Abstract
A major barrier to remission from an alcohol use disorder (AUD) is the continued risk of relapse during abstinence. Assessing the neuroadaptations after chronic alcohol and repeated abstinence is important to identify mechanisms that may contribute to relapse. In this study, we used a rhesus macaque model of long-term alcohol use and repeated abstinence, providing a platform to extend mechanistic findings from rodents to primates. The central amygdala (CeA) displays elevated GABA release following chronic alcohol in rodents and in abstinent male macaques, highlighting this neuroadaptation as a conserved mechanism that may underlie excessive alcohol consumption. Here, we determined circulating interleukin-1β (IL-1β) levels, CeA transcriptomic changes, and the effects of IL-1β and corticotropin releasing factor (CRF) signaling on CeA GABA transmission in male controls and abstinent drinkers. While no significant differences in peripheral IL-1β or the CeA transcriptome were observed, pathway analysis identified several canonical immune-related pathways. We addressed this potential dysregulation of CeA immune signaling in abstient drinkers with an electrophysiological approach. We found that IL-1β decreased CeA GABA release in controls while abstinent drinkers were less sensitive to IL-1β’s effects, suggesting adaptations in the neuromodulatory role of IL-1β. In contrast, CRF enhanced CeA GABA release similarly in controls and abstinent drinkers, consistent with rodent studies. Notably, CeA CRF expression was inversely correlated with intoxication, suggesting that CRF levels during abstinence may predict future intoxication. Together, our findings highlight conserved and divergent actions of chronic alcohol on neuroimmune and stress signaling on CeA GABA transmission across rodents and macaques.
Similar content being viewed by others
Introduction
Alcohol use disorder (AUD) is a chronically relapsing disease characterized by a preoccupation in obtaining and excessively drinking alcohol, resulting in the emergence of a withdrawal syndrome when abstinent that includes, but is not limited to, dysphoria, sleep disturbances, and increases in anxiety and irritability (ICD-10 [1] and DSM-V [2]). Alcohol preoccupation/anticipation, binge/intoxication, and withdrawal/negative affect are three stages conceptualized as a feed-forward cycle that becomes more intense over time, and ultimately leads to the pathological state known as addiction/dependence [3]. Pharmacotherapy and abstinence are used to treat AUD, however the continual risk of relapse prevents many from achieving long-term remission [4, 5]. Thus, there is a need to understand the neural mechanisms that promote relapse to develop more efficacious therapies for AUD.
The development and maintenance of an AUD involves long-lasting neuroadaptations in stress and neuroimmune systems across several brain regions. Extensive preclinical evidence points to a critical role of the central nucleus of the amygdala (CeA) in stress-related disorders, anxiety, and the reinforcing effects of alcohol as well as other drugs of abuse [6, 7]. The CeA, a predominantly GABAergic nucleus, is the major output of the amygdala complex, and is intricately connected to neighboring amygdala nuclei and other addiction-related brain regions [6]. Notably, the CeA is a major extra-hypothalamic source of the brain stress peptide corticotropin-releasing factor (CRF) [8], which is implicated in several alcohol dependence-related behaviors. Long-term alcohol consumption results in persistent dysregulation of CRF signaling in rodents, including increased CRF release during withdrawal in dependent rodents, which contributes to dependence-associated alcohol intake, relapse to alcohol drinking, and withdrawal-induced anxiety [9,10,11,12,13,14,15,16,17]. In addition to stress signaling, the role of neuroimmune signaling in alcohol dependence-related behaviors, such as alcohol reinforcement and associated negative affect, is becoming increasingly evident [18,19,20]. In line with this, acute and chronic alcohol exposure alters both circulating and brain pro-inflammatory cytokine levels [21,22,23]. This includes an upregulation of the interleukin-1 system (IL-1), a prominent pro-inflammatory pathway responsible for the initiation and regulation of immune responses, in the CeA [24]. Human genetic and preclinical studies suggest a critical role for IL-1β signaling in alcohol drinking and dependence [25,26,27,28,29,30]. Together, these findings highlight the central role of CeA stress and neuroimmune signaling in regulating AUD-related behaviors.
At a cellular-level, acute and chronic alcohol increase CeA GABA transmission in rodents [20, 31, 32]. Indeed, potentiated CeA GABA transmission is a hallmark of dependence and dampening this aberrant CeA activity abolishes dependence-related behaviors in rodents [20, 33,34,35,36]. CRF also increases CeA GABA transmission in control and alcohol dependent rodents, primarily by increasing GABA release [31, 32, 37]. In addition, we recently reported that IL-1β modulates CeA GABA transmission displaying bi-directional effects, and its neuromodulatory role is not significantly altered by alcohol dependence [24]. While there is substantial preclinical evidence supporting a role of CeA CRF and emerging evidence for IL-1β signaling in AUD, further assessment of the translational potential of these findings to primates is needed.
The rhesus macaque model of alcohol self-administration provides an important translational link between rodent and human studies. Macaques more closely resemble the alcohol consummatory behavior [38,39,40], alcohol metabolism, endocrine physiology [11] and brain structure [41] of humans compared to rodents. Using this macaque model, we reported altered synaptic transmission following chronic alcohol drinking in multiple brain regions [42,43,44,45]. Notably, CeA neurons from male macaques with a history of chronic alcohol self-administration and repeated abstinence displayed an elevated level of basal GABA release [45]. Moreover, acute alcohol application significantly increased GABA release in controls, but not in abstinent drinkers, suggesting a tolerance to alcohol-enhanced GABA release in macaques with a history of chronic alcohol self-administration and repeated abstinence [45]. These findings replicate some observations in rodent models of alcohol dependence, highlighting both conserved mechanisms of action for alcohol and the specifity of the CeA to chronic alcohol. This provides a unique opportunity to validate cellular-level mechanistic findings from rodent studies in macaques to help bridge the translational gap to humans.
Given the increasing evidence for a prominent role for neuroimmune responses in the neurobiological consequences of chronic alcohol exposure, as well as stress signaling, we sought to determine the effects of long-term alcohol self-administration and abstinence on CeA GABA synapses and their responsivity to IL-1β and CRF. These results are the first to demonstrate distinct synaptic adaptations and sensitivity to IL-1β and CRF in the CeA of male abstinent rhesus macaques.
Materials and methods
For full details see Supplementary Materials.
Animal information
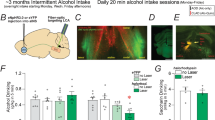
Adult male rhesus macaques (Macaca mulatta) were assigned as alcohol naïve control (n = 6) or alcohol drinker (n = 13), as previously reported [45, 46]. All procedures were in accordance with NIH guidelines for the Care and Use of Laboratory Animals and approved by the Oregon National Primate Research Center Institutional Animal Care and Use Committee. Details for the cohorts used in this study can be found through the Monkey Alcohol and Tissue Research Resource (MATRR.com; Fig. 1A–E and Supplementary Table 1).
A Schematic of the experimental timeline. Following induction, daily 22-h open access sessions with water and alcohol concurrently available occurred. After approximately 14 months of open-access self-administration, animals experienced the first of three abstinence phases, each lasting approximately one month (28–46 days). Alcohol was available during two post-abstinence phases lasting ~3 months. Necropsy was performed after 35–46 days of abstinence. B Average daily alcohol intake during each open-access phase with color-coding for drinking phenotype (white circles—low drinkers, grey circles—high drinkers, red circles—very high drinkers). C Average daily alcohol intake during the third open access period categorized by drinking phenotype. D Average blood ethanol concentration (BEC) during each open-access period. E Average BEC during the third open-access phase categorized by drinking phenotype. F Coronal brain slice depicting the central nucleus of the amygdala (CeA) (in red) from the Scannable Brain Atlas [80]. G Representative image of a recorded CeA neuron (left) and sIPSC traces from a control and abstinent drinker (right). H, I Baseline sIPSC frequency and amplitude averaged within individual controls and abstinent drinkers. J, K Baseline sIPSC frequency and amplitude for each cell from controls and abstinent drinkers. L sIPSC frequency is inversely correlated with BEC in open-access phase 1 (left) but not in open access phase 2 (middle) or open-access phase 3 (right). Low (white circles), high (grey circles), and very heavy drinkers (red circles) are denoted. *p < 0.05, **p < 0.01 by unpaired t test.
Blood plasma measures
Blood was collected, as previously described [47], to measure circulating IL-1β and interleukin-1 receptor 1 antagonist (IL-1ra; Fig. 2) [48] as well as blood ethanol concentration [47] (BEC; Fig. 1D, E).
Self-administration and abstinence
Alcohol induction, open-access to 4% ethanol (v/v), and abstinence occurred as previously described [46, 49] (Fig. 1A). Subjects were categorized based on previous drinking criteria [38] (Fig. 1B–E).
RNA sequencing and differential expression analysis
The CeA was microdissected, RNA was extracted (Qiagen Sciences, Germantown, MD, USA), and samples were sequenced using HiSeq 2500 or a NovaSeq 6000. Differential expression analysis was performed [50]. Gene expression correlations were conducted with spontaneous inhibitory postsynaptic currents (sIPSC) frequency values.
Electrophysiological recordings
Necropsy and tissue collection procedures and electrophysiological recordings were conducted as previously reported [45, 51, 52]. A total of 17 cells were recorded from six individual ethanol-naïve controls; a total of 46 cells were recorded from 13 individual abstinent drinkers. Recombinant IL-1β and CRF were purchased from Biolegend (San Diego, CA) and Tocris Bioscience (Bristol, United Kingdom).
Statistical analysis for spontaneous inhibitory postsyanptic currents
Spontaneous events were analyzed using Mini Analysis, Synaptosoft Inc. Drug effects were calculated from the maximum effect in a 3-min bin between minute 6 and 15 following drug application. Analyses were performed using Prism (GraphPad Software, La Jolla, CA). Data are presented separately for both the individual subjects (i.e., average of cells per macaque) and individual cells by group, which consistently displayed similar trends.
Results
Abstinent alcohol drinkers have enhanced CeA GABA release
The CeA GABA system critically regulates alcohol dependence-related behaviors [17, 20, 34,35,36]. We first assessed basal CeA GABA transmission following long-term alcohol self-administration and repeated forced abstinence (Fig. 1A–E and Supplementary Table 2). Abstinent drinkers had a significantly higher CeA average sIPSC frequency (4.26 ± 0.36 Hz) compared to controls (2.61 ± 0.50 Hz; t(17) = 2.63, p < 0.05 by unpaired t test; Fig. 1F–H), consistent with our previous observations of increased GABA release in macaque and rodent models of dependence [20, 32, 45]. No differences were found between drinking categories, so all abstinent drinkers were combined for these analyses. Thus, above a threshold of 2.0 g alcohol/kg/day there is no difference in CeA GABA release, such that the threshold to trigger synaptic adaptation was achieved in even the lowest drinker. Of note, we found an inverse correlation between sIPSC frequencies and average BECs during the first open-access phase, but no relationships in the second and third open-access phases (Fig. 1L and Supplementary Tables 3 and 4), though future studies are needed to elucidate whether there is a causal link between the two factors.
Given the cellular heterogeneity in the CeA, averaging data from CeA neurons within individuals may mask important effects. Therefore, the data were also examined on a cellular basis by averaging data from CeA neurons from all abstinent drinkers and all controls. Similar to the subject-grouped data, the average sIPSC frequency of all CeA neurons was significantly higher in abstinent drinkers (4.71 ± 0.41 Hz) compared to ethanol-naïve controls (2.33 ± 0.30 Hz; t(57)=2.99, p < 0.01 by unpaired t test; Fig. 1J). There were no differences in sIPSC amplitude, rise or decay time between controls and abstinent drinkers when compared at the subject level or cellular level (Fig. 1I, K; see Supplementary Table 2 for full summary). An increase in sIPSC frequency is associated with greater probability of GABAergic synaptic vesicle fusion and/or more synapses, while changes in amplitude, rise time or decay time are typically linked to postsynaptic GABAA receptor function. Therefore, long-term alcohol self-administration and repeated abstinence is associated with persistent pre-synaptic adaptations in the CeA of macaques, leading to an enhanced basal inhibitory tone.
Long-term alcohol exposure and abstinence does not alter peripheral IL-1β levels or the CeA transcriptome
Alterations in peripheral and central IL-1β have been observed in humans with AUD [53, 54], suggesting that chronic alcohol exposure induces persistent inflammatory responses. We sought to determine if similar immune responses occur in the macaque model. We measured circulating levels of IL-1β and interleukin-1 receptor antagonist (IL-1ra), an endogenous antagonist of the IL-1 receptor [29, 55], following necropsy and found no difference between controls and abstinent drinkers (IL-1β: t(16) = 1.15, p = 0.27; IL-1ra: t(16) = 1.10, p = 0.29; Fig. 2A, B). In addition, peripheral IL-1β and IL-1ra levels collected at necropsy did not correlate with ethanol intake or BEC during any of the open-access periods, total lifetime alcohol intake, or baseline sIPSC frequency (data not shown). This suggests that the peripheral IL-1β system is not significantly altered in abstinence following long-term alcohol exposure and repeated abstinence in macaques.
To examine local CeA molecular adaptations, we sequenced mRNA isolated from the contralateral CeA tissue than was used for ex vivo electrophysiology to compare the CeA transcriptome between controls and abstinent drinkers. We detected 17,108 genes and found no significant differentially expressed genes (DEGs) between control and abstinent drinkers after false discovery rate (FDR) correction (Fig. 2C, D). This was unsurprising given the high degree of variability and overlap in samples from controls and abstinent drinkers (Supplementary Fig. 1). Although not statistically significant, subtle yet concordant changes in genes belonging to the same pathways can help to reveal biological implications of abstinent drinking. To identify potential pathways that may be dysregulated in abstinent drinkers, we performed Ingenuity Pathway Analysis using significant DEGs prior to FDR correction. We identified several canonical pathways related to immune signaling including the Th1 and Th2 pathways, IL-17 signaling, CD40 signaling, among others supporting a stronger immune response in the CeA of abstinent drinkers compared to controls (Fig. 2C, Supplementary Fig. 2). Together, these findings highlight the complex interaction between individual genetic makeup and alcohol exposure, and further underscores the importance of validating findings from preclinical rodent studies in macaques.
IL-1β reduces CeA GABA release in controls and to a lesser degree in abstinent drinkers
In our previous study using a mouse model of alcohol dependence, we observed increased CeA microglial and neuronal IL-1β levels in dependent mice compared to non-dependent and control mice [24]. In addition, IL-1β bi-directionally regulated CeA GABA transmission, and alcohol dependence did not alter this pattern of neuromodulation [24]. To determine whether these observations are conserved across species, we tested the effects of IL-1β (50 ng/mL [24, 29, 56]) on CeA sIPSCs in the macaque. We found a trend toward IL-1β decreasing CeA sIPSC frequency from 4.84 ± 1.60 Hz to 3.14 ± 1.34 Hz in controls (t(2) = 3.55, p = 0.071 by paired t test; Fig. 3A, B). Similar to the subject data, IL-1β significantly reduced the average sIPSC frequency of individual CeA neurons from control macaques (t(7) = 2.69, p < 0.05 by paired t test; Fig. 3D). There were no other differences in sIPSC characteristics at the subject or cellular levels (Fig. 3C, E; rise and decay time data not shown). These data suggest that IL-1β dampens CeA GABAergic pre-synaptic activity in ethanol-naïve control macaques.
A Representative image of a recorded CeA neuron from a control macaque (left) and sIPSC traces during baseline conditions and during IL-1β (50 ng/mL) application (right). B, C Average sIPSC frequency and amplitude before and during IL-1β application for each control macaque. D, E sIPSC frequency and amplitude before and during IL-1β application for each cell from control macaques. F Representative image of a recorded CeA neuron from an abstinent drinker (left) and sIPSC traces during baseline conditions and during IL-1β application (right). G, H Average sIPSC frequency and amplitude before and during IL-1β application for each drinker macaque. Colored circles represent cells from the same subject. I, J sIPSC frequency and amplitude before and during IL-1β application for all cells from abstinent drinkers. K, L Comparison of IL-1β effects on sIPSC frequency and amplitude between controls and abstinent drinkers. M, N Comparison of IL-1β effects on sIPSC frequency and amplitude by cell between controls and abstinent drinkers. *p < 0.05, ****p < 0.0001 by one-sample, paired, or unpaired t test.
IL-1β had similar effects in the CeA of abstinent drinkers. There was a trend toward IL-1β decreasing sIPSC frequency by subject (t(9) = 2.02, p = 0.074 by paired t-test; Fig. 3F, G), and IL-1β significantly reduced the average sIPSC frequency of individual CeA neurons (t(15) = 2.30, p < 0.05 by paired t-test; Fig. 3I). No differences in other sIPSC characteristics were observed (sIPSC amplitude: Fig. 3H, J; rise and decay time data not shown). Additionally, correlation analyses revealed no relationship between IL-1β’s effect on sIPSC frequencies and average BECs or ethanol intake for individual subjects during each of the three open-access phases (Supplementary Tables 3 and 4).
To assess potential differences in the magnitude of IL-1β’s effect in controls and abstinent drinkers, we normalized the effect of IL-1β to a baseline period for each cell. At the subject level, there was a trend toward a difference in IL-1β’s effects (t(11) = 2.06, p = 0.063 by unpaired t-test), with IL-1β decreasing sIPSC frequency to 59.6 ± 8.8% (t(2) = 4.6, p < 0.05 by one-sample t-test) of baseline in controls and to 88.1 ± 7.0% (t(9) = 1.7, p = 0.12 by one-sample t-test) in abstinent alcohol drinkers (Fig. 3K). At the cellular level, the magnitude of IL-1β’s effect was significantly smaller in the abstinent drinkers (controls: 60.7 ± 4.1%, t(7) = 9.57, p < 0.0001 by one-sample t-test; abstinent drinkers: 86.6 ± 6.6%, t(15) = 2.02, p = 0.06 by one-sample t-test; t(22) = 2.60, p < 0.05 by unpaired t-test; Fig. 3M). This may be in part due to the greater diversity of responses to IL-1β observed in abstinent drinkers, with some cells demonstrating an increase or no change in sIPSC frequency. Overall, these data suggest that CeA GABAergic synapses are, on average, less sensitive to the dampening/inhibitory effects of IL-1β following long-term alcohol abstinence.
CRF enhances CeA GABA release similarly in controls and abstinent drinkers
Given the role of CeA CRF signaling in escalated alcohol drinking [34], and that CRF (200 nM [34, 57]) regulates CeA GABA transmission in both alcohol-naïve controls and alcohol-dependent rodents [34, 57], here we examined the impact of CRF on macaque CeA. We found that CRF had similar effects in the CeA of controls and abstinent drinkers, increasing the sIPSC frequency in both groups at the subject (control: t(2) = 3.92, p = 0.059; abstinent drinker: t(11) = 2.87, p < 0.05 by paired t-test) and cellular levels (control: t(5) = 3.98, p < 0.05; abstinent drinker: t(13) = 2.55, p < 0.05 by paired t-test; Fig. 4A–J). Correlation analyses revealed no relationship between CRF’s effects on subject sIPSC frequencies and average BECs or ethanol intake during each of the three open-access phases (Supplementary Tables 3 and 4). The magnitude of CRF’s effects on the sIPSC frequency were also similar across both groups (subject: t(13) = 0.29, p = 0.78 by unpaired t-test; cell: t(17) = 0.41, p = 0.69 by unpaired t test; Fig. 4K, M). Thus, CRF-induced potentiation of CeA GABA release is not altered after repeated abstinence, consistent with the similar expression levels of the CRF signaling pathway in controls and abstinent drinkers (Figs. 2 and 5).
A Representative image of a recorded CeA neuron from a control macaque (left) and sIPSC traces during baseline conditions and after CRF (200 nM) application (right). B, C Average sIPSC frequency and amplitude before and during CRF application for each control macaque. D, E sIPSC frequency and amplitude before and after CRF application for each cell from control macaques. F Representative image of a recorded CeA neuron from an abstinent drinker (left) and sIPSC traces during baseline conditions and during CRF application (right). G, H Average sIPSC frequency and amplitude before and during CRF application for each drinker macaque. Colored circles represent cells from the same subject. I, J sIPSC frequency and amplitude before and during CRF application for each cell from drinker macaques. K, L Comparison of CRF effects on sIPSC frequency and amplitude between control and abstinent drinkers. M, N Comparison of CRF effects on sIPSC frequency and amplitude by cell between controls and abstinent drinkers. *p < 0.05, **p < 0.01, ***p < 0.001 by one-sample, paired, or unpaired t test.
A Expression of the CRF signaling pathway is not significantly different between controls and drinkers. B CRF expression trends toward a significant correlation with sIPSC frequency. C CRF expression is inversely correlated with blood ethanol concentration from all open-access phases in low (white circles), high (grey circles), and very heavy drinkers (red circles).
Notably, our RNA sequencing results showed that CRF gene expression in the CeA of control and abstinent drinkers trended (r2 = 0.2; p = 0.059) toward a correlation with sIPSC frequency (Fig. 5B), suggesting that CRF may partly contribute to basal differences in sIPSC frequency. This was in line with findings in rodents demonstrating that CRF increases CeA GABA release [58], and that alcohol dependence increased both CeA CRF expression and GABA release [34]. Interestingly, CeA CRF expression inversely correlated with average BEC for each of the three open-access phases (Fig. 5C). Given no significant differences in expression of CRF between controls and abstinent drinkers, these data suggest that CeA CRF levels may be predictive of future drinking levels.
Discussion
In this study, we sought to determine the impact of long-term alcohol drinking with cycles of abstinence and relapse on CeA neuroimmune and stress signaling at GABA synapses in male rhesus macaques. These studies were motivated by previous findings in rodent models of AUD supporting a critical role of CeA IL-1β and CRF in regulating GABAergic transmission and AUD-related behaviors. We found elevated CeA GABA release in abstinent drinkers, replicating a central finding in rodent studies that has been directly linked to escalated alcohol intake. This conserved adaptation points to the sensitivity of the CeA to alcohol, highlighting the importance of conducting cross-species studies. In the present study, we also identified an inverse relationship between the BECs achieved during the initial open access alcohol drinking phase and the degree of CeA synaptic adaptation after repeated cycles of long-term alcohol use and abstinence. In contrast to findings in rodents and humans, we observed no changes in the peripheral or local CeA IL-1β system in this macaque model. Moreover, there were no significant differences in the CeA transcriptome between controls and abstinent drinkers due to the high degree of variability and overlap, suggesting a complex interaction between individual genetic makeup and alcohol intake. Despite the lack of striking single-gene transcriptomic differences, pathway analysis of significant differentially expressed genes prior to FDR correction identified several immune-related signaling pathways in the CeA impacted by abstinence after long-term alcohol self-administration. This CeA neuroimmune dysregulation is supported by observed synaptic adaptations in sensitivity to the effects of IL-1β on GABA release, where IL-1β uniformly dampened CeA GABA release in controls and displayed more heterogenous responses with an overall reduced inhibition of GABA release in abstinent drinkers. These findings contrast with CeA CRF stress signaling which increased CeA GABA release and was unaltered by alcohol, consistent with no overall differences in expression levels of the CeA CRF signaling pathway. Notably, CeA CRF expression trended, but did not reach significance, toward a direct correlation with basal GABA release, suggesting that CRF may mechanistically underlie differences in CeA GABA release. In addition, we identified a novel inverse relationship between BECs and CeA CRF levels, suggesting that its expression in protracted abstinence may predict the amount of alcohol consumed during relapse. Together, these findings are the first to demonstrate distinct synaptic adaptations in response to IL-1β and CRF in the CeA of abstinent macaque males with a history of alcohol drinking and abstinence.
Acute and chronic alcohol increase CeA GABA release during acute withdrawal on the order of hours, days, or a couple of weeks in rodents [20, 31, 32], highlighting the sensitivity of CeA GABA synapses to the direct and indirect actions of alcohol. Notably, the current study and our previous work, revealed enhancement of CeA GABA release in macaques during protracted (several weeks) abstinence following long-term alcohol use and repeated abstinence [45]. These findings demonstrate the persistence of this conserved neuroadaptation. Of note, in the current study, we combined data from low, heavy, and very heavy drinkers as no significant differences were observed among the groups. Since spontaneous inhibitory post-synaptic current characteristics did not directly correlate with alcohol intake, heightened CeA GABA release may instead reflect a “primed” CeA state that occurs after long-term alcohol exposure regardless of daily ethanol intakes above a threshold amount. Indeed, heightened CeA GABA transmission has been linked with dependence-related escalation of alcohol drinking in rodents [20, 33,34,35,36], suggesting that this neuroadaptation may confer persistent vulnerability to relapsing alcohol use. The lack of differential gene expression in the CeA between controls and abstinent drinkers suggests that changes in CeA GABA transmission may be due to either 1) upstream GABA projections into the CeA or 2) local neuromodulator signaling, such as IL-1, regulating GABA release and/or recycling mechanisms. Identifying key regulators underlying alcohol’s potentiation of CeA GABA release may provide novel targets for therapeutic intervention to decrease relapse to heavy drinking.
Neuroimmune signaling is of significant interest, as alcohol elicits immune responses which can persist with repeated alcohol exposure and may contribute to the development and maintenance of an AUD. Plasma/circulating cytokine levels represent an opportunity to identify potential biomarkers predictive of disease-related conditions/states such as degree of alcohol use or alcohol-induced tissue damage. IL-1β, a prominent proinflammatory cytokine responsible for the initiation and maintenance of the neuroimmune response, has been investigated as a biomarker in several diseases [48, 59,60,61,62] and is peripherally elevated in patients with an AUD [63]. However, we did not observe changes in circulating IL-1β and IL-1ra levels in macaques at necropsy following long-term alcohol use and repeated abstinence, which differs from rodent models of alcohol dependence [20, 24, 64]. While these findings demonstrate potential differences in alcohol-induced peripheral immune responses across species, cytokine signaling is highly dynamic [65]. How IL-1β signaling adapts across alcohol exposure and abstinence remains unclear, which may also depend on the type of alcohol exposure/administration/paradigm. It is possible that protracted abstinence normalizes peripheral IL-1β levels, in which case long-term abstinence may reverse alcohol-induced peripheral inflammation. In line with this, alcohol abstinence in humans with AUD largely reverses plasma cytokine abnormalities [66]. Notably, these alcohol-induced plasma cytokines abnormalities are not observed in heavy drinking individuals without alcoholic hepatitis, suggesting that alcohol-induced peripheral tissue damage may be an important factor contributing to elevations in plasma cytokines [66]. Together, this suggests that drinking levels in this macaque model do not lead to persistent changes in peripheral IL-1β, although peripheral IL-1 receptor 1 gene expression is downregulated in female macaques following chronic heavy drinking [67], and likely do not induce tissue damage, which may be predicted by elevated peripheral IL-1β.
Neuroimmune signaling is increasingly being recognized as a key regulator of synaptic transmission under both physiological and pathological conditions [68]. IL-1R1 is expressed in the rodent CeA under basal conditions [69], while IL-1β is induced in CeA neurons and microglia following systemic excitotoxic or inflammatory responses, as well as during intoxication in alcohol-dependent rodents [24, 70, 71]. Of note, we did not observe any differences in IL-1β levels in abstinent drinkers in our CeA transcriptomic data. This may be due to differences in the timepoint at which we measured IL1-β levels. Alterations in the IL-1 system induced by chronic ethanol may have normalized during the protracted abstinence period, 35–46 days after the last self-administration session. We have also previously shown that IL-1β plays a neuromodulatory role in the mouse under basal conditions, IL-1ra modulates CeA GABAergic transmission in naïve mice suggesting that basal IL-1 (α and/or β) levels are sufficient to tonically modulate CeA neurons [24]. Moreover, IL-1β displays dual effects on GABA transmission, leading to inhibition of some and disinhibition of other CeA neurons in both control and dependent mice. In contrast, here we found that IL-1β uniformly disinhibited CeA neurons in ethanol-naive macaques, suggesting that this neuromodulatory role of IL-1β is not conserved across species. It is important to note that the sample size (both in terms of subjects and cells) of the control group is limited, which may mask potential inter-individual variability. Interestingly, we observed a reduction in CeA GABA synapse sensitivity to the effects of IL-1β in abstinent drinkers, similar to the dependent mice. This was due to the development of heterogeneity in responses to IL-1β, perhaps due to IL-1β acting at both the synapse (i.e. pre-synaptic terminals) and network (i.e. regulation of action potential firing) levels to ultimately modulate GABA release. While overall CeA IL-1β and IL-1ra gene expression was unaltered in the current study, it is still possible that IL-1β and IL-1ra protein expression and/or IL-1β release are changed (similar to our observations with NHP CeA GABA in this study). Alternatively, there could be functional changes in other IL-1 effectors, including the IL-1R1 or downstream intracellular PI3K/AKT and MyD88/p38 MAPK cascades [55, 72, 73]. Regardless, IL-1 signaling is important for regulating behavior under physiological conditions [74, 75]. Therefore, despite no change in IL-1β/IL-1ra, a decrease in GABA synapse sensitivity to IL-1 could alter behavior through downstream mechanisms.
The macaques studied here encompasses a greater degree of genetic variability, compared to isogenic rodent models, perhaps contributing to the complex interaction of alcohol and genetic makeup. In the mouse model of alcohol dependence, we observed a significant increase in CeA IL-1β levels [24], suggesting a local, central neuroimmune response to chronic alcohol exposure, although only a trend toward increased microglial numbers in the CeA [76]. While we did not observe significant differences in the CeA transcriptome between controls and abstinent drinkers, pathway analysis identified several immune-related signaling pathways, supporting a potential dysregulation of CeA neuroimmune signaling. This is consistent with the observed neuroadaptations in IL-1β’s effect on CeA GABA release in abstinent drinkers, and suggests its potential contribution to CeA dysregulation and further investigation.
In addition to neuroimmune signaling, CeA CRF stress signaling also plays a critical role in AUD-related behaviors in preclinical rodent studies [9,10,11,12,13,14,15,16,17, 77, 78]. CRF signaling facilitates CeA GABA transmission similarly across ethanol-naïve macaques and rodents. However, divergent neuroadaptations in CRF effects were observed in macaques and rodents following chronic alcohol exposure, wherein CRF’s facilitatory effect was unchanged in abstinent macaques but potentiated in dependent rats [34]. Note, dependent rats were generated using an ethanol inhalation model and CRF effects were examined during acute (2–8 h) of withdrawal. Interestingly, we also found that terminal CeA CRF expression indirectly correlates with average blood ethanol concentration from each of the three open access periods. Since overall CeA CRF expression was not different between controls and abstinent drinkers, this suggests that long-term alcohol exposure may not significantly impact the expression of the CeA CRF system; though, it is also possible that protracted abstinence, when CRF levels were measured, normalizes CRF expression. Thus, CeA CRF levels may be predictive of an alcohol intake pattern that leads to a preferred blood ethanol concentration setpoint in these male macaques, wherein high levels of CeA CRF correlated with lower average BECs achieved. While this is counterintuitive to rodent studies that suggest that dampening CeA CRF levels or CRF1 receptor activity decreases alcohol consumption after short abstinence periods of several hours [16, 17, 34], these findings provide insight into potential mechanistic and temporal differences contributing to the so far low clinical efficacy of CRF1 antagonists for treatment of AUD [79], further underscoring the importance of studies in macaques.
Overall, our results provide support for the macaque model of alcohol self-administration as a critical translational tool to study mechanistic links between rodent and human studies. Here we recapitulated that inhibitory signaling is increased in the CeA of abstinent male macaques with a history of chronic alcohol self-administration. Application of IL-1β and CRF uniformly decreased and increased CeA GABA transmission, respectively, in ethanol-naïve controls. However, the CeA GABAergic synapses of abstinent drinkers displayed a shift in their sensitivity to IL-1β with an overall decreased sensitivity to its inhibitory effect, and no change in the effects of CRF on GABA transmission. These divergent synaptic adaptations point to a potentially critical role of CeA neuroimmune signaling to the state of the brain vulnerable to relapsing drug use, and to the need for future studies in animal models that more closely resemble human alcohol use to elucidate the underlying specific mechanisms contributing to AUD.
References
World Health Organization, International statistical classification of diseases and related health problems. (2016).
American Psychiatric Association, Diagnostic and statistical manual of mental disorders. (2013).
Wise RA, Koob GF. The development and maintenance of drug addiction. Neuropsychopharmacology. 2014;39:254–62.
Batra A, Muller CA, Mann K, Heinz A. Alcohol dependence and harmful use of alcohol. Dtsch Arztebl Int. 2016;113:301–10.
Schellekens AF, de Jong CA, Buitelaar JK, Verkes RJ. Co-morbid anxiety disorders predict early relapse after inpatient alcohol treatment. Eur Psychiatry. 2015;30:128–36.
Gilpin NW, Herman MA, Roberto M. The central amygdala as an integrative hub for anxiety and alcohol use disorders. Biol Psychiatry. 2015;77:859–69.
Koob GF. Neuroadaptive mechanisms of addiction: studies on the extended amygdala. Eur Neuropsychopharmacol. 2003;13:442–52.
Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34.
Adinoff B, et al. Dissection of hypothalamic-pituitary-adrenal axis pathology in 1-month-abstinent alcohol-dependent men, part 2: response to ovine corticotropin-releasing factor and naloxone. Alcohol Clin Exp Res. 2005;29:528–37.
Breese GR, Sinha R, Heilig M. Chronic alcohol neuroadaptation and stress contribute to susceptibility for alcohol craving and relapse. Pharm Ther. 2011;129:149–71.
Jimenez VA, Grant KA. Studies using macaque monkeys to address excessive alcohol drinking and stress interactions. Neuropharmacology. 2017;122:127–35.
Tunstall BJ, Carmack SA, Koob GF, Vendruscolo LF. Dysregulation of brain stress systems mediates compulsive alcohol drinking. Curr Opin Behav Sci. 2017;13:85–90.
Merlo Pich E, et al. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J Neurosci. 1995;15:5439–47.
Rassnick S, Heinrichs SC, Britton KT, Koob GF. Microinjection of a corticotropin-releasing factor antagonist into the central nucleus of the amygdala reverses anxiogenic-like effects of ethanol withdrawal. Brain Res. 1993;605:25–32.
Overstreet DH, Knapp DJ, Breese GR. Modulation of multiple ethanol withdrawal-induced anxiety-like behavior by CRF and CRF1 receptors. Pharm Biochem Behav. 2004;77:405–13.
Funk CK, Zorrilla EP, Lee MJ, Rice KC, Koob GF. Corticotropin-releasing factor 1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biol Psychiatry. 2007;61:78–86.
Varodayan FP, de Guglielmo G, Logrip ML, George O, Roberto M. Alcohol dependence disrupts amygdalar L-type voltage-gated calcium channel mechanisms. J Neurosci. 2017;37:4593–603.
Blednov YA, et al. Neuroimmune regulation of alcohol consumption: behavioral validation of genes obtained from genomic studies. Addict Biol. 2012;17:108–20.
Woelfer M, Kasties V, Kahlfuss S, Walter M. The role of depressive subtypes within the neuroinflammation hypothesis of major depressive disorder. Neuroscience. 2019;403:93–110.
Patel, RR, et al. IL-10 normalizes aberrant amygdala GABA transmission and reverses anxiety-like behavior and dependence-induced escalation of alcohol intake. Prog Neurobiol. 101952 (2020).
Roberto, M, Patel, RR & Bajo, M. Ethanol and cytokines in the central nervous system. Handbook of Experimental Pharmacology. (2017).
Gano A, Doremus-Fitzwater TL, Deak T. Sustained alterations in neuroimmune gene expression after daily, but not intermittent, alcohol exposure. Brain Res. 2016;1646:62–72.
Erickson EK, Grantham EK, Warden AS, Harris RA. Neuroimmune signaling in alcohol use disorder. Pharm Biochem Behav. 2019;177:34–60.
Patel RR, et al. IL-1beta expression is increased and regulates GABA transmission following chronic ethanol in mouse central amygdala. Brain Behav Immun. 2019;75:208–19.
Pastor IJ, Laso FJ, Romero A, Gonzalez-Sarmiento R. Interleukin-1 gene cluster polymorphisms and alcoholism in Spanish men. Alcohol Alcohol. 2005;40:181–6.
Mulligan MK, et al. Toward understanding the genetics of alcohol drinking through transcriptome meta-analysis. Proc Natl Acad Sci USA. 2006;103:6368–73.
Marshall SA, et al. IL-1 receptor signaling in the basolateral amygdala modulates binge-like ethanol consumption in male C57BL/6J mice. Brain Behav Immun. 2016;51:258–67.
Breese GR, et al. Repeated lipopolysaccharide (LPS) or cytokine treatments sensitize ethanol withdrawal-induced anxiety-like behavior. Neuropsychopharmacology. 2008;33:867–76.
Bajo M, et al. IL-1 interacts with ethanol effects on GABAergic transmission in the mouse central amygdala. Front Pharm. 2015;6:49.
Bajo M, et al. Innate immune factors modulate ethanol interaction with GABAergic transmission in mouse central amygdala. Brain Behav Immun. 2014;40:191–202.
Roberto M, Madamba SG, Moore SD, Tallent MK, Siggins GR. Ethanol increases GABAergic transmission at both pre- and postsynaptic sites in rat central amygdala neurons. Proc Natl Acad Sci USA. 2003;100:2053–8.
Roberto M, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Increased GABA release in the central amygdala of ethanol-dependent rats. J Neurosci. 2004;24:10159–66.
Roberto M, Gilpin NW, Siggins GR. The central amygdala and alcohol: role of gamma-aminobutyric acid, glutamate, and neuropeptides. Cold Spring Harb Perspect Med. 2012;2:a012195.
Roberto M, et al. Corticotropin releasing factor-induced amygdala gamma-aminobutyric acid release plays a key role in alcohol dependence. Biol Psychiatry. 2010;67:831–9.
Hyytia P, Koob GF. GABAA receptor antagonism in the extended amygdala decreases ethanol self-administration in rats. Eur J Pharm. 1995;283:151–9.
Gilpin NW, Roberto M. Neuropeptide modulation of central amygdala neuroplasticity is a key mediator of alcohol dependence. Neurosci Biobehav Rev. 2012;36:873–88.
Herman MA, Contet C, Justice NJ, Vale W, Roberto M. Novel subunit-specific tonic GABA currents and differential effects of ethanol in the central amygdala of CRF receptor-1 reporter mice. J Neurosci. 2013;33:3284–98.
Baker EJ, Farro J, Gonzales S, Helms C, Grant KA. Chronic alcohol self-administration in monkeys shows long-term quantity/frequency categorical stability. Alcohol Clin Exp Res. 2014;38:2835–43.
Baker EJ, et al. Identifying future drinkers: behavioral analysis of monkeys initiating drinking to intoxication is predictive of future drinking classification. Alcohol Clin Exp Res. 2017;41:626–36.
Mello NK, Mendelson JH. Drinking patterns during work-contingent and noncontingent alcohol acquisition. Psychosom Med. 1972;34:139–64.
Miranda-Dominguez O, et al. Bridging the gap between the human and macaque connectome: a quantitative comparison of global interspecies structure-function relationships and network topology. J Neurosci. 2014;34:5552–63.
Nimitvilai S, et al. Orbitofrontal neuroadaptations and cross-species synaptic biomarkers in heavy-drinking macaques. J Neurosci. 2017;37:3646–60.
Pleil KE, et al. Effects of chronic alcohol consumption on neuronal function in the non-human primate BNST. Addict Biol. 2016;21:1151–67.
Welsh JP, et al. Bidirectional plasticity in the primate inferior olive induced by chronic ethanol intoxication and sustained abstinence. Proc Natl Acad Sci USA. 2011;108:10314–9.
Jimenez VA, et al. Synaptic adaptations in the central amygdala and hypothalamic paraventricular nucleus associated with protracted ethanol abstinence in male rhesus monkeys. Neuropsychopharmacology. 2019;44:982–93.
Allen DC, Gonzales SW, Grant KA. Effect of repeated abstinence on chronic ethanol self-administration in the rhesus monkey. Psychopharmacol (Berl). 2018;235:109–20.
Jimenez VA, Helms CM, Cornea A, Meshul CK, Grant KA. An ultrastructural analysis of the effects of ethanol self-administration on the hypothalamic paraventricular nucleus in rhesus macaques. Front Cell Neurosci. 2015;9:260.
Sureshchandra S, et al. Dose-dependent effects of chronic alcohol drinking on peripheral immune responses. Sci Rep. 2019;9:7847.
Vivian JA, et al. Induction and maintenance of ethanol self-administration in cynomolgus monkeys (Macaca fascicularis): long-term characterization of sex and individual differences. Alcohol Clin Exp Res. 2001;25:1087–97.
Team, RCR: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. (2017).
Cuzon Carlson VC, Grant KA, Lovinger DM. Synaptic adaptations to chronic ethanol intake in male rhesus monkey dorsal striatum depend on age of drinking onset. Neuropharmacology. 2018;131:128–42.
Cuzon Carlson VC, et al. Synaptic and morphological neuroadaptations in the putamen associated with long-term, relapsing alcohol drinking in primates. Neuropsychopharmacology. 2011;36:2513–28.
Coleman LG Jr, Zou J, Qin L, Crews FT. HMGB1/IL-1beta complexes regulate neuroimmune responses in alcoholism. Brain Behav Immun. 2018;72:61–77.
Achur RN, Freeman WM, Vrana KE. Circulating cytokines as biomarkers of alcohol abuse and alcoholism. J Neuroimmune Pharm. 2010;5:83–91.
Krumm B, Xiang Y, Deng J. Structural biology of the IL-1 superfamily: key cytokines in the regulation of immune and inflammatory responses. Protein Sci. 2014;23:526–38.
Bajo, M, et al. Role of MyD88 in IL-1beta and ethanol modulation of GABAergic transmission in the central amygdala. Brain Sci. 2019;9:361.
Varodayan FP, Logrip ML, Roberto M. P/Q-type voltage-gated calcium channels mediate the ethanol and CRF sensitivity of central amygdala GABAergic synapses. Neuropharmacology. 2017;125:197–206.
Wolfe, SA, et al. Molecular, morphological, and functional characterization of corticotropin-releasing factor receptor 1-expressing neurons in the central nucleus of the amygdala. eNeuro. 2019;6:ENEURO.0087-19.2019.
Idris A, Ghazali NB, Koh D. Interleukin 1beta-A potential salivary biomarker for cancer progression? Biomark Cancer. 2015;7:25–29.
Su C, Zhao K, Xia H, Xu Y. Peripheral inflammatory biomarkers in Alzheimer’s disease and mild cognitive impairment: a systematic review and meta-analysis. Psychogeriatrics. 2019;19:300–9.
Choi J, et al. Serum alpha-synuclein and IL-1beta are increased and correlated with measures of disease severity in children with epilepsy: potential prognostic biomarkers? BMC Neurol. 2020;20:85.
Barr T, et al. Alcohol consumption modulates host defense in Rhesus Macaques by altering gene expression in circulating leukocytes. J Immunol. 2016;196:182–95.
Khoruts A, Stahnke L, McClain CJ, Logan G, Allen JI. Circulating tumor necrosis factor, interleukin-1 and interleukin-6 concentrations in chronic alcoholic patients. Hepatology. 1991;13:267–76.
Xu, J, et al. Blockade of IL-17 signaling reverses alcohol-induced liver injury and excessive alcohol drinking in mice. JCI Insight. 2020;5:e131277.
Doremus-Fitzwater TL, et al. Intoxication- and withdrawal-dependent expression of central and peripheral cytokines following initial ethanol exposure. Alcohol Clin Exp Res. 2014;38:2186–98.
Li W, et al. Alcohol abstinence ameliorates the dysregulated immune profiles in patients with alcoholic hepatitis: a prospective observational study. Hepatology. 2017;66:575–90.
Sureshchandra S, Rais M, Stull C, Grant K, Messaoudi I. Transcriptome profiling reveals disruption of innate immunity in chronic heavy ethanol consuming female Rhesus Macaques. PLoS One. 2016;11:e0159295.
Nistico R, et al. Synaptoimmunology—roles in health and disease. Mol Brain. 2017;10:26.
Frost P, Barrientos RM, Makino S, Wong ML, Sternberg EM. IL-1 receptor type I gene expression in the amygdala of inflammatory susceptible Lewis and inflammatory resistant Fischer rats. J Neuroimmunol. 2001;121:32–39.
Eriksson C, Tehranian R, Iverfeldt K, Winblad B, Schultzberg M. Increased expression of mRNA encoding interleukin-1beta and caspase-1, and the secreted isoform of interleukin-1 receptor antagonist in the rat brain following systemic kainic acid administration. J Neurosci Res. 2000;60:266–79.
Konsman JP, et al. Central nervous action of interleukin-1 mediates activation of limbic structures and behavioural depression in response to peripheral administration of bacterial lipopolysaccharide. Eur J Neurosci. 2008;28:2499–510.
O’Neill LA, Greene C. Signal transduction pathways activated by the IL-1 receptor family: ancient signaling machinery in mammals, insects, and plants. J Leukoc Biol. 1998;63:650–7.
Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: back to the future. Immunity. 2013;39:1003–18.
Krueger JM. The role of cytokines in sleep regulation. Curr Pharm Des. 2008;14:3408–16.
Depino AM, et al. Learning modulation by endogenous hippocampal IL-1: blockade of endogenous IL-1 facilitates memory formation. Hippocampus. 2004;14:526–35.
Warden AS, Wolfe SA, Khom S, Varodayan FP, Patel RR, Steinman MQ, et al. Microglia control escalation of drinking in alcohol dependent mice: Genomic and synaptic drivers. Biol Psychiatry. 2020;88:910–21.
Olive MF, Koenig HN, Nannini MA, Hodge CW. Elevated extracellular CRF levels in the bed nucleus of the stria terminalis during ethanol withdrawal and reduction by subsequent ethanol intake. Pharm Biochem Behav. 2002;72:213–20.
Chu K, Koob GF, Cole M, Zorrilla EP, Roberts AJ. Dependence-induced increases in ethanol self-administration in mice are blocked by the CRF1 receptor antagonist antalarmin and by CRF1 receptor knockout. Pharm Biochem Behav. 2007;86:813–21.
Pomrenze MB, Fetterly TL, Winder DG, Messing RO. The corticotropin releasing factor receptor 1 in alcohol use disorder: still a valid drug target? Alcohol Clin Exp Res. 2017;41:1986–99.
Bakker R, Tiesinga P, Kotter R. The scalable brain atlas: instant web-based access to public brain atlases and related content. Neuroinformatics. 2015;13:353–66.
Acknowledgements
We thank Tim Carlson and Samuel Shin for their assistance and hospitality and Steven Gonzales for exceptional technical support and data processing. Short read sequencing assays were performed by the OHSU Massively Parallel Sequencing Shared Resource. This is manuscript number 30119 from The Scripps Research Institute. This research was supported in part by grants from the National Institutes of Health: INIA U01 AA013498 (MR), AA025408 (FPV), AA019431 (KAG), AA013641 (KAG), AA006420 (MR), AA15566 (MR), AA027700 (MR), AA021491 (MR) and INIA U01 AA013510 (KAG). The Oregon National Primate Research Center cores (Primate Genetics and Bioinformatics & Biostatistics) were supported by P51 OD011092 and P60 AA010760. The authors have no disclosures.
Author information
Authors and Affiliations
Contributions
RRP, FPV, MAH, KAG, and MR designed and conceived the project. KAG, VJ, NW and VCC generated macaque subjects for this study. RRP, FPV, and MAH collected electrophysiology data. RRP, FPV, MAH, MB and MR analyzed electrophysiology data. RA, LG, and SSF collected and analyzed the sequencing data. RRP, FPV, MR drafted the figures and manuscript. RRP, FPV, MAH, VJ, RA, MB, VCC, SSF, KAG, MR edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Patel, R.R., Varodayan, F.P., Herman, M.A. et al. Synaptic effects of IL-1β and CRF in the central amygdala after protracted alcohol abstinence in male rhesus macaques. Neuropsychopharmacol. 47, 847–856 (2022). https://doi.org/10.1038/s41386-021-01231-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-021-01231-y