Abstract
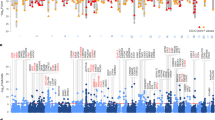
Psychiatric diseases are strongly influenced by genetics, but genetically guided treatments have been slow to develop, and precise molecular mechanisms remain mysterious. Although individual locations in the genome tend to not contribute powerfully to psychiatric disease incidence, genome-wide association studies (GWAS) have now successfully linked hundreds of specific genetic loci to psychiatric disorders [1,2,3]. Here, building upon results from well-powered GWAS of four phenotypes relevant to psychiatry, we motivate an exploratory workflow leading from GWAS screening, through causal testing in animal models using methods such as optogenetics, to new therapies in human beings. We focus on schizophrenia and the dopamine D2 receptor (DRD2), hot flashes and the neurokinin B receptor (TACR3), cigarette smoking and receptors bound by nicotine (CHRNA5, CHRNA3, CHRNB4), and alcohol use and enzymes that help to break down alcohol (ADH1B, ADH1C, ADH7). A single genomic locus may not powerfully determine disease at the level of the population, but the same locus may nevertheless represent a potent treatment target suitable for population-wide therapeutic approaches.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 13 print issues and online access
$259.00 per year
only $19.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Notes
Throughout this manuscript we refer to genome-wide studies, singular and plural, as GWAS. GWAS have historically been conducted using array-based (“chip”) technology but sequencing-based genome-wide studies are increasingly common as sequencing costs decrease.
Note that the presence of significant variants in the DRD2 gene does not necessarily imply that variation in DRD2 genotypes influences schizophrenia risk; functional studies would be needed to conclusively establish this link.
The linking of loci to genes constitutes an entire field of research. There is no field-wide consensus regarding the best approach, since much remains unknown about the function of individual genes and their regulation by genetic elements within and outside of genes. Leading approaches include nearest gene, MAGMA gene-level p value, and numerous fine-mapping approaches.
References
Visscher PM, Brown MA, McCarthy MI, Yang J. Five years of GWAS discovery. Am J Hum Genet. 2012;90:7–24.
Visscher PM, Wray NR, Zhang Q, Sklar P, McCarthy MI, Brown MA, et al. 10 years of GWAS discovery: biology, function, and translation. Am J Hum Genet. 2017;101:5–22.
Abdellaoui A, Yengo L, Verweij KJH, Visscher PM. 15 years of GWAS discovery: realizing the promise. Am J Hum Genet. 2023;110:179–94.
Trubetskoy V, Pardiñas AF, Qi T, Panagiotaropoulou G, Awasthi S, Bigdeli TB, et al. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature. 2022;604:502–8.
Crandall CJ, Manson JE, Hohensee C, Horvath S, Wactawski-Wende J, LeBlanc ES, et al. Association of genetic variation in the tachykinin receptor 3 locus with hot flashes and night sweats in the Women’s Health Initiative Study. Menopause. 2017;24:252–61.
Meijsen JJ, Shen H, Vemuri M, Rasgon NL, Koenen KC, Duncan LE. Shared genetic influences on depression and menopause symptoms. Psychol Med. 2023;53:2241–51.
Saunders GRB, Wang X, Chen F, Jang S-K, Liu M, Wang C, et al. Genetic diversity fuels gene discovery for tobacco and alcohol use. Nature. 2022;612:720–4.
Seeman P. Atypical antipsychotics: mechanism of action. Focus. 2004;2:48–58.
Kapur S, Remington G. Dopamine D2 receptors and their role in atypical antipsychotic action: still necessary and may even be sufficient. Biol Psychiatry. 2001;50:873–83.
Huhn M, Nikolakopoulou A, Schneider-Thoma J, Krause M, Samara M, Peter N, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet. 2019;394:939–51.
Zalocusky KA, Ramakrishnan C, Lerner TN, Davidson TJ, Knutson B, Deisseroth K. Nucleus accumbens D2R cells signal prior outcomes and control risky decision-making. Nature. 2016;531:642–6.
Hunter M, Gentry-Maharaj A, Ryan A, Burnell M, Lanceley A, Fraser L, et al. Prevalence, frequency and problem rating of hot flushes persist in older postmenopausal women: impact of age, body mass index, hysterectomy, hormone therapy use, lifestyle and mood in a cross-sectional cohort study of 10 418 British women aged 54–65. BJOG. Int J Obstet Gynaecol. 2012;119:40–50.
UK Parliament H of CC report. Menopause and the workplace. 2022.
Rance NE, Dacks PA, Mittelman-Smith MA, Romanovsky AA, Krajewski-Hall SJ. Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: a novel hypothesis on the mechanism of hot flushes. Front Neuroendocrinol. 2013;34:211–27.
Padilla SL, Johnson CW, Barker FD, Patterson MA, Palmiter RD. A neural circuit underlying the generation of hot flushes. Cell Rep. 2018;24:271–7.
Fraser GL, Lederman S, Waldbaum A, Kroll R, Santoro N, Lee M, et al. A phase 2b, randomized, placebo-controlled, double-blind, dose-ranging study of the neurokinin 3 receptor antagonist fezolinetant for vasomotor symptoms associated with menopause. Menopause. 2020;27:382–92.
Prague JK, Roberts RE, Comninos AN, Clarke S, Jayasena CN, Nash Z, et al. Neurokinin 3 receptor antagonism as a novel treatment for menopausal hot flushes: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389:1809–20.
Astellas Pharma Global Development, Inc. A randomized, placebo-controlled, double-blind phase 3 clinical study to investigate the long-term safety of fezolinetant in women suffering from vasomotor symptoms (hot flashes) associated with menopause. clinicaltrials.gov; 2023.
Tahara A, Takamatsu H, Ohtake A, Tanaka-Amino K, Kaku S. Effects of neurokinin 3 receptor antagonist fezolinetant on hot flash-like symptoms in ovariectomized rats. Eur J Pharm. 2021;905:174207.
Schuckit MA. Comorbidity between substance use disorders and psychiatric conditions. Addiction. 2006;101:76–88.
Abdellaoui A, Smit DJA, van den Brink W, Denys D, Verweij KJH. Genomic relationships across psychiatric disorders including substance use disorders. Drug Alcohol Depend. 2021;220:108535.
Sinnott-Armstrong N, Naqvi S, Rivas M, Pritchard JK. GWAS of three molecular traits highlights core genes and pathways alongside a highly polygenic background. ELife. 2021;10:e58615.
Howard DM, Adams MJ, Clarke T-K, Hafferty JD, Gibson J, Shirali M, et al. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat Neurosci. 2019;22:343–52.
Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–22.
Ochoa D, Karim M, Ghoussaini M, Hulcoop DG, McDonagh EM, Dunham I. Human genetics evidence supports two-thirds of the 2021 FDA-approved drugs. Nat Rev Drug Discov. 2022;21:551–551.
O’Connor LJ, Schoech AP, Hormozdiari F, Gazal S, Patterson N, Price AL. Extreme polygenicity of complex traits is explained by negative selection. Am J Hum Genet. 2019;105:456–76.
Keller MC, Miller G. Resolving the paradox of common, harmful, heritable mental disorders: which evolutionary genetic models work best? Behav Brain Sci. 2006;29:385–404.
Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–91.
Acknowledgements
We would like to thank the Psychiatric Genomics Consortium (PGC) for the vision, perseverance, and international collaboration necessary to create a well-powered psychiatric GWAS. We thank Katy Werwath, Kevina Wang, Ciera Stafford, and Hanyang Shen for help with this manuscript.
Funding
This work was supported by NIMH grants to LD (R01 MH123486, R21 MH125358) and the Jaswa Innovator Award. KD is supported by NIDA, NIMH, and the BRAIN Initiative. Funding bodies had no role in the design of the study, in the collection and analysis of data, or in decisions regarding publication.
Author information
Authors and Affiliations
Contributions
LD and KD were responsible for the conception, drafting, and revision of the work.
Corresponding authors
Ethics declarations
Competing interests
LD has no competing interests. KD is a cofounder and consultant for Maplight Therapeutics.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Duncan, L., Deisseroth, K. Are novel treatments for brain disorders hiding in plain sight?. Neuropsychopharmacol. 49, 276–281 (2024). https://doi.org/10.1038/s41386-023-01636-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-023-01636-x
This article is cited by
-
Dissecting the shared genetic landscape of anxiety, depression, and schizophrenia
Journal of Translational Medicine (2024)