social novelty test: OR, 0.941 [95% CI 0.904–0.979], p = 0.003). The findings validate that the cognitive abilities of subjects play a pivotal role in predicting their social status.
The level of ACh in the prelimbic cortex (PL) is correlated with mice effortful pushes in tube test
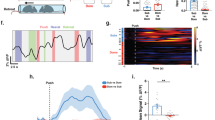
Cognition have been associated with the ACh release in the prefrontal cortex [29, 44, 45]. However, the dynamic release of ACh in the prefrontal cortex during social competition remains elusive. We conducted fiber photometry recordings in freely behaving mice during their participation in the tube test (Fig. 2A). The AAV encoding G protein–coupled receptor-activation–based ACh sensor (GRABACh3.0) [46] was injected in the PL, a subregion of the prefrontal cortex, to monitor the real-time ACh activities (Fig. 2B, C). We aligned ACh activities with epochs of pushing, resistance and retreat [47]. The ACh signaling was significantly elevated during the push epochs (Fig. 2D, E, S2A; 2D, paired t test, ACh3.0, p = 0.0195; EYFP, p = 0.6126). but no significant change in ACh activity was detected in the resistance(Fig. 2F, S2B; paired t test, 2 F, ACh3.0, p = 0.2730; EYFP, p = 0.6100) and retreat epochs (Fig. 2G, S2C; 2 G, paired t test, ACh3.0, p = 0.7216; EYFP, p = 0.2672). In another experiment which mice pushed against a block in the tube, the findings show that mice pushed against the block also acompanied with increased ACh activity in the PL (Fig. S2D–G). Additionally, the locomotion of mice did not exert any influence on the ACh activity in the PL (Fig. S2H–K). Importantly, the administration of an acetylcholinesterase inhibitor (AChEI), donepezil (1.0 mg/kg) [48] did not result in any significant changes in level of basal aggression (Fig. S2L; unpaired t test, p = 0.936) and muscle strength (Fig. S2M; unpaired t test, p = 0.6616). Collectively, our findings indicate that in vivo ACh activities in the PL correlates with mice effortful pushes in tube test.
A Fiber photometry recording of ACh release in the PL during dominance tube test. B, C AAV-Syn-GRABACh3.0 were injected in the PL of C57 mice. D Example fluorescence trace from the PL region of a dominant mouse during tube test. Different behavioral epochs are indicated by colored shading. E Heat map (upper) and plot (middle) of fluorescence changes across animals aligned to the initiation of push. Yellow bold line and gray shadow indicate mean and SEM, respectively. Lower, quantification of change in fluorescence signals before and after push initiation (paired t test: ACh3.0 t(9) = 2.838, p = 0.0195; EYFP t(12) = 0.5199, p = 0.6126; 10 events from three ACh3.0-expressing mice, 13 events from three EYFP-expressing mice). F Heat map (upper) and plot (middle) of fluorescence changes across animals aligned to the initiation of resistance. Lower, quantification of change in fluorescence signals before and after resistance initiation (paired t test: ACh3.0 t(14) = 1.141, p = 0.273; EYFP t(14) = 0.5218, p = 0.61; 15 events from three ACh3.0-expressing mice, 15 events from three EYFP-expressing mice). G Heat map (upper) and plot (middle) of fluorescence changes across animals aligned to the initiation of retreat. Lower, quantification of change in fluorescence signals before and after retreat initiation. (paired t test: ACh3.0 t(2) = 0.4099, p = 0.7216; EYFP t(4) = 1.288, p = 0.2672; 3 events from three ACh3.0-expressing mice, 5 events from three EYFP-expressing mice). The data are presented as the mean ± s.e.m. *p < 0.05, n.s. not significant.
ACh enhances the excitability of the PL neurons through M1
The neural activities of the prefrontal cortex is crucial for social hierarchy [17]. The ACh from cholinergic neurons located in the basal forebrain influences neural activities of the prefrontal cortex [28]. We set out to investigate the impact of ACh on neural activities within the PL. AAV-Dio-ChR2-EYFP was injected into basal forebrain of Chat-Cre mice, virus was specifically expressed in cholinergic neurons of the basal forebrain, and EYFP-containing terminals were observed within the PL after 4 weeks (Fig. 3A). Subsequently, slices contain the PL were cuted for patch recording (Fig. 3B). Recordings from layer V pyramidal neurons of the PL demonstrate that brief blue light stimulation of ChR2-containing terminals consistently evoked depolarization, which was reversed by the application of a selective M1 inhibitor VU0255035 (M1i, 5 μM) (Fig. 3C; paired t test, p < 0.001). Furthermore, optical enhancement of ACh release in the PL increased the amplitude of the miniature excitory postsynaptic currents (mEPSCs) within the PL neurons, which was reversed by the M1i (Fig. 3D; Two-way ANOVA; amplitude, F1,46 = 9.935, p = 0.0029; frequency, F1,46 = 1.036, p = 0.3141). This effect was mimicked by bath perfusion of ACh (1 mM) [49] (Fig. 3E; Two-way ANOVA; amplitude, F1,48 = 15.15, p < 0.0001; frequency, F1,48 = 0.9563, p = 0.3330). Taken together, our results indicate that ACh release in the PL enhances the excitability of neurons through M1.
A Schematic representation showing viral injection in mice and the recording configuration in acute slices. B Virus expression in HDB cholinergic neurons (left) and the fiber in the PL (right). C Representative traces and summarized data showing depolarized potentials in pyramidal cells of the PL layer 5 (paired t test, t(6) = 11.63, p < 0.001; n = 7 cells from three mice). D Sample traces and summarized data showing the effects of photostimulation on mEPSCs recorded from pyramidal cells of the PL layer 5 in response to M1i (Two-way ANOVA with Bonferroni’s multiple comparisons test, Baseline vs. Light p < 0.001; M1i vs. M1i+Light p å 0.9999; Light, n = 12 from four mice, Light+M1i, n = 13 from four mice). E Sample traces and summarized data showing the effects of bath application of ACh on mEPSCs recorded from pyramidal cells of the PL layer 5 in response to M1i (Two-way ANOVA with Bonferroni’s multiple comparisons test, Baseline vs. ACh p < 0.001; M1i vs. M1i+ ACh p å 0.9999; ACh, n = 13 from four mice, ACh+M1i, n = 13 from four mice). The data are presented as the mean ± s.e.m. ***p < 0.001; n.s. not significant.
Cognitive impairment induced lose in social competition in tube test
Next, we conducted an investigation into the role of the PL M1 in spatial memory and social novelty (Fig. 4A). In Y-maze test, micro-injection of M1i (5 μM) into the PL reduced duration (Fig. 4B; unpaired t test, t(22) = 2.944, p = 0.0075) and frequency of exploration (Fig. 4C; unpaired t test, t(22) = 2.602, p = 0.0163) in the novel arm compared to the control mice. In addition, the application of M1i into the PL does not exert any influence on the duration spent in the social zone during the three-chamber sociability test (Fig. 4D, E; 4D, two-way ANOVA; chamber main effect: F1,44 = 0.5696, p = 0.4545; M1i main effect: F1,44 = 337.7, p < 0.001; no interaction; 4 F, unpaired t test, t(22) = 0.8548, p = 0.0163), while in social novelty test, inhibition of M1 reduced mice preference for strange individuals (Fig. 4F–H; 4 G, two-way ANOVA; chamber main effect: F1,44 = 2.135, p = 0.1511; M1i main effect: F1,44 = 19.84, p < 0.001; interaction: F1,44 = 7.609, p = 0.0084; 4H, unpaired t test, t(22) = 4.529, p = 0.0075). The results suggest that the blockade of M1 in the PL impaired both spatial memory and social novelty in mice.
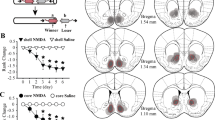
A The timeline of experiments. B, C Micro-injections of M1i in the PL of mice decrease the time spent exploring the novel arm (B, unpaired t test, t(22) = 2.944, p = 0.0075; n = 12 mice/group) and the percentage of entries into the novel arm (C, unpaired t test, t(22) = 2.602, p = 0.0163; n = 12 mice/group) during the Y-maze test trial phase. D Time spent in the empty chamber and mouse chamber for ACSF and M1i injection mice in sociability test (Bonferroni’s multiple comparisons test; (S–E) Saline, p < 0.001, M1i, p < 0.001). E Inhibition of M1 has no effect on mice preference for the social stimulus (unpaired t test, t(22) = 0.8548, p = 0.0163; n = 12 mice/group). F Examples of ACSF and M1i injection mice performing the three-chamber social novelty test. Heat map indicates time in location. G Time spent in the familiar mouse chamber and stranger mouse chamber for ACSF and M1i injection mice in social novelty test (Bonferroni’s multiple comparisons test; (S–E) Saline, p < 0.001, M1i, p = 0.0742). H Inhibition of M1 decrease mice preference for the familiar mouse (unpaired t test, t(22) = 4.529, p = 0.0075; n = 12 mice/group). I Schematic for injection of M1i in rank tube test. For experiment groups, in every cage, only the identified dominant mouse received M1i injection. For control groups, the dominant mouse received ACSF. J The timeline of experiments. K Social rank change of a cage after M1i (left) or ACSF (right) treatment in the dominant mouse. L Average rank change after M1i (red) or ACSF (gray) injection in dominant mice (Wilcoxon signed-rank test, Test1, ACSF vs. M1i p = 0.024; Test2, ACSF vs. M1i p = 0.034; Test3, ACSF vs. M1i p = 0.180; n = 6 mice/group). M Representative confocal images of AAV-U6-M1shRNA-CMV-RFP (red) distribution in the PL. N The protein levels of M1 were decreased in the PL of mice injected with an AAV expressing M1-shRNA. unpaired t test, t(10) = 4.355, p = 0.0014; (n = 6 mice/group). O The timeline of experiments. P M1shRNA-mice spent less time in the novel arm during the test trial phase compared with control mice. unpaired t test, t(30) = 2.343, p = 0.0260; (n = 16 mice/group). Q M1shRNA-mice showed significant decrease in the percentage of entries into the novel arm during the test trial phase compared with control mice. unpaired t test, t(30) = 3.491, p = 0.0015; (n = 16 mice/group). R Time spent in the empty chamber and mouse chamber for mCherry- and M1shRNA-mice in sociability test (Bonferroni’s multiple comparisons test; (S–E) mCherry, p < 0.001, M1shRNA, p < 0.001). S Interference of M1 expression in the PL has no effect on mice preference for the social stimulus. unpaired t test, t(30) = 0.09571, p = 0.9244; (n = 16 mice/group). T Examples of control and M1shRNA-mice performing the three-chamber social novelty test. Heat map indicates time in location. U Time spent in the familiar mouse chamber and stranger mouse chamber for control and M1shRNA-mice in social novelty test (Bonferroni’s multiple comparisons test; (S–E) mCherry, p < 0.001, M1shRNA, p = 0.0703). V Interference of M1 expression in the PL decreases mice preference for the familiar mouse. unpaired t test, t(30) = 2.530, p = 0.0169; (n = 16 mice/group). W Social rank for control (gray) and M1shRNA-mice (red) in a cage. unpaired t test, t(30) = 3.189, p = 0.0033; (n = 16 mice/group). The data are presented as the mean ± s.e.m. *p < 0.05, **p < 0.01, ***p < 0.001, n.s. not significant.
Next, dominant individuals were administered either ACSF or M1i. The social hierarchy was confirmed at 6 h, 24 h, and 48 h post drug delivery (Fig. 4I, J). We found that antagonism of M1 disrupts expression of social dominance (Fig. 4K). Notably, dominance returns when the effect of the drug is removed (Fig. 4L; Wilcoxon signed-rank test, Test1, ACSF vs. M1i p = 0.024; Test2, ACSF vs. M1i p = 0.034; Test3, ACSF vs. M1i p = 0.180). These data indicate that M1 is required to maintain a dominant status in a stable social hierarchy.
We further identified whether M1 modulates individual social dominance via their cognitive influence. The expression of M1 in the PL were interfered by injection of the AAV encoding M1shRNA [50] (Fig. 4M). We confirmed a high efficiency of M1 knockdown in the PL of mice after AAV-U6-M1shRNA-CMV-RFP injection (Fig. 4N, S3; 4 N, unpaired t test, t(10) = 5.469, p < 0.001). After virus expression, cognitive performance tests were conducted on the mice. Subsequently, mice were mixed raised in cage with two shRNA-mice and two control mice, the tube test was conducted two weeks later to allow for the re-establishment of social hierarchy among these mice (Fig. 4O). In the cognitive performance tests, M1 knockdown in the PL reduced duration and frequency of exploration in the novel arm compared to the control mice (Fig. 4P, Q; 4 P, unpaired t test, t(30) = 2.343, p = 0.0260; 4Q, unpaired t test, t(30) = 3.491, p = 0.0015). Meanwhile, shRNA-mice did not exert any difference on the duration spent in the social zone during the three-chamber sociability test compared with control mice (Fig. 4R, S; 4 R, two-way ANOVA; chamber main effect: F1,60 = 0.5658, p = 0.4549; shRNA main effect: F1, 60 = 97.51, p < 0.001; no interaction; 4 S, unpaired t test, t(30) = 0.09571, p = 0.9244), However, they demonstrated a reduced preference for strange individuals (Fig. 4T–V; 4 T, two-way ANOVA; chamber main effect: F1,60 = 0.2612, p = 0.6112; shRNA main effect: F1,60 = 35.61, p < 0.001; interaction: F1,60 = 8.586, p = 0.0048; 4 V, unpaired t test, t(30) = 2.530, p = 0.0169). Two weeks after mixed raising, most shRNA-mice occupied a subordinate position within the cage (Fig. 4W; unpaired t test, t(30) = 3.189, p = 0.0033). These findings suggest that down regulation of M1 in the PL impairs cognitive performance and contributes to a decline in tube test ranks.
Elevating ACh level induced winning against previously dominant opponents through M1 in the PL
We further investigate whether the elevation of ACh levels in the PL of subordinate mice could potentially influence their social hierarchy. Donepezil (1.0 mg/kg), or saline was intraperitoneally injected into rank 4 mice with ACSF or M1i (5 μM) [51] micro-injection into the PL (Fig. 5A). Mice establishing a stable social hierarchy through the examination of dominance-subordinate relationships during the pretest phase of tube test training. Subsequently, the social rank was confirmed at 6 h, 24 h and 48 h following drug delivery (Fig. 5B). Consistent with the aforementioned findings, our study reveals that elevated ACh levels in subordinate mice induced winning in the tube test, and this effect is attenuated by administration of M1i in the PL (Fig. 5C, D; 5D, Wilcoxon signed-rank test, Test1, Phy vs. Saline p = 0.020, Phy+M1i vs. Saline p = 0.066; Test2, Phy vs. Saline p = 0.023, Phy+M1i vs. Saline p = 0.059; Test3, Phy vs. Saline p = 0.060, Phy+M1i vs. Saline p = 0.317).
A Schematic for drugs delivery. B The timeline of experiments. C Social rank change of a cage after drugs treatment in the rank-4 mouse. D Average rank change after drugs treatment in the rank-4 mice. (Wilcoxon signed-rank test, Test1, Phy vs. Saline p = 0.020, Phy+M1i vs. Saline p = 0.066; Test2, Phy vs. Saline p = 0.023, Phy+M1i vs. Saline p = 0.059; Test3, Phy vs. Saline p = 0.060, Phy+M1i vs. Saline p = 0.317; n = 6 mice/group). The data are presented as the mean ± s.e.m. *p < 0.05, n.s. not significant.
Discussion
The association between social hierarchy and cognitive abilities has been extensively corroborated by numerous studies; however, the causal relationship between social hierarchy and cognitive abilities remains unidentified. Here, we define that higher cognitive abilities are crucial for mice to attain a higher social status, and the modulation of M1 in the PL can influence mice’s social hierarchy through the impact on cognitive abilities.
Animals in the wild constantly encounter environmental fluctuations. Augmenting cognitive abilities could potentially optimize individuals’ resource acquisition and elevate their social status. While dominance with better cognitive abilities has been implicated in many studies [6, 9, 42, 43, 52], others have failed to reveal the correlation between social rank and cognitive abilities [53, 54]. These inconsistent findings may be attributed to variations in species selection and the diverse behavioral tasks. According to our data, the relationship was consistently observed in mice, as well as in tasks assessing spatial working memory and recognition abilities. Meanwhile, cognitive performances have been suggested to determine social success [10, 55]. However, for cognitive or behavioral attributes, which are highly plastic, measures should be conducted prior to the establishment of the hierarchy in order to prevent the potential confounding factor that attribute expression is merely a consequence of social rank; hence, assessing the predictive ability of cognitive performances for individual social rank remains challenging and has not been demonstrated in these studies. In our study, mice were socially isolated prior to behavioral tests in order to mitigate the influence of social hierarchy. Consequently, our study further revealed that mice exhibiting higher cognition possess the ability to acquired social dominance upon integration into a group setting. Moreover, by modulating the cholinergic system to manipulate cognitive flexibility in mice of varying social ranks, we insure that higher cognitive abilities can qualify mice to win a competition and achieve a higher status in a social hierarchy. This is consistent with previous research indicating that forthcoming dominants exhibit significantly better performance than their cage-mates in the Morris water maze working memory task prior to the establishment of social dominance [41].
Through fiber photometry recording, we observed a significant release of ACh in the PL during mice pushing against opponents. Moreover, mice pushed against the block also accompanied with increased ACh activity in mPFC. Furthermore, no significant change in ACh activity was detected when mice walked through the tube without an opponent. Using a pharmacological approach, we identified that elevating the ACh level does not seem to boost dominance by enhancing basal aggression level or physical strength. These findings suggest that ACh activity in the PL was generally enhanced during effortful pushes. The prefrontal cortex has been implicated in the neural processes underlying cost-benefit analysis and effort-based decision-making [56,57,58,59,60]. We propose that ACh activity may influence these cognitive processes mediated by the prefrontal cortex and serve as a neurobiological basis for dominance-related personality traits, such as perseverance or competitive drive.
Recording from the PL pyramidal neurons indicated that these neurons are activated during effortful behaviors in social competition [18]. In the PL, M1 is widely distributed throughout somata and dendrites including spines [61]. Therefore, the activation of ACh in the PL may serve as a crucial regulator via M1 during social competition. However, ACh in the PL may release from cholinergic fibers originating from basal forebrain ChAT neurons as well as ChAT interneurons within the PL, further investigation is required to determine the specific source of ACh that is relevant in the processing. Although our recording results during tube test indicates the enhanced release of ACh in social competition. The electrophysiological and pharmacological results identified the ACh involved in rank modulation through M1 in the PL. However, further investigation is needed to determine whether the PL neurons in dominance mice exhibit heightened sensitivity to ACh or increased expression of M1, which contribute to social dominance. Furthermore, different GABAergic interneurons in mPFC exhibit distinct, or even opposite, outcomes in dominance tube test [62]. Further investigation is warranted to elucidate the precise involvement of specific ACh receptors in modulating GABAergic interneurons during social competition.
Cholinergic signaling in the mPFC exerts crucial influence on circuit dynamics underlying cognitive processing [29]. Enhanced activity of basal forebrain cholinergic axon fibers in the mPFC resulted in improved object recognition memory in mice [44]. ChAT interneurons directly elicit neuronal excitation across cortical layers and contribute to attentional processes [63]. Intra-mPFC infusion of α4β2 or α7 nAChR agonist enhanced object recognition memory [64, 65]. Our finding revealed that inhibition of M1 in the PL resulted in impaired cognitive performance in mice. The M1 subtype is the predominant muscarinic receptor in the cortex [66]. In the mPFC, activation of postsynaptic M1 mediates a slow membrane depolarization and increases neuronal firing frequency [67, 68]. Previous study has shown that M1 promote phosphorylation and membrane insertion of AMPA receptor GluA1 subunit and enhanced synaptic delivery [69, 70]. In consistent, our finding indicated that ACh release induced depolarized membrane potentials and increased the amplitude of sEPSC in pyramidal neurons through M1. Previous work has shown that synaptic strength in the pyramidal neurons of the dorsal mPFC is linked with social hierarchy status, and alterations in synaptic strength via manipulation of AMPAR expression can change an individual’s status consistent with the results seen in dominant relative to subordinate mice in the tube test for dominance, supporting the role of mPFC synaptic strength in the relationship to dominance behavior.
Conclusively, our results provide compelling evidence that cognitive abilities are key regulators in determining intermale social hierarchy. Given the association between an deficiency in dominance and various mental disorders [71], our findings have the potential to provide valuable insights into the treatment of these psychiatric conditions.
References
Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005;308:648–52.
Yeh SR, Fricke RA, Edwards DH. The effect of social experience on serotonergic modulation of the escape circuit of crayfish. Science. 1996;271:366–9.
Rushworth MF, Kolling N, Sallet J, Mars RB. Valuation and decision-making in frontal cortex: one or many serial or parallel systems? Curr Opin Neurobiol. 2012;22:946–55.
Mooney SJ, Peragine DE, Hathaway GA, Holmes MM. A game of thrones: neural plasticity in mammalian social hierarchies. Soc Neurosci. 2014;9:108–17.
Sandi C, Haller J. Stress and the social brain: behavioural effects and neurobiological mechanisms. Nat Rev Neurosci. 2015;16:290–304.
Boogert NJ, Reader SM, Laland KN. The relation between social rank, neophobia and individual learning in starlings. Anim Behav. 2006;72:1229–39.
Barnard CJ, Luo N. Acquisition of dominance status affects maze learning in mice. Behav Process. 2002;60:53–9.
Francia N, Cirulli F, Chiarotti F, Antonelli A, Aloe L, Alleva E. Spatial memory deficits in middle-aged mice correlate with lower exploratory activity and a subordinate status: role of hippocampal neurotrophins. Eur J Neurosci. 2006;23:711–28.
Pravosudov VV, Mendoza SP, Clayton NS. The relationship between dominance, corticosterone, memory, and food caching in mountain chickadees (Poecile gambeli). Hormones Behav. 2003;44:93–102.
Seyfarth RM, Cheney DL. What are big brains for? Proc Natl Acad Sci USA. 2002;99:4141–2.
Fernald RD. Cognitive skills needed for social hierarchies. Cold Spring Harb Symposia Quant Biol. 2014;79:229–36.
Tibbetts EA, Pardo-Sanchez J, Weise C. The establishment and maintenance of dominance hierarchies. Philos Trans R Soc Lond Ser B, Biol Sci. 2022;377:20200450.
Reader SM, Laland KN. Social intelligence, innovation, and enhanced brain size in primates. Proc Natl Acad Sci USA. 2002;99:4436–41.
Nicol CJ, Pope SJ. The effects of demonstrator social status and prior foraging success on social learning in laying hens. Anim Behav. 1999;57:163–71.
Zink CF, Tong Y, Chen Q, Bassett DS, Stein JL, Meyer-Lindenberg A. Know your place: neural processing of social hierarchy in humans. Neuron. 2008;58:273–83.
Dulka BN, Bress KS, Grizzell JA, Cooper MA. Social dominance modulates stress-induced neural activity in medial prefrontal cortex projections to the basolateral amygdala. Neuroscience. 2018;388:274–83.
Wang F, Zhu J, Zhu H, Zhang Q, Lin Z, Hu H. Bidirectional control of social hierarchy by synaptic efficacy in medial prefrontal cortex. Science. 2011;334:693–7.
Zhou T, Zhu H, Fan Z, Wang F, Chen Y, Liang H, et al. History of winning remodels thalamo-PFC circuit to reinforce social dominance. Science. 2017;357:162–8.
Kolb B, Buhrmann K, McDonald R, Sutherland RJ. Dissociation of the medial prefrontal, posterior parietal, and posterior temporal cortex for spatial navigation and recognition memory in the rat. Cereb cortex. 1994;4:664–80.
Wang GW, Cai JX. Disconnection of the hippocampal-prefrontal cortical circuits impairs spatial working memory performance in rats. Behav Brain Res. 2006;175:329–36.
Spellman T, Rigotti M, Ahmari SE, Fusi S, Gogos JA, Gordon JA. Hippocampal-prefrontal input supports spatial encoding in working memory. Nature. 2015;522:309–14.
Miller EK, Freedman DJ, Wallis JD. The prefrontal cortex: categories, concepts and cognition. Philos Trans R Soc Lond Ser B Biol Sci. 2002;357:1123–36.
Abbas AI, Sundiang MJM, Henoch B, Morton MP, Bolkan SS, Park AJ, et al. Somatostatin interneurons facilitate hippocampal-prefrontal synchrony and prefrontal spatial encoding. Neuron. 2018;100:926–39e3.
Tamura M, Spellman TJ, Rosen AM, Gogos JA, Gordon JA. Hippocampal-prefrontal theta-gamma coupling during performance of a spatial working memory task. Nat Commun. 2017;8:2182.
Bicks LK, Koike H, Akbarian S, Morishita H. Prefrontal cortex and social cognition in mouse and man. Front Psychol. 2015;6:1805.
Blakemore SJ. The social brain in adolescence. Nat Rev Neurosci. 2008;9:267–77.
Xing B, Mack NR, Guo KM, Zhang YX, Ramirez B, Yang SS, et al. A subpopulation of prefrontal cortical neurons is required for social memory. Biol psychiatry. 2021;89:521–31.
Ballinger EC, Ananth M, Talmage DA, Role LW. Basal forebrain cholinergic circuits and signaling in cognition and cognitive decline. Neuron. 2016;91:1199–218.
Picciotto MR, Higley MJ, Mineur YS. Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron. 2012;76:116–29.
Shirey JK, Brady AE, Jones PJ, Davis AA, Bridges TM, Kennedy JP, et al. A selective allosteric potentiator of the M1 muscarinic acetylcholine receptor increases activity of medial prefrontal cortical neurons and restores impairments in reversal learning. J Neurosci. 2009;29:14271–86.
Digby GJ, Noetzel MJ, Bubser M, Utley TJ, Walker AG, Byun NE, et al. Novel allosteric agonists of M1 muscarinic acetylcholine receptors induce brain region-specific responses that correspond with behavioral effects in animal models. J Neurosci. 2012;32:8532–44.
Higashino K, Ago Y, Umeki T, Hasebe S, Onaka Y, Hashimoto H, et al. Rivastigmine improves isolation rearing-induced prepulse inhibition deficits via muscarinic acetylcholine receptors in mice. Psychopharmacology. 2016;233:521–8.
Gulledge AT, Bucci DJ, Zhang SS, Matsui M, Yeh HH. M1 receptors mediate cholinergic modulation of excitability in neocortical pyramidal neurons. J Neurosci. 2009;29:9888–902.
Williams SR, Fletcher LN. A dendritic substrate for the cholinergic control of neocortical output neurons. Neuron. 2019;101:486–99e4.
Xing B, Mack NR, Zhang YX, McEachern EP, Gao WJ. Distinct roles for prefrontal Dopamine D1 and D2 neurons in social hierarchy. J Neurosci. 2022;42:313–24.
Fan Z, Zhu H, Zhou T, Wang S, Wu Y, Hu H. Using the tube test to measure social hierarchy in mice. Nat Protoc. 2019;14:819–31.
Shen Y, Hua L, Yeh CK, Shen L, Ying M, Zhang Z, et al. Ultrasound with microbubbles improves memory, ameliorates pathology and modulates hippocampal proteomic changes in a triple transgenic mouse model of Alzheimer’s disease. Theranostics. 2020;10:11794–819.
Pan L, Zheng L, Wu X, Zhu Z, Wang S, Lu Y, et al. A short period of early life oxytocin treatment rescues social behavior dysfunction via suppression of hippocampal hyperactivity in male mice. Mol Psychiatry. 2022;27:4157–71.
Chen YH, Wu JL, Hu NY, Zhuang JP, Li WP, Zhang SR, et al. Distinct projections from the infralimbic cortex exert opposing effects in modulating anxiety and fear. J Clin Investig. 2021;131:e145692.
Zhang SR, Wu JL, Chen H, Luo R, Chen WJ, Tang LJ, et al. ErbB4 knockdown in serotonergic neurons in the dorsal raphe induces anxiety-like behaviors. Neuropsychopharmacol. 2020;45:1698–706.
Jaafari Suha A, Hosseinmardi N, Janahmadi M. Spatial working memory is disparately interrelated with social status through different developmental stages in rats. Behav Brain Res. 2022;416:113547.
Langley EJG, van Horik JO, Whiteside MA, Beardsworth CE, Madden JR. The relationship between social rank and spatial learning in pheasants, Phasianus colchicus: cause or consequence? PeerJ. 2018;6:e5738.
Langley EJG, van Horik JO, Whiteside MA, Madden JR. Group social rank is associated with performance on a spatial learning task. R Soc Open Sci. 2018;5:171475.
Sun Q, Zhang J, Li A, Yao M, Liu G, Chen S, et al. Acetylcholine deficiency disrupts extratelencephalic projection neurons in the prefrontal cortex in a mouse model of Alzheimer’s disease. Nat Commun. 2022;13:998.
Pezze MA, Marshall HJ, Cassaday HJ. Scopolamine impairs appetitive but not aversive trace conditioning: role of the medial prefrontal cortex. J Neurosci. 2017;37:6289–98.
Jing M, Li Y, Zeng J, Huang P, Skirzewski M, Kljakic O, et al. An optimized acetylcholine sensor for monitoring in vivo cholinergic activity. Nat Methods. 2020;17:1139–46.
Noh K, Cho WH, Lee BH, Kim DW, Kim YS, Park K, et al. Cortical astrocytes modulate dominance behavior in male mice by regulating synaptic excitatory and inhibitory balance. Nat Neurosci. 2023;26:1541–54.
Karvat G, Kimchi T. Acetylcholine elevation relieves cognitive rigidity and social deficiency in a mouse model of autism. Neuropsychopharmacol. 2014;39:831–40.
Proulx E, Suri D, Heximer SP, Vaidya VA, Lambe EK. Early stress prevents the potentiation of muscarinic excitation by calcium release in adult prefrontal cortex. Biol Psychiatry. 2014;76:315–23.
Wohleb ES, Wu M, Gerhard DM, Taylor SR, Picciotto MR, Alreja M, et al. GABA interneurons mediate the rapid antidepressant-like effects of scopolamine. J Clin Investig. 2016;126:2482–94.
Zhu H, Yan H, Tang N, Li X, Pang P, Li H, et al. Impairments of spatial memory in an Alzheimer’s disease model via degeneration of hippocampal cholinergic synapses. Nat Commun. 2017;8:1676.
Chou YJ, Ma YK, Lu YH, King JT, Tasi WS, Yang SB, et al. Potential cross-species correlations in social hierarchy and memory between mice and young children. Commun Biol. 2022;5:230.
Varholick JA, Bailoo JD, Palme R, Wurbel H. Phenotypic variability between Social Dominance Ranks in laboratory mice. Sci Rep. 2018;8:6593.
Varholick JA, Pontiggia A, Murphy E, Daniele V, Palme R, Voelkl B, et al. Social dominance hierarchy type and rank contribute to phenotypic variation within cages of laboratory mice. Sci Rep. 2019;9:13650.
Taborsky B, Oliveira RF. Social competence: an evolutionary approach. Trends Ecol Evolut. 2012;27:679–88.
Fujii N, Hihara S, Nagasaka Y, Iriki A. Social state representation in prefrontal cortex. Soc Neurosci. 2009;4:73–84.
Friedman A, Homma D, Gibb LG, Amemori K-I, Rubin SJ, Hood AS, et al. A corticostriatal path targeting striosomes controls decision-making under conflict. Cell. 2015;161:1320–33.
Hillman KL, Bilkey DK. Neural encoding of competitive effort in the anterior cingulate cortex. Nat Neurosci. 2012;15:1290–7.
Holroyd CB, McClure SM. Hierarchical control over effortful behavior by rodent medial frontal cortex: a computational model. Psychol Rev. 2015;122:54–83.
Hosokawa T, Watanabe M. Prefrontal neurons represent winning and losing during competitive video shooting games between monkeys. J Neurosci. 2012;32:7662–71.
Oda S, Tsuneoka Y, Yoshida S, Adachi-Akahane S, Ito M, Kuroda M, et al. Immunolocalization of muscarinic M1 receptor in the rat medial prefrontal cortex. J Comp Neurol. 2018;526:1329–50.
Zhang C, Zhu H, Ni Z, Xin Q, Zhou T, Wu R, et al. Dynamics of a disinhibitory prefrontal microcircuit in controlling social competition. Neuron. 2022;110:516–31e6.
Obermayer J, Luchicchi A, Heistek TS, de Kloet SF, Terra H, Bruinsma B, et al. Prefrontal cortical ChAT-VIP interneurons provide local excitation by cholinergic synaptic transmission and control attention. Nat Commun. 2019;10:5280.
Esaki H, Deyama S, Izumi S, Katsura A, Nishikawa K, Nishitani N, et al. Varenicline enhances recognition memory via alpha7 nicotinic acetylcholine receptors in the medial prefrontal cortex in male mice. Neuropharmacology. 2023;239:109672.
Esaki H, Izumi S, Fukao A, Ito S, Nishitani N, Deyama S, et al. Nicotine enhances object recognition memory via stimulating alpha4beta2 and alpha7 nicotinic acetylcholine receptors in the medial prefrontal cortex of mice. Biol Pharm Bull. 2021;44:1007–13.
Levey AI, Kitt CA, Simonds WF, Price DL, Brann MR. Identification and localization of muscarinic acetylcholine receptor proteins in brain with subtype-specific antibodies. J Neurosci. 1991;11:3218–26.
Kurowski P, Gawlak M, Szulczyk P. Muscarinic receptor control of pyramidal neuron membrane potential in the medial prefrontal cortex (mPFC) in rats. Neuroscience. 2015;303:474–88.
Satake T, Mitani H, Nakagome K, Kaneko K. Individual and additive effects of neuromodulators on the slow components of afterhyperpolarization currents in layer V pyramidal cells of the rat medial prefrontal cortex. Brain Res. 2008;1229:47–60.
Zhao LX, Chen MW, Qian Y, Yang QH, Ge YH, Chen HZ, et al. M1 muscarinic receptor activation rescues beta-amyloid-induced cognitive impairment through AMPA receptor GluA1 subunit. Neuroscience. 2019;408:239–47.
Zhao LX, Ge YH, Li JB, Xiong CH, Law PY, Xu JR, et al. M1 muscarinic receptors regulate the phosphorylation of AMPA receptor subunit GluA1 via a signaling pathway linking cAMP-PKA and PI3K-Akt. FASEB J. 2019;33:6622–31.
Fan Z, Chang J, Liang Y, Zhu H, Zhang C, Zheng D, et al. Neural mechanism underlying depressive-like state associated with social status loss. Cell. 2023;186:560–76e17.
Funding
This work was supported by grants from the Guangdong Basic and Applied Basic Research Foundation (2022A1515011987) to J-LW and Scientific Research Starting Foundation for High-level Talents of Meizhou People’s Hospital (KYQD202301 to W-JC, KYQD202302 to J-LW).
Author information
Authors and Affiliations
Contributions
W-JC performed the experiments, HC and Z-ML contributed to experiments, W-YH and J-LW designed the study, J-LW supervised the work. W-JC wrote manuscript with help of J-LW. All authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, WJ., Chen, H., Li, ZM. et al. Acetylcholine muscarinic M1 receptors in the rodent prefrontal cortex modulate cognitive abilities to establish social hierarchy. Neuropsychopharmacol. 49, 974–982 (2024). https://doi.org/10.1038/s41386-023-01785-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-023-01785-z